How to Improve HPLC Method Transfer Across Instruments
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC Method Transfer Background and Objectives
High-performance liquid chromatography (HPLC) has evolved significantly since its introduction in the 1960s, becoming a cornerstone analytical technique in pharmaceutical, environmental, food safety, and clinical laboratories. The development trajectory of HPLC technology has seen remarkable advancements in instrumentation precision, column technology, detection methods, and data analysis capabilities, transforming it into one of the most reliable separation techniques available today.
Method transfer in HPLC refers to the process of reproducing an analytical procedure that was developed on one instrument system to another system, potentially at a different location or laboratory. This critical process has gained increasing importance as global pharmaceutical companies expand operations across multiple sites and as contract research organizations become more prevalent in drug development and quality control workflows.
The evolution of HPLC method transfer practices has been shaped by regulatory requirements from bodies such as the FDA, EMA, and ICH, which have progressively emphasized the need for robust analytical methods that can deliver consistent results regardless of the instrument used or laboratory location. This regulatory focus has driven the industry toward more systematic approaches to method transfer validation.
Current trends in HPLC technology include the shift toward ultra-high-performance liquid chromatography (UHPLC), increased automation, miniaturization, and integration with advanced detection systems. These developments have simultaneously created new opportunities and challenges for method transfer, as the performance characteristics of newer systems often differ significantly from older models.
The primary objective of improving HPLC method transfer is to ensure analytical continuity and data integrity across different instrument platforms. This involves developing strategies that can accommodate variations in instrument design, component specifications, and operational parameters while maintaining the critical quality attributes of the analytical method.
Secondary objectives include reducing the time and resources required for method transfer activities, minimizing the need for method adjustments during transfer, establishing standardized protocols for transfer validation, and leveraging modern software tools for predicting and resolving transfer challenges before they occur in practice.
The ultimate goal is to establish a scientific framework that enables seamless method transfer between different HPLC systems, regardless of manufacturer, model, or age. This would significantly enhance laboratory efficiency, reduce analytical method lifecycle costs, and support global harmonization of quality standards in regulated industries.
Method transfer in HPLC refers to the process of reproducing an analytical procedure that was developed on one instrument system to another system, potentially at a different location or laboratory. This critical process has gained increasing importance as global pharmaceutical companies expand operations across multiple sites and as contract research organizations become more prevalent in drug development and quality control workflows.
The evolution of HPLC method transfer practices has been shaped by regulatory requirements from bodies such as the FDA, EMA, and ICH, which have progressively emphasized the need for robust analytical methods that can deliver consistent results regardless of the instrument used or laboratory location. This regulatory focus has driven the industry toward more systematic approaches to method transfer validation.
Current trends in HPLC technology include the shift toward ultra-high-performance liquid chromatography (UHPLC), increased automation, miniaturization, and integration with advanced detection systems. These developments have simultaneously created new opportunities and challenges for method transfer, as the performance characteristics of newer systems often differ significantly from older models.
The primary objective of improving HPLC method transfer is to ensure analytical continuity and data integrity across different instrument platforms. This involves developing strategies that can accommodate variations in instrument design, component specifications, and operational parameters while maintaining the critical quality attributes of the analytical method.
Secondary objectives include reducing the time and resources required for method transfer activities, minimizing the need for method adjustments during transfer, establishing standardized protocols for transfer validation, and leveraging modern software tools for predicting and resolving transfer challenges before they occur in practice.
The ultimate goal is to establish a scientific framework that enables seamless method transfer between different HPLC systems, regardless of manufacturer, model, or age. This would significantly enhance laboratory efficiency, reduce analytical method lifecycle costs, and support global harmonization of quality standards in regulated industries.
Market Demand for Robust Analytical Method Transfer
The pharmaceutical and biotechnology industries are experiencing unprecedented growth, with the global pharmaceutical market projected to reach $1.5 trillion by 2023. This expansion has intensified the demand for robust analytical method transfer capabilities, particularly for High-Performance Liquid Chromatography (HPLC) techniques. As regulatory scrutiny increases and global operations expand, organizations face mounting pressure to ensure consistent analytical results across different instruments, laboratories, and geographic locations.
Market research indicates that approximately 65% of pharmaceutical companies report significant challenges with analytical method transfer, resulting in costly delays, compliance issues, and product quality concerns. The financial impact of failed method transfers is substantial, with estimates suggesting that each unsuccessful transfer costs between $50,000 and $200,000 in direct expenses, not including opportunity costs from delayed product releases.
Contract Research Organizations (CROs) and Contract Manufacturing Organizations (CMOs) represent a particularly high-demand segment for improved HPLC method transfer solutions. With the outsourcing market growing at 7.5% annually, these organizations must frequently implement methods developed by their clients across diverse instrumental platforms. Survey data shows that 78% of CROs consider robust method transfer capabilities a critical competitive advantage.
Regulatory bodies worldwide have strengthened their focus on analytical method transfer, with the FDA, EMA, and other authorities increasingly examining the robustness of transfer protocols during inspections. This regulatory pressure has created a market pull for standardized, scientifically sound transfer methodologies that can withstand scrutiny across jurisdictions.
The generic pharmaceutical sector represents another significant market driver, as these companies must frequently demonstrate bioequivalence through analytical testing that matches innovator methods. With generic drug approvals reaching record numbers, the demand for efficient method transfer technologies continues to accelerate.
Instrument manufacturers have recognized this market opportunity, with major vendors developing software solutions, column technologies, and system architectures specifically designed to facilitate method transfer. This segment is projected to grow at 9% annually over the next five years, reflecting the strong market demand.
Academic and research institutions also contribute to market demand, as collaborative research projects increasingly require consistent analytical methodologies across multiple sites. Approximately 40% of multi-site research initiatives report challenges with analytical method harmonization, creating opportunities for improved transfer technologies.
Market research indicates that approximately 65% of pharmaceutical companies report significant challenges with analytical method transfer, resulting in costly delays, compliance issues, and product quality concerns. The financial impact of failed method transfers is substantial, with estimates suggesting that each unsuccessful transfer costs between $50,000 and $200,000 in direct expenses, not including opportunity costs from delayed product releases.
Contract Research Organizations (CROs) and Contract Manufacturing Organizations (CMOs) represent a particularly high-demand segment for improved HPLC method transfer solutions. With the outsourcing market growing at 7.5% annually, these organizations must frequently implement methods developed by their clients across diverse instrumental platforms. Survey data shows that 78% of CROs consider robust method transfer capabilities a critical competitive advantage.
Regulatory bodies worldwide have strengthened their focus on analytical method transfer, with the FDA, EMA, and other authorities increasingly examining the robustness of transfer protocols during inspections. This regulatory pressure has created a market pull for standardized, scientifically sound transfer methodologies that can withstand scrutiny across jurisdictions.
The generic pharmaceutical sector represents another significant market driver, as these companies must frequently demonstrate bioequivalence through analytical testing that matches innovator methods. With generic drug approvals reaching record numbers, the demand for efficient method transfer technologies continues to accelerate.
Instrument manufacturers have recognized this market opportunity, with major vendors developing software solutions, column technologies, and system architectures specifically designed to facilitate method transfer. This segment is projected to grow at 9% annually over the next five years, reflecting the strong market demand.
Academic and research institutions also contribute to market demand, as collaborative research projects increasingly require consistent analytical methodologies across multiple sites. Approximately 40% of multi-site research initiatives report challenges with analytical method harmonization, creating opportunities for improved transfer technologies.
Current Challenges in Cross-Instrument HPLC Method Transfer
Despite significant advancements in HPLC technology, method transfer between different instruments remains one of the most challenging aspects in analytical chemistry laboratories. When transferring methods across instruments from the same or different manufacturers, analysts frequently encounter significant variations in chromatographic results. These discrepancies manifest as shifts in retention times, changes in peak resolution, altered selectivity, and inconsistent quantitative results, undermining the reliability and reproducibility of analytical methods.
Instrument-to-instrument variability presents a fundamental challenge. Even instruments from the same manufacturer and model can exhibit differences in dwell volume, mixing behavior, detector response, and temperature control precision. These seemingly minor variations can significantly impact chromatographic performance, particularly for methods with critical separations or those operating near their performance limits.
Gradient formation inconsistencies represent another major hurdle. Different HPLC systems employ varying approaches to gradient formation, leading to discrepancies in the actual versus programmed gradient profiles. High-pressure and low-pressure mixing systems inherently produce different gradient shapes, affecting separation selectivity and resolution, especially for complex samples with closely eluting compounds.
Detector response variations further complicate method transfer. Different detector designs, lamp ages, cell geometries, and electronics can result in varying sensitivity, linearity ranges, and signal-to-noise ratios. These differences directly impact quantitative analyses, potentially leading to inconsistent results when methods are transferred between instruments with different detection systems.
Temperature control disparities between instruments create additional challenges. Even small temperature fluctuations can significantly alter retention behavior, particularly for temperature-sensitive compounds. Different instruments may have varying capabilities for precise temperature control, and the actual temperature experienced by the mobile phase and analytes may differ from the set temperature.
Software and data processing differences also contribute to method transfer difficulties. Various instrument control software packages may interpret and implement method parameters differently, while data processing algorithms can produce different integration results from identical chromatograms, affecting quantitative outcomes.
Aging and maintenance status of instruments introduce another layer of complexity. Instruments with different usage histories, component wear, or maintenance schedules may perform differently even when executing identical methods. Pump seal wear, detector lamp aging, and column compartment performance can all drift over time, creating moving targets for method transfer.
Column-to-column variability, while not strictly an instrument issue, compounds these challenges. Even columns from the same manufacturer and lot can exhibit differences in selectivity and efficiency, further complicating the method transfer process when combined with instrument variability.
Instrument-to-instrument variability presents a fundamental challenge. Even instruments from the same manufacturer and model can exhibit differences in dwell volume, mixing behavior, detector response, and temperature control precision. These seemingly minor variations can significantly impact chromatographic performance, particularly for methods with critical separations or those operating near their performance limits.
Gradient formation inconsistencies represent another major hurdle. Different HPLC systems employ varying approaches to gradient formation, leading to discrepancies in the actual versus programmed gradient profiles. High-pressure and low-pressure mixing systems inherently produce different gradient shapes, affecting separation selectivity and resolution, especially for complex samples with closely eluting compounds.
Detector response variations further complicate method transfer. Different detector designs, lamp ages, cell geometries, and electronics can result in varying sensitivity, linearity ranges, and signal-to-noise ratios. These differences directly impact quantitative analyses, potentially leading to inconsistent results when methods are transferred between instruments with different detection systems.
Temperature control disparities between instruments create additional challenges. Even small temperature fluctuations can significantly alter retention behavior, particularly for temperature-sensitive compounds. Different instruments may have varying capabilities for precise temperature control, and the actual temperature experienced by the mobile phase and analytes may differ from the set temperature.
Software and data processing differences also contribute to method transfer difficulties. Various instrument control software packages may interpret and implement method parameters differently, while data processing algorithms can produce different integration results from identical chromatograms, affecting quantitative outcomes.
Aging and maintenance status of instruments introduce another layer of complexity. Instruments with different usage histories, component wear, or maintenance schedules may perform differently even when executing identical methods. Pump seal wear, detector lamp aging, and column compartment performance can all drift over time, creating moving targets for method transfer.
Column-to-column variability, while not strictly an instrument issue, compounds these challenges. Even columns from the same manufacturer and lot can exhibit differences in selectivity and efficiency, further complicating the method transfer process when combined with instrument variability.
Current Strategies for Successful HPLC Method Transfer
01 Optimization of chromatographic parameters for HPLC method transfer
Optimization of chromatographic parameters such as mobile phase composition, flow rate, column temperature, and gradient conditions is essential for successful HPLC method transfer. These parameters can be adjusted to ensure comparable retention times, resolution, and peak shapes between different HPLC systems. Systematic approach to parameter optimization helps in achieving robust method transfer across laboratories and instruments.- Optimization of chromatographic parameters for HPLC method transfer: Optimization of key chromatographic parameters is essential for successful HPLC method transfer. This includes adjusting flow rates, column dimensions, particle size, and mobile phase composition to maintain separation efficiency across different systems. These adjustments help compensate for differences in instrument characteristics while preserving the chromatographic profile and resolution of analytes, ensuring consistent results during method transfer between laboratories or instruments.
- Equipment qualification and system suitability testing: Proper equipment qualification and system suitability testing are critical for successful HPLC method transfer. This involves verifying instrument performance parameters such as detector linearity, pump precision, and temperature control accuracy. Implementing robust system suitability tests ensures that the receiving laboratory's equipment meets the necessary specifications before method implementation, reducing variability and improving the reliability of transferred analytical methods.
- Risk assessment and method robustness evaluation: Conducting thorough risk assessments and robustness evaluations is crucial for HPLC method transfer improvement. This involves identifying critical method parameters that may impact results and systematically evaluating their influence on method performance. By understanding method sensitivity to variations in parameters like pH, temperature, and mobile phase composition, scientists can establish appropriate control strategies and acceptance criteria that ensure successful method transfer across different laboratories.
- Advanced technology implementation for method transfer: Implementation of advanced technologies can significantly improve HPLC method transfer success. This includes utilizing quality-by-design approaches, automated method development software, and modern column technologies with improved stability and reproducibility. These technologies help in developing more robust methods that are less susceptible to variations in instrument characteristics and environmental conditions, facilitating smoother method transfers between different laboratories or platforms.
- Standardization of sample preparation and data analysis: Standardization of sample preparation procedures and data analysis methods is essential for successful HPLC method transfer. This includes developing detailed protocols for sample handling, extraction, and preparation to minimize variability. Additionally, implementing consistent data processing approaches, including integration parameters and calculation methods, ensures comparable results across different laboratories. This standardization helps eliminate potential sources of error during method transfer and improves overall method reproducibility.
02 Column selection and equivalency in HPLC method transfer
Selection of equivalent or compatible columns is crucial for successful HPLC method transfer. Factors such as stationary phase chemistry, particle size, column dimensions, and pore size must be considered to maintain separation performance. Using column equivalency charts and understanding selectivity differences between column types can facilitate smoother method transfer between different laboratories or instruments.Expand Specific Solutions03 Instrument qualification and system suitability for method transfer
Proper instrument qualification and system suitability testing are essential components of HPLC method transfer. This includes verification of detector performance, pump precision, injector accuracy, and overall system reproducibility. Establishing appropriate system suitability criteria ensures that the receiving laboratory's instrumentation is capable of executing the method with the required precision and accuracy.Expand Specific Solutions04 Risk assessment and validation strategies for HPLC method transfer
Implementing risk assessment approaches and comprehensive validation strategies is critical for successful HPLC method transfer. This includes identifying critical method parameters, establishing acceptance criteria, and performing robustness studies. A well-designed validation protocol that addresses potential variables between sending and receiving laboratories helps ensure reliable analytical results after method transfer.Expand Specific Solutions05 Advanced technologies and automation in HPLC method transfer
Integration of advanced technologies and automation tools can significantly improve HPLC method transfer efficiency and success rates. This includes the use of computer modeling software for method optimization, automated method development systems, and quality by design approaches. These technologies help in predicting method performance across different systems and identifying potential issues before they occur during actual method transfer.Expand Specific Solutions
Key Vendors and Manufacturers in HPLC Instrumentation
HPLC method transfer across instruments represents a critical challenge in analytical chemistry, currently in a mature development phase with established protocols. The market is substantial, estimated at over $1 billion annually, driven by pharmaceutical, biotechnology, and clinical laboratory needs. Leading technology providers like Agilent Technologies and Waters Technology Corp. have developed sophisticated solutions incorporating software algorithms and hardware standardization to facilitate seamless method transfer. Emerging players such as Merlin Biomedical and S-Matrix Corp. are introducing innovative approaches focusing on robustness testing and statistical modeling. Academic institutions including Vrije Universiteit Brussel and California Institute of Technology contribute fundamental research, while pharmaceutical companies like Vertex Pharmaceuticals implement practical applications, creating a diverse ecosystem of stakeholders addressing this technical challenge.
Agilent Technologies, Inc.
Technical Solution: Agilent has developed a comprehensive HPLC method transfer toolkit that includes both hardware and software solutions. Their Intelligent System Emulation Technology (ISET) allows instruments to emulate the behavior of other HPLC systems by adjusting parameters like gradient delay volume, mixing behavior, and injection characteristics. This technology enables seamless method transfer between different instrument platforms without method modification. Agilent's Method Transfer Reference Solution Kit provides standard samples specifically designed to evaluate critical method transfer parameters. Their OpenLab CDS software includes built-in method transfer tools that automatically adjust method parameters based on instrument characteristics and provide detailed comparison reports to verify successful transfers. Agilent also offers specialized columns with consistent manufacturing specifications to minimize column-to-column variability during method transfer.
Strengths: Comprehensive ecosystem approach combining hardware emulation, software tools, and reference standards; ability to emulate competitor systems; strong technical support network. Weaknesses: Premium pricing structure may be prohibitive for smaller labs; some advanced features require investment in latest-generation instruments; complexity of full implementation may require significant training.
Waters Technology Corp.
Technical Solution: Waters has pioneered the Arc HPLC System specifically designed to facilitate method transfer across different instrument platforms. The system features Gradient SmartStart technology that precisely controls gradient formation and delivery, ensuring consistent retention times regardless of system configuration. Waters' Method Transfer Application Kit includes specialized software algorithms that automatically adjust critical parameters like dwell volume, mixing behavior, and detector settings to match the characteristics of the original method. Their Empower Chromatography Data Software includes a Method Transfer Agent that performs automated method scaling and optimization when transferring between different column dimensions or flow rates. Waters also employs Ultra Performance Convergence Chromatography (UPC²) technology that combines the principles of SFC and HPLC to provide more robust methods that are inherently easier to transfer across platforms.
Strengths: Purpose-built systems specifically designed for method transfer applications; extensive method transfer knowledge base and documentation; strong focus on regulatory compliance aspects of method transfer. Weaknesses: Solutions often work best within the Waters ecosystem; may require significant method development expertise to optimize transfers between non-Waters systems; premium pricing structure.
Critical Parameters and Variables Affecting Method Transfer
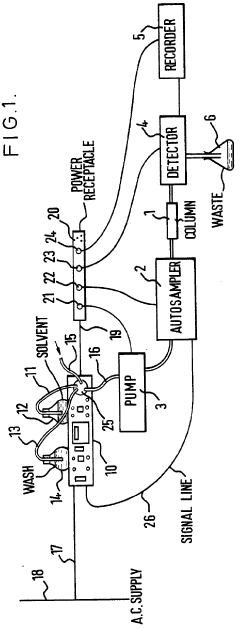
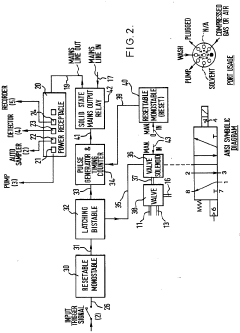
High-performance liquid chromatography with a controllable transverse flow inducer
PatentActiveEP3322978A1
Innovation
- The use of a controllable transverse flow inducer, which generates micro-scale vortices through alternating current electrokinetics, allowing for orthogonal flow induction independent of axial velocity, reducing dispersion by combining pressure and electro-osmotic flow, and enabling retention modulation without permanent surface charges.
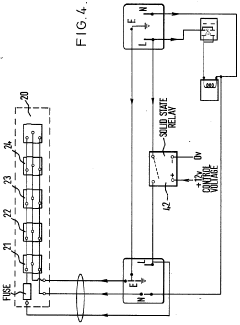
Improvements in or relating to high performance liquid chromatography systems
PatentInactiveGB2174016A
Innovation
- An ancillary apparatus that includes a valve system for selecting between mobile phase and wash liquid sources, a timing mechanism initiated by a trigger pulse from the HPLC system, and an electric switch to disconnect power to the HPLC system units after a predetermined wash liquid supply, ensuring a controlled shutdown and washout process.
Regulatory Considerations for HPLC Method Transfer
Regulatory compliance represents a critical dimension in HPLC method transfer processes across different instruments. The pharmaceutical and biotechnology industries operate under stringent regulatory frameworks established by authorities such as the FDA, EMA, and ICH, which mandate specific requirements for analytical method validation and transfer.
The ICH Q2(R1) guideline provides comprehensive recommendations for analytical method validation, including specificity, linearity, accuracy, precision, detection limit, quantitation limit, range, and robustness. These parameters must be carefully evaluated during method transfer to ensure consistent performance across different instruments.
FDA's guidance on analytical procedures and method validation emphasizes the importance of establishing acceptance criteria before initiating method transfer. These criteria should be scientifically justified and appropriate for the intended analytical application. The guidance also recommends documenting all aspects of method transfer, including any deviations and their impact on method performance.
Risk assessment frameworks, such as those outlined in ICH Q9, should be integrated into HPLC method transfer protocols. This approach helps identify critical method parameters that may be affected by instrument variations and establishes appropriate control strategies to mitigate risks.
Data integrity considerations have gained increased regulatory attention in recent years. When transferring HPLC methods, organizations must ensure compliance with ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available). This includes maintaining audit trails for all data generated during method transfer activities.
Regulatory agencies increasingly expect companies to implement a lifecycle approach to analytical method management, as described in USP <1220> and the proposed ICH Q14 guideline. This approach encompasses method development, validation, transfer, and continuous verification throughout the method's lifecycle.
For global organizations, navigating regional regulatory differences presents additional challenges. While harmonization efforts have made progress, differences still exist in requirements for method transfer across different jurisdictions. Companies must develop comprehensive transfer protocols that satisfy the most stringent requirements applicable to their products.
The qualification status of sending and receiving laboratories must be verified before method transfer. Both laboratories should operate under appropriate quality systems, with trained personnel and qualified instruments. Documentation of instrument qualification (IQ/OQ/PQ) is essential for regulatory compliance during method transfer activities.
The ICH Q2(R1) guideline provides comprehensive recommendations for analytical method validation, including specificity, linearity, accuracy, precision, detection limit, quantitation limit, range, and robustness. These parameters must be carefully evaluated during method transfer to ensure consistent performance across different instruments.
FDA's guidance on analytical procedures and method validation emphasizes the importance of establishing acceptance criteria before initiating method transfer. These criteria should be scientifically justified and appropriate for the intended analytical application. The guidance also recommends documenting all aspects of method transfer, including any deviations and their impact on method performance.
Risk assessment frameworks, such as those outlined in ICH Q9, should be integrated into HPLC method transfer protocols. This approach helps identify critical method parameters that may be affected by instrument variations and establishes appropriate control strategies to mitigate risks.
Data integrity considerations have gained increased regulatory attention in recent years. When transferring HPLC methods, organizations must ensure compliance with ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available). This includes maintaining audit trails for all data generated during method transfer activities.
Regulatory agencies increasingly expect companies to implement a lifecycle approach to analytical method management, as described in USP <1220> and the proposed ICH Q14 guideline. This approach encompasses method development, validation, transfer, and continuous verification throughout the method's lifecycle.
For global organizations, navigating regional regulatory differences presents additional challenges. While harmonization efforts have made progress, differences still exist in requirements for method transfer across different jurisdictions. Companies must develop comprehensive transfer protocols that satisfy the most stringent requirements applicable to their products.
The qualification status of sending and receiving laboratories must be verified before method transfer. Both laboratories should operate under appropriate quality systems, with trained personnel and qualified instruments. Documentation of instrument qualification (IQ/OQ/PQ) is essential for regulatory compliance during method transfer activities.
Quality Risk Management in Method Transfer Processes
Quality Risk Management (QRM) represents a systematic approach to identifying, assessing, controlling, and reviewing risks associated with HPLC method transfer processes. Implementing QRM principles in method transfer activities ensures that potential issues are proactively addressed rather than reactively managed after problems occur. The ICH Q9 guideline provides a comprehensive framework for applying risk management to pharmaceutical quality processes, including analytical method transfers.
Risk identification constitutes the initial phase of QRM in method transfer. This involves systematically cataloging all potential failure points across the transfer process, from instrument differences to environmental variations between sending and receiving laboratories. Critical parameters that may impact method performance must be identified through techniques such as failure mode and effects analysis (FMEA) or hazard analysis and critical control points (HACCP).
Risk assessment follows identification, quantifying the probability and severity of each identified risk. For HPLC method transfers, this assessment typically evaluates the likelihood of method failure against the impact on data quality and ultimately patient safety. Prioritization matrices help teams focus resources on high-risk areas that could significantly affect method transferability, such as differences in column chemistry, detector sensitivity, or data processing algorithms.
Risk control measures form the practical implementation of QRM in method transfer. These include developing robust transfer protocols with predefined acceptance criteria, conducting thorough system suitability tests, and implementing appropriate training programs for analysts at receiving sites. Preventive actions might include specifying tighter control ranges for critical parameters or implementing additional system checks when transferring between significantly different instrument models.
Continuous risk review represents the final component of effective QRM. This involves monitoring the performance of transferred methods over time, collecting data on method robustness across different instruments, and updating risk assessments based on accumulated knowledge. Establishing key performance indicators for method transfer success enables organizations to quantitatively track improvement in their transfer processes.
Documentation plays a crucial role throughout the QRM process for method transfers. Comprehensive risk management plans, detailed risk assessment reports, and clear mitigation strategies provide transparency and traceability. This documentation not only satisfies regulatory expectations but also creates an institutional knowledge base that improves future method transfer activities.
Risk identification constitutes the initial phase of QRM in method transfer. This involves systematically cataloging all potential failure points across the transfer process, from instrument differences to environmental variations between sending and receiving laboratories. Critical parameters that may impact method performance must be identified through techniques such as failure mode and effects analysis (FMEA) or hazard analysis and critical control points (HACCP).
Risk assessment follows identification, quantifying the probability and severity of each identified risk. For HPLC method transfers, this assessment typically evaluates the likelihood of method failure against the impact on data quality and ultimately patient safety. Prioritization matrices help teams focus resources on high-risk areas that could significantly affect method transferability, such as differences in column chemistry, detector sensitivity, or data processing algorithms.
Risk control measures form the practical implementation of QRM in method transfer. These include developing robust transfer protocols with predefined acceptance criteria, conducting thorough system suitability tests, and implementing appropriate training programs for analysts at receiving sites. Preventive actions might include specifying tighter control ranges for critical parameters or implementing additional system checks when transferring between significantly different instrument models.
Continuous risk review represents the final component of effective QRM. This involves monitoring the performance of transferred methods over time, collecting data on method robustness across different instruments, and updating risk assessments based on accumulated knowledge. Establishing key performance indicators for method transfer success enables organizations to quantitatively track improvement in their transfer processes.
Documentation plays a crucial role throughout the QRM process for method transfers. Comprehensive risk management plans, detailed risk assessment reports, and clear mitigation strategies provide transparency and traceability. This documentation not only satisfies regulatory expectations but also creates an institutional knowledge base that improves future method transfer activities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!