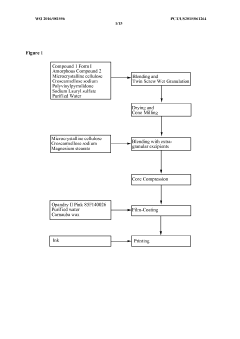
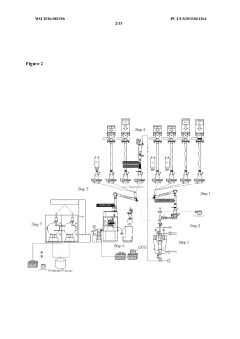
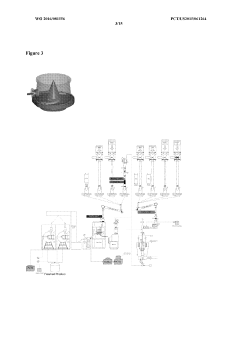
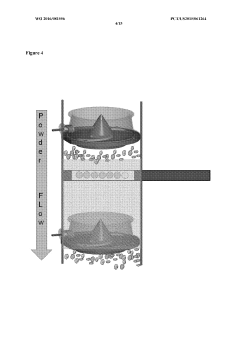
Validate HPLC Calibration for Consistent Results
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC Calibration Background and Objectives
High-Performance Liquid Chromatography (HPLC) has evolved significantly since its introduction in the late 1960s, becoming a cornerstone analytical technique in pharmaceutical, chemical, environmental, and food industries. The technology has progressed from rudimentary systems with limited detection capabilities to sophisticated instruments featuring advanced detectors, automation, and integration with mass spectrometry. This evolution has been driven by increasing demands for higher sensitivity, greater accuracy, and improved reproducibility in analytical measurements.
Calibration represents the critical foundation of reliable HPLC analysis, establishing the relationship between instrument response and analyte concentration. Historically, calibration approaches have transitioned from simple single-point methods to comprehensive multi-point strategies that account for non-linearity and matrix effects. The industry has witnessed a parallel development in calibration standards, reference materials, and validation protocols to ensure measurement integrity.
Current trends in HPLC calibration focus on implementing automated calibration systems, developing robust statistical models for calibration curve evaluation, and establishing traceability to international standards. The integration of machine learning algorithms for calibration optimization and error prediction represents an emerging frontier in this field. Additionally, regulatory bodies worldwide have progressively strengthened requirements for calibration validation, particularly in regulated industries like pharmaceuticals and clinical diagnostics.
The primary objective of HPLC calibration validation is to ensure consistent, accurate, and reliable analytical results across different analyses, instruments, operators, and laboratories. This encompasses verifying linearity across the working range, confirming detection and quantification limits, assessing precision through repeatability and intermediate precision studies, and determining accuracy through recovery experiments.
Secondary objectives include minimizing systematic errors, reducing measurement uncertainty, ensuring compliance with regulatory requirements, and facilitating method transfer between laboratories. Proper calibration validation also aims to establish appropriate calibration intervals, identify potential sources of drift or instability, and implement effective corrective actions when calibration parameters deviate from acceptable limits.
From a business perspective, validated HPLC calibration delivers significant value by reducing costly analytical errors, minimizing batch rejections, supporting regulatory compliance, and enabling confident decision-making based on analytical data. The economic impact of calibration failures can be substantial, particularly in pharmaceutical manufacturing where product quality decisions rely heavily on chromatographic analysis.
The technological trajectory points toward integrated calibration systems with real-time monitoring capabilities, predictive maintenance features, and automated documentation for regulatory compliance. The ultimate goal remains achieving maximum reliability and reproducibility in analytical measurements while minimizing human intervention and potential error sources.
Calibration represents the critical foundation of reliable HPLC analysis, establishing the relationship between instrument response and analyte concentration. Historically, calibration approaches have transitioned from simple single-point methods to comprehensive multi-point strategies that account for non-linearity and matrix effects. The industry has witnessed a parallel development in calibration standards, reference materials, and validation protocols to ensure measurement integrity.
Current trends in HPLC calibration focus on implementing automated calibration systems, developing robust statistical models for calibration curve evaluation, and establishing traceability to international standards. The integration of machine learning algorithms for calibration optimization and error prediction represents an emerging frontier in this field. Additionally, regulatory bodies worldwide have progressively strengthened requirements for calibration validation, particularly in regulated industries like pharmaceuticals and clinical diagnostics.
The primary objective of HPLC calibration validation is to ensure consistent, accurate, and reliable analytical results across different analyses, instruments, operators, and laboratories. This encompasses verifying linearity across the working range, confirming detection and quantification limits, assessing precision through repeatability and intermediate precision studies, and determining accuracy through recovery experiments.
Secondary objectives include minimizing systematic errors, reducing measurement uncertainty, ensuring compliance with regulatory requirements, and facilitating method transfer between laboratories. Proper calibration validation also aims to establish appropriate calibration intervals, identify potential sources of drift or instability, and implement effective corrective actions when calibration parameters deviate from acceptable limits.
From a business perspective, validated HPLC calibration delivers significant value by reducing costly analytical errors, minimizing batch rejections, supporting regulatory compliance, and enabling confident decision-making based on analytical data. The economic impact of calibration failures can be substantial, particularly in pharmaceutical manufacturing where product quality decisions rely heavily on chromatographic analysis.
The technological trajectory points toward integrated calibration systems with real-time monitoring capabilities, predictive maintenance features, and automated documentation for regulatory compliance. The ultimate goal remains achieving maximum reliability and reproducibility in analytical measurements while minimizing human intervention and potential error sources.
Market Demand for Reliable HPLC Analysis
The High-Performance Liquid Chromatography (HPLC) analysis market has witnessed substantial growth over the past decade, driven primarily by increasing demands for accurate analytical methods across pharmaceutical, biotechnology, food and beverage, environmental, and clinical research sectors. The global HPLC market was valued at approximately $4.5 billion in 2022 and is projected to reach $6.7 billion by 2027, growing at a CAGR of 8.2%.
Pharmaceutical and biopharmaceutical industries represent the largest market segment, accounting for nearly 55% of the total HPLC market. These industries require stringent quality control measures to ensure product safety and efficacy, creating a persistent demand for reliable HPLC calibration solutions. The implementation of regulatory frameworks such as FDA's 21 CFR Part 11 and GMP guidelines has further intensified the need for validated analytical methods.
Contract research organizations (CROs) and contract manufacturing organizations (CMOs) have emerged as significant consumers of HPLC technology, with their market share expanding from 12% to 18% between 2018 and 2022. This growth correlates with the increasing outsourcing of analytical testing services by pharmaceutical companies seeking cost-effective solutions while maintaining quality standards.
Regional analysis indicates that North America dominates the HPLC market with a 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is experiencing the fastest growth rate at 10.5% annually, primarily driven by expanding pharmaceutical manufacturing capabilities in China and India, coupled with increasing regulatory oversight in these regions.
The demand for automated calibration systems has grown by 15% annually since 2020, reflecting industry's push toward reducing human error and improving operational efficiency. Organizations are increasingly seeking integrated solutions that offer seamless documentation, traceability, and compliance reporting capabilities to meet regulatory requirements while optimizing laboratory workflows.
Market research indicates that 78% of laboratory managers consider calibration reliability as a critical factor when purchasing HPLC systems, ranking it above initial acquisition costs. This preference underscores the significant financial implications of calibration failures, which can result in product recalls, regulatory penalties, and damaged reputation.
The COVID-19 pandemic has accelerated the adoption of remote monitoring and calibration verification technologies, with a 22% increase in demand for cloud-connected HPLC systems that enable real-time performance monitoring and predictive maintenance. This trend aligns with the broader industry movement toward digitalization and laboratory automation.
Pharmaceutical and biopharmaceutical industries represent the largest market segment, accounting for nearly 55% of the total HPLC market. These industries require stringent quality control measures to ensure product safety and efficacy, creating a persistent demand for reliable HPLC calibration solutions. The implementation of regulatory frameworks such as FDA's 21 CFR Part 11 and GMP guidelines has further intensified the need for validated analytical methods.
Contract research organizations (CROs) and contract manufacturing organizations (CMOs) have emerged as significant consumers of HPLC technology, with their market share expanding from 12% to 18% between 2018 and 2022. This growth correlates with the increasing outsourcing of analytical testing services by pharmaceutical companies seeking cost-effective solutions while maintaining quality standards.
Regional analysis indicates that North America dominates the HPLC market with a 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is experiencing the fastest growth rate at 10.5% annually, primarily driven by expanding pharmaceutical manufacturing capabilities in China and India, coupled with increasing regulatory oversight in these regions.
The demand for automated calibration systems has grown by 15% annually since 2020, reflecting industry's push toward reducing human error and improving operational efficiency. Organizations are increasingly seeking integrated solutions that offer seamless documentation, traceability, and compliance reporting capabilities to meet regulatory requirements while optimizing laboratory workflows.
Market research indicates that 78% of laboratory managers consider calibration reliability as a critical factor when purchasing HPLC systems, ranking it above initial acquisition costs. This preference underscores the significant financial implications of calibration failures, which can result in product recalls, regulatory penalties, and damaged reputation.
The COVID-19 pandemic has accelerated the adoption of remote monitoring and calibration verification technologies, with a 22% increase in demand for cloud-connected HPLC systems that enable real-time performance monitoring and predictive maintenance. This trend aligns with the broader industry movement toward digitalization and laboratory automation.
Current HPLC Calibration Challenges
High-performance liquid chromatography (HPLC) calibration faces significant challenges in today's analytical laboratories despite its widespread use as a gold standard for quantitative analysis. The primary challenge stems from the inherent complexity of maintaining consistent calibration across multiple instruments, operators, and environmental conditions. Laboratories frequently encounter drift in calibration curves over time, leading to questionable data reliability and potential compliance issues with regulatory standards.
System suitability failures represent a persistent obstacle, with approximately 30% of HPLC downtime attributed to calibration-related issues according to recent industry surveys. These failures often manifest as retention time shifts, peak area inconsistencies, or detector response variations that compromise analytical precision. The root causes typically include column degradation, mobile phase composition changes, temperature fluctuations, and instrument component wear.
Method transfer between different HPLC systems presents another significant challenge. Even when using identical protocols, variations in instrument design, detector sensitivity, and fluidic path configurations can lead to systematic differences in calibration parameters. This becomes particularly problematic in multi-site operations or collaborative research environments where data comparability is essential.
The increasing regulatory scrutiny, especially in pharmaceutical and clinical diagnostics sectors, has elevated the importance of robust calibration validation. Current guidelines from FDA, USP, and ICH demand comprehensive documentation of calibration procedures with stringent acceptance criteria. Meeting these requirements while maintaining operational efficiency creates tension in many laboratories.
Modern HPLC applications involving complex biological matrices introduce additional calibration challenges. Matrix effects can significantly impact detector response, creating non-linear calibration relationships that are difficult to model consistently. The presence of endogenous compounds with similar chromatographic properties to analytes of interest further complicates accurate calibration.
Automation paradoxically introduces both solutions and new challenges. While automated calibration systems reduce human error, they may mask underlying system problems if not properly validated. The "black box" nature of some automated calibration software limits troubleshooting capabilities when deviations occur.
Resource constraints compound these technical challenges. The high cost of certified reference standards, especially for specialized compounds, often leads to compromises in calibration frequency or range coverage. Additionally, the time-intensive nature of comprehensive calibration protocols creates pressure to abbreviate procedures, potentially sacrificing accuracy for throughput.
System suitability failures represent a persistent obstacle, with approximately 30% of HPLC downtime attributed to calibration-related issues according to recent industry surveys. These failures often manifest as retention time shifts, peak area inconsistencies, or detector response variations that compromise analytical precision. The root causes typically include column degradation, mobile phase composition changes, temperature fluctuations, and instrument component wear.
Method transfer between different HPLC systems presents another significant challenge. Even when using identical protocols, variations in instrument design, detector sensitivity, and fluidic path configurations can lead to systematic differences in calibration parameters. This becomes particularly problematic in multi-site operations or collaborative research environments where data comparability is essential.
The increasing regulatory scrutiny, especially in pharmaceutical and clinical diagnostics sectors, has elevated the importance of robust calibration validation. Current guidelines from FDA, USP, and ICH demand comprehensive documentation of calibration procedures with stringent acceptance criteria. Meeting these requirements while maintaining operational efficiency creates tension in many laboratories.
Modern HPLC applications involving complex biological matrices introduce additional calibration challenges. Matrix effects can significantly impact detector response, creating non-linear calibration relationships that are difficult to model consistently. The presence of endogenous compounds with similar chromatographic properties to analytes of interest further complicates accurate calibration.
Automation paradoxically introduces both solutions and new challenges. While automated calibration systems reduce human error, they may mask underlying system problems if not properly validated. The "black box" nature of some automated calibration software limits troubleshooting capabilities when deviations occur.
Resource constraints compound these technical challenges. The high cost of certified reference standards, especially for specialized compounds, often leads to compromises in calibration frequency or range coverage. Additionally, the time-intensive nature of comprehensive calibration protocols creates pressure to abbreviate procedures, potentially sacrificing accuracy for throughput.
Established HPLC Calibration Protocols
01 Calibration standards and reference materials for HPLC consistency
The use of certified reference materials and calibration standards is crucial for maintaining HPLC calibration consistency. These standards provide known concentrations of analytes that allow for accurate instrument calibration and verification. Regular use of these standards helps establish calibration curves, verify system performance, and ensure measurement traceability. Implementing proper storage and handling protocols for these reference materials further enhances calibration reliability.- Calibration methods for HPLC systems: Various calibration methods are employed to ensure consistency in HPLC analysis. These methods include using standard reference materials, multi-point calibration curves, and automated calibration procedures. Proper calibration methodology helps maintain accuracy across different analytical runs and reduces systematic errors. Regular calibration with certified standards ensures that the HPLC system provides reliable and reproducible results over time.
- Internal standard techniques for improved consistency: Internal standard techniques involve adding known compounds to samples to compensate for variations in injection volume, detector response, and other operational parameters. This approach normalizes results against the internal standard response, significantly improving measurement consistency. The technique is particularly valuable for complex matrices where matrix effects might otherwise impact calibration stability. Internal standards with similar chemical properties to the analytes of interest provide the most reliable calibration consistency.
- Automated calibration systems and software solutions: Automated calibration systems incorporate software algorithms that continuously monitor and adjust HPLC parameters to maintain calibration consistency. These systems can perform scheduled calibration checks, apply drift corrections, and alert operators when calibration falls outside acceptable limits. Advanced software solutions can track calibration history, predict maintenance needs, and apply appropriate correction factors to ensure consistent analytical results. Integration with laboratory information management systems further enhances calibration traceability and compliance.
- Temperature and environmental control for calibration stability: Maintaining stable environmental conditions is crucial for HPLC calibration consistency. Temperature fluctuations can significantly affect retention times, peak areas, and detector response. Specialized temperature control systems for columns, mobile phases, and detectors help minimize these variations. Environmental monitoring systems track and record conditions during calibration and analysis, allowing for appropriate corrections when necessary. Proper thermal insulation of critical components further contributes to calibration stability across different operating conditions.
- Quality control procedures for long-term calibration consistency: Comprehensive quality control procedures are essential for maintaining HPLC calibration consistency over extended periods. These include regular system suitability tests, periodic verification with certified reference materials, and statistical monitoring of calibration parameters. Control charts help visualize trends and detect calibration drift before it affects analytical results. Scheduled preventive maintenance of critical components such as pumps, injectors, and detectors ensures that the physical basis for calibration remains stable. Documentation of all calibration activities supports regulatory compliance and facilitates troubleshooting when inconsistencies arise.
02 Automated calibration systems for HPLC
Automated calibration systems improve HPLC calibration consistency by reducing human error and standardizing the calibration process. These systems can automatically prepare calibration samples, inject standards, analyze results, and generate calibration curves. They often include software that monitors calibration status, schedules routine calibrations, and alerts users when calibration parameters drift outside acceptable limits. Automation ensures reproducible calibration procedures across different operators and time periods.Expand Specific Solutions03 Temperature control for HPLC calibration stability
Maintaining consistent temperature conditions is essential for HPLC calibration stability. Temperature fluctuations can affect retention times, peak shapes, and detector responses, leading to calibration inconsistencies. Advanced temperature control systems for column compartments, sample storage, and mobile phase reservoirs help minimize these variations. Some systems incorporate temperature compensation algorithms that adjust calibration parameters based on ambient temperature changes, ensuring consistent analytical results regardless of environmental conditions.Expand Specific Solutions04 Calibration verification and system suitability testing
Regular calibration verification and system suitability testing are critical for maintaining HPLC calibration consistency. These procedures involve analyzing control samples with known properties to confirm that the system is operating within specified parameters. System suitability tests evaluate key performance indicators such as retention time reproducibility, peak resolution, and signal-to-noise ratio. Implementing standardized protocols for these tests helps detect calibration drift early and ensures consistent analytical performance over time.Expand Specific Solutions05 Data management and statistical analysis for calibration consistency
Robust data management systems and statistical analysis tools are essential for monitoring and maintaining HPLC calibration consistency. These systems track calibration history, identify trends, and apply statistical methods to evaluate calibration stability over time. Advanced software can automatically flag outliers, calculate uncertainty values, and determine when recalibration is necessary. Implementing control charts and other statistical process control techniques helps analysts visualize calibration performance and make data-driven decisions about system maintenance and method adjustments.Expand Specific Solutions
Key HPLC Instrument Manufacturers and Solution Providers
The HPLC calibration validation market is currently in a growth phase, with increasing demand driven by stringent regulatory requirements in pharmaceutical and analytical industries. The competitive landscape features established analytical instrument manufacturers like Agilent Technologies, Horiba Ltd., and Roche Diagnostics, who dominate with comprehensive calibration solutions. Pharmaceutical companies such as Janssen Pharmaceutica and Sunshine Lake Pharma represent key end-users driving innovation in this space. The technology has reached moderate maturity, with companies like F. Hoffmann-La Roche and Toshiba developing advanced automated calibration systems. However, opportunities remain for specialized players like Cytonome/ST to introduce disruptive technologies addressing precision and efficiency challenges in HPLC calibration validation.
Roche Diagnostics GmbH
Technical Solution: Roche Diagnostics has developed a robust HPLC calibration validation framework specifically designed for pharmaceutical and clinical applications. Their approach incorporates a hierarchical calibration strategy with primary and secondary standards to ensure traceability and minimize calibration drift. The system employs automated calibration verification using intelligent algorithms that detect deviations from expected response patterns. Roche's calibration technology includes temperature-controlled sample compartments and column ovens that maintain stability within ±0.1°C, significantly reducing environmental variables that affect chromatographic performance. Their validation protocol implements a comprehensive system suitability assessment including resolution, tailing factor, theoretical plate count, and precision measurements before sample analysis. The calibration software features advanced statistical tools for evaluating linearity across the analytical range with automatic flagging of outliers and drift patterns. Roche's system also incorporates regular preventive maintenance schedules synchronized with calibration cycles to ensure consistent instrument performance.
Strengths: Exceptional calibration stability with documented drift less than 1% over extended periods; comprehensive compliance with regulatory requirements including 21 CFR Part 11; advanced data visualization tools for trend analysis. Weaknesses: System complexity may increase validation time; higher operational costs due to proprietary consumables and standards; limited flexibility for method customization in some applications.
Roche Diagnostics Operations, Inc.
Technical Solution: Roche Diagnostics Operations has implemented an integrated HPLC calibration validation system designed specifically for high-throughput clinical and pharmaceutical environments. Their technology employs a risk-based calibration approach that allocates validation resources according to the criticality of analytical parameters. The system features intelligent calibration verification using automated comparison of current performance against historical baseline data to detect subtle shifts in system behavior. Their validation protocol incorporates comprehensive detector linearity testing across multiple wavelengths with automatic correction factors for optimal quantitative accuracy. Roche's calibration software includes advanced statistical tools for evaluating method robustness under varying conditions, including deliberate perturbations of critical parameters. The system implements automated calibration curve assessment with intelligent outlier detection and handling protocols. Their technology includes continuous monitoring of critical system parameters including pressure profiles, baseline noise, and detector response factors with real-time alerts when values drift beyond established control limits. The validation approach also incorporates regular proficiency testing with blinded samples to verify the entire analytical process from sample preparation through data analysis.
Strengths: Excellent integration with laboratory workflow automation; comprehensive compliance documentation for regulated environments; advanced data analytics for performance trending and predictive maintenance. Weaknesses: Significant initial investment in implementation and validation; system complexity may require dedicated support personnel; potential vendor lock-in for consumables and service.
Critical Parameters for Successful HPLC Calibration
Process of conducting high throughput testing high performance liquid chromatography
PatentWO2016081556A1
Innovation
- A process involving pre-weighed samples in HDPE bottles, with solutions added and mixed, followed by centrifugation, and loading the supernatant onto an HPLC column for analysis, which can be used to correlate with process analytical technology (PAT) measurements for continuous manufacturing and API concentration measurement.
High-performance liquid chromatography with a controllable transverse flow inducer
PatentActiveEP3322978A1
Innovation
- The use of a controllable transverse flow inducer, which generates micro-scale vortices through alternating current electrokinetics, allowing for orthogonal flow induction independent of axial velocity, reducing dispersion by combining pressure and electro-osmotic flow, and enabling retention modulation without permanent surface charges.
Regulatory Compliance Requirements for HPLC Methods
High Performance Liquid Chromatography (HPLC) methods must adhere to stringent regulatory frameworks established by various international and national authorities. The FDA's Code of Federal Regulations (21 CFR Part 11) mandates electronic record integrity and electronic signatures for HPLC calibration data. Similarly, the European Medicines Agency (EMA) enforces GMP Annex 11, which outlines computerized system validation requirements applicable to HPLC calibration processes.
The International Conference on Harmonisation (ICH) guidelines, particularly ICH Q2(R1), provide comprehensive frameworks for analytical method validation, including specificity, linearity, range, accuracy, precision, detection limit, and quantitation limit. These parameters form the cornerstone of HPLC calibration validation protocols across pharmaceutical and biotechnology industries.
USP <1058> Analytical Instrument Qualification categorizes HPLC validation into four critical phases: Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). Each phase requires meticulous documentation and verification to ensure regulatory compliance.
For laboratories seeking ISO/IEC 17025 accreditation, additional requirements apply to calibration procedures, including uncertainty measurements and traceability to international standards. This standard emphasizes the technical competence of testing laboratories and ensures global recognition of calibration results.
The pharmaceutical industry must also comply with product-specific monographs in pharmacopeias (USP, EP, JP) that often specify HPLC method parameters and system suitability requirements. These monographs serve as legally binding standards for product quality assessment.
Data integrity principles, encapsulated in the ALCOA+ framework (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available), must be incorporated into all HPLC calibration procedures. Regulatory inspections increasingly focus on data governance systems that protect calibration data throughout its lifecycle.
Method transfer regulations require robust protocols when HPLC calibration methods move between laboratories or facilities. The receiving laboratory must demonstrate equivalent performance to the originating laboratory through comparative testing and statistical analysis.
Continuous compliance monitoring through periodic review of calibration data, trend analysis, and preventive maintenance schedules is mandated by most regulatory frameworks. This ensures ongoing validity of calibration parameters and early detection of potential compliance issues.
The International Conference on Harmonisation (ICH) guidelines, particularly ICH Q2(R1), provide comprehensive frameworks for analytical method validation, including specificity, linearity, range, accuracy, precision, detection limit, and quantitation limit. These parameters form the cornerstone of HPLC calibration validation protocols across pharmaceutical and biotechnology industries.
USP <1058> Analytical Instrument Qualification categorizes HPLC validation into four critical phases: Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). Each phase requires meticulous documentation and verification to ensure regulatory compliance.
For laboratories seeking ISO/IEC 17025 accreditation, additional requirements apply to calibration procedures, including uncertainty measurements and traceability to international standards. This standard emphasizes the technical competence of testing laboratories and ensures global recognition of calibration results.
The pharmaceutical industry must also comply with product-specific monographs in pharmacopeias (USP, EP, JP) that often specify HPLC method parameters and system suitability requirements. These monographs serve as legally binding standards for product quality assessment.
Data integrity principles, encapsulated in the ALCOA+ framework (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available), must be incorporated into all HPLC calibration procedures. Regulatory inspections increasingly focus on data governance systems that protect calibration data throughout its lifecycle.
Method transfer regulations require robust protocols when HPLC calibration methods move between laboratories or facilities. The receiving laboratory must demonstrate equivalent performance to the originating laboratory through comparative testing and statistical analysis.
Continuous compliance monitoring through periodic review of calibration data, trend analysis, and preventive maintenance schedules is mandated by most regulatory frameworks. This ensures ongoing validity of calibration parameters and early detection of potential compliance issues.
Quality Control Strategies for Long-term HPLC Performance
Quality control strategies for maintaining long-term HPLC performance require systematic approaches that ensure reliable and consistent analytical results. Implementing a comprehensive validation protocol for HPLC calibration serves as the foundation for sustainable analytical excellence. These strategies must address both routine operation and preventive maintenance to maximize instrument uptime and data integrity.
Regular system suitability testing (SST) represents a cornerstone of effective quality control, providing objective evidence that the chromatographic system performs within established parameters. SST protocols typically include evaluations of retention time reproducibility, peak area precision, theoretical plate count, and tailing factor measurements. Establishing control charts for these parameters enables trend analysis and early detection of system drift before it impacts analytical results.
Calibration verification schedules should be established based on risk assessment, considering factors such as sample complexity, method criticality, and regulatory requirements. For high-throughput environments, more frequent calibration checks may be necessary, while stable methods may require less frequent verification. Implementation of bracketing calibration approaches, where standards are analyzed before and after sample batches, provides continuous assurance of system performance.
Reference standard management constitutes another vital component of long-term HPLC performance. Certified reference materials should be stored according to manufacturer specifications, with clear expiration tracking and regular verification against primary standards. The establishment of internal working standards with defined acceptance criteria helps minimize variability introduced by reference material changes.
Preventive maintenance schedules tailored to usage patterns significantly extend instrument lifetime and performance stability. Critical components requiring regular attention include pump seals, check valves, autosampler needles, and detector lamps. Documenting maintenance activities in electronic laboratory information management systems facilitates compliance and enables correlation between maintenance events and system performance.
Environmental control measures further support consistent HPLC performance. Temperature fluctuations, electromagnetic interference, and vibration can all impact chromatographic separation. Monitoring and controlling these variables through dedicated HVAC systems, vibration-dampening workstations, and electromagnetic shielding help maintain stable baseline performance.
Data management strategies complete the quality control framework by ensuring that calibration data is properly archived, readily retrievable, and protected against unauthorized modification. Implementing audit trails for calibration activities supports data integrity and facilitates troubleshooting when performance deviations occur.
Regular system suitability testing (SST) represents a cornerstone of effective quality control, providing objective evidence that the chromatographic system performs within established parameters. SST protocols typically include evaluations of retention time reproducibility, peak area precision, theoretical plate count, and tailing factor measurements. Establishing control charts for these parameters enables trend analysis and early detection of system drift before it impacts analytical results.
Calibration verification schedules should be established based on risk assessment, considering factors such as sample complexity, method criticality, and regulatory requirements. For high-throughput environments, more frequent calibration checks may be necessary, while stable methods may require less frequent verification. Implementation of bracketing calibration approaches, where standards are analyzed before and after sample batches, provides continuous assurance of system performance.
Reference standard management constitutes another vital component of long-term HPLC performance. Certified reference materials should be stored according to manufacturer specifications, with clear expiration tracking and regular verification against primary standards. The establishment of internal working standards with defined acceptance criteria helps minimize variability introduced by reference material changes.
Preventive maintenance schedules tailored to usage patterns significantly extend instrument lifetime and performance stability. Critical components requiring regular attention include pump seals, check valves, autosampler needles, and detector lamps. Documenting maintenance activities in electronic laboratory information management systems facilitates compliance and enables correlation between maintenance events and system performance.
Environmental control measures further support consistent HPLC performance. Temperature fluctuations, electromagnetic interference, and vibration can all impact chromatographic separation. Monitoring and controlling these variables through dedicated HVAC systems, vibration-dampening workstations, and electromagnetic shielding help maintain stable baseline performance.
Data management strategies complete the quality control framework by ensuring that calibration data is properly archived, readily retrievable, and protected against unauthorized modification. Implementing audit trails for calibration activities supports data integrity and facilitates troubleshooting when performance deviations occur.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!