Measure Peak Area in HPLC for Quantitative Analysis
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC Quantitative Analysis Background and Objectives
High-Performance Liquid Chromatography (HPLC) has evolved significantly since its inception in the 1960s, becoming an indispensable analytical technique in pharmaceutical, environmental, food, and clinical laboratories. The measurement of peak area in HPLC represents a cornerstone methodology for quantitative analysis, enabling precise determination of compound concentrations in complex mixtures.
The historical trajectory of HPLC quantitative analysis shows remarkable technological advancement, from rudimentary UV detection systems to today's sophisticated diode array, fluorescence, and mass spectrometric detection methods. This evolution has been driven by increasing demands for higher sensitivity, improved selectivity, and enhanced reproducibility in analytical measurements across various industries.
Peak area measurement specifically has transitioned from manual integration using planimeters to sophisticated automated algorithms capable of handling complex chromatographic profiles. This progression reflects the broader trend toward digitalization and automation in analytical chemistry, significantly improving both accuracy and throughput in quantitative analysis.
Current technological trends in HPLC peak area measurement include machine learning approaches for peak deconvolution, cloud-based data processing systems, and the integration of quantitative analysis with broader laboratory information management systems. These developments aim to address persistent challenges in peak integration, particularly for co-eluting compounds and peaks with irregular shapes.
The primary technical objectives in this field include enhancing the accuracy of peak area determination through improved baseline correction algorithms, developing more robust integration methods for complex matrices, and establishing standardized approaches to peak area measurement that reduce inter-laboratory variability. Additionally, there is significant focus on developing methods that can handle increasingly lower detection limits while maintaining quantitative reliability.
Regulatory considerations have become increasingly important in HPLC quantitative analysis, with organizations such as the FDA, USP, and ICH establishing guidelines for validation of analytical methods, including specific requirements for peak area measurement and integration. These regulatory frameworks have substantially influenced the development trajectory of quantitative HPLC methodologies.
The convergence of advanced data processing capabilities with improved hardware has created new possibilities for peak area measurement, including real-time analysis and decision-making in process analytical technology applications. This represents a paradigm shift from traditional post-analysis data processing to integrated analytical workflows that provide immediate quantitative feedback.
The historical trajectory of HPLC quantitative analysis shows remarkable technological advancement, from rudimentary UV detection systems to today's sophisticated diode array, fluorescence, and mass spectrometric detection methods. This evolution has been driven by increasing demands for higher sensitivity, improved selectivity, and enhanced reproducibility in analytical measurements across various industries.
Peak area measurement specifically has transitioned from manual integration using planimeters to sophisticated automated algorithms capable of handling complex chromatographic profiles. This progression reflects the broader trend toward digitalization and automation in analytical chemistry, significantly improving both accuracy and throughput in quantitative analysis.
Current technological trends in HPLC peak area measurement include machine learning approaches for peak deconvolution, cloud-based data processing systems, and the integration of quantitative analysis with broader laboratory information management systems. These developments aim to address persistent challenges in peak integration, particularly for co-eluting compounds and peaks with irregular shapes.
The primary technical objectives in this field include enhancing the accuracy of peak area determination through improved baseline correction algorithms, developing more robust integration methods for complex matrices, and establishing standardized approaches to peak area measurement that reduce inter-laboratory variability. Additionally, there is significant focus on developing methods that can handle increasingly lower detection limits while maintaining quantitative reliability.
Regulatory considerations have become increasingly important in HPLC quantitative analysis, with organizations such as the FDA, USP, and ICH establishing guidelines for validation of analytical methods, including specific requirements for peak area measurement and integration. These regulatory frameworks have substantially influenced the development trajectory of quantitative HPLC methodologies.
The convergence of advanced data processing capabilities with improved hardware has created new possibilities for peak area measurement, including real-time analysis and decision-making in process analytical technology applications. This represents a paradigm shift from traditional post-analysis data processing to integrated analytical workflows that provide immediate quantitative feedback.
Market Demand for Accurate Peak Area Measurement
The global market for High-Performance Liquid Chromatography (HPLC) analysis continues to expand significantly, driven by increasing demands for accurate quantitative analysis across multiple industries. The pharmaceutical sector represents the largest market segment, where precise peak area measurement directly impacts drug development, quality control, and regulatory compliance. According to recent market analyses, the global HPLC market is projected to grow at a compound annual growth rate of 5.2% through 2028, with the software and data analysis segment showing particularly strong growth.
Accurate peak area measurement in HPLC has become increasingly critical as regulatory bodies worldwide implement more stringent requirements for analytical method validation. The FDA, EMA, and other regulatory authorities now demand higher levels of precision and reproducibility in quantitative analyses, particularly for pharmaceutical products where safety margins are narrow and dosing accuracy is paramount.
The biotechnology sector presents another rapidly growing market for advanced HPLC peak area measurement solutions. As biopharmaceuticals become more complex, the need for highly accurate quantification of proteins, peptides, and other biomolecules has intensified. This sector requires peak area measurement technologies capable of handling complex chromatograms with overlapping peaks and variable baselines.
Environmental monitoring represents an emerging market with substantial growth potential. Increasingly strict regulations regarding pollutants and contaminants in water, soil, and air samples necessitate highly sensitive and accurate quantitative analysis. The ability to precisely measure trace amounts of compounds in complex environmental matrices has become essential for regulatory compliance and public safety assurance.
The food and beverage industry demonstrates growing demand for accurate HPLC analysis, particularly in quality control applications and detection of contaminants or adulterants. As consumers and regulators focus more on food safety and authenticity, manufacturers require more sophisticated analytical capabilities, including precise peak area measurement for quantification of nutrients, additives, and potential contaminants.
Clinical diagnostics represents another significant market segment, where HPLC is increasingly used for therapeutic drug monitoring, vitamin analysis, and biomarker detection. The accuracy of peak area measurement directly impacts clinical decision-making, making advanced quantification technologies particularly valuable in this sector.
Market research indicates a growing preference for integrated software solutions that offer automated peak integration, baseline correction, and statistical analysis capabilities. End users increasingly demand systems that reduce manual intervention, minimize subjective interpretation, and provide audit trails for regulatory compliance. This trend is particularly pronounced in regulated industries where data integrity is paramount.
Accurate peak area measurement in HPLC has become increasingly critical as regulatory bodies worldwide implement more stringent requirements for analytical method validation. The FDA, EMA, and other regulatory authorities now demand higher levels of precision and reproducibility in quantitative analyses, particularly for pharmaceutical products where safety margins are narrow and dosing accuracy is paramount.
The biotechnology sector presents another rapidly growing market for advanced HPLC peak area measurement solutions. As biopharmaceuticals become more complex, the need for highly accurate quantification of proteins, peptides, and other biomolecules has intensified. This sector requires peak area measurement technologies capable of handling complex chromatograms with overlapping peaks and variable baselines.
Environmental monitoring represents an emerging market with substantial growth potential. Increasingly strict regulations regarding pollutants and contaminants in water, soil, and air samples necessitate highly sensitive and accurate quantitative analysis. The ability to precisely measure trace amounts of compounds in complex environmental matrices has become essential for regulatory compliance and public safety assurance.
The food and beverage industry demonstrates growing demand for accurate HPLC analysis, particularly in quality control applications and detection of contaminants or adulterants. As consumers and regulators focus more on food safety and authenticity, manufacturers require more sophisticated analytical capabilities, including precise peak area measurement for quantification of nutrients, additives, and potential contaminants.
Clinical diagnostics represents another significant market segment, where HPLC is increasingly used for therapeutic drug monitoring, vitamin analysis, and biomarker detection. The accuracy of peak area measurement directly impacts clinical decision-making, making advanced quantification technologies particularly valuable in this sector.
Market research indicates a growing preference for integrated software solutions that offer automated peak integration, baseline correction, and statistical analysis capabilities. End users increasingly demand systems that reduce manual intervention, minimize subjective interpretation, and provide audit trails for regulatory compliance. This trend is particularly pronounced in regulated industries where data integrity is paramount.
Current Challenges in HPLC Peak Integration
Despite significant advancements in HPLC technology, peak integration remains one of the most challenging aspects of quantitative analysis. Current integration algorithms often struggle with complex chromatographic scenarios, leading to inconsistent results across different laboratories and analytical platforms. The fundamental challenge lies in accurately defining peak boundaries, especially in cases where peaks exhibit tailing, fronting, or partial overlap with neighboring signals.
Baseline drift presents a persistent obstacle in peak area measurement. Environmental factors, gradient elution conditions, and detector instability can all contribute to non-linear baselines that complicate accurate integration. While modern software offers baseline correction algorithms, these often require manual optimization and can introduce subjective variations in quantitative results.
Peak shouldering and co-elution represent another significant challenge. When compounds elute with minimal separation, traditional integration methods may fail to accurately delineate individual peak areas. Current approaches like perpendicular drop, tangential skim, or exponential skim methods each introduce their own biases and limitations, particularly when dealing with compounds of vastly different concentrations or spectral properties.
Matrix effects in complex samples further complicate peak integration. Biological samples, environmental extracts, and pharmaceutical formulations often contain interfering compounds that can distort peak shapes and alter retention behaviors. Current integration strategies struggle to compensate for these matrix-induced variations, leading to reduced accuracy in quantitative determinations.
Noise discrimination represents another significant challenge. Modern ultra-sensitive HPLC detectors can detect trace analytes but also amplify baseline noise. Integration algorithms must distinguish between genuine small peaks and random signal fluctuations. Current threshold-based approaches often require compromise between sensitivity and specificity, potentially missing low-concentration analytes or integrating noise as false positives.
Reproducibility across different chromatographic systems remains problematic. Method transfer between instruments, even of the same model, can result in subtle changes in peak characteristics that affect integration results. Current standardization efforts have not fully addressed the need for robust integration parameters that maintain consistency across platforms.
The increasing automation of analytical workflows has highlighted limitations in existing integration algorithms. High-throughput environments require minimal manual intervention, yet current automated integration often fails with complex samples, necessitating time-consuming manual review and adjustment. This creates a bottleneck in analytical productivity and introduces potential human bias into supposedly objective quantitative results.
Baseline drift presents a persistent obstacle in peak area measurement. Environmental factors, gradient elution conditions, and detector instability can all contribute to non-linear baselines that complicate accurate integration. While modern software offers baseline correction algorithms, these often require manual optimization and can introduce subjective variations in quantitative results.
Peak shouldering and co-elution represent another significant challenge. When compounds elute with minimal separation, traditional integration methods may fail to accurately delineate individual peak areas. Current approaches like perpendicular drop, tangential skim, or exponential skim methods each introduce their own biases and limitations, particularly when dealing with compounds of vastly different concentrations or spectral properties.
Matrix effects in complex samples further complicate peak integration. Biological samples, environmental extracts, and pharmaceutical formulations often contain interfering compounds that can distort peak shapes and alter retention behaviors. Current integration strategies struggle to compensate for these matrix-induced variations, leading to reduced accuracy in quantitative determinations.
Noise discrimination represents another significant challenge. Modern ultra-sensitive HPLC detectors can detect trace analytes but also amplify baseline noise. Integration algorithms must distinguish between genuine small peaks and random signal fluctuations. Current threshold-based approaches often require compromise between sensitivity and specificity, potentially missing low-concentration analytes or integrating noise as false positives.
Reproducibility across different chromatographic systems remains problematic. Method transfer between instruments, even of the same model, can result in subtle changes in peak characteristics that affect integration results. Current standardization efforts have not fully addressed the need for robust integration parameters that maintain consistency across platforms.
The increasing automation of analytical workflows has highlighted limitations in existing integration algorithms. High-throughput environments require minimal manual intervention, yet current automated integration often fails with complex samples, necessitating time-consuming manual review and adjustment. This creates a bottleneck in analytical productivity and introduces potential human bias into supposedly objective quantitative results.
Established Peak Area Measurement Techniques
01 HPLC peak area quantification methods
High-Performance Liquid Chromatography (HPLC) peak area is used for quantitative analysis of compounds in various samples. The peak area correlates with the concentration of the analyte, allowing for precise measurement of compound quantities. This method involves integrating the area under chromatographic peaks and comparing them with calibration standards to determine concentrations. Advanced algorithms are employed to ensure accurate peak integration even in complex chromatograms with overlapping peaks.- HPLC peak area quantification methods: High-Performance Liquid Chromatography (HPLC) peak area is used for quantitative analysis of compounds in various samples. The peak area is directly proportional to the concentration of the analyte, allowing for accurate determination of compound quantities. This method involves integrating the area under chromatographic peaks and comparing them with calibration standards to determine concentrations of target compounds in complex mixtures.
- HPLC peak area normalization techniques: Peak area normalization is a technique used in HPLC analysis to express the relative content of components in a sample. This method calculates the percentage of each peak area relative to the total area of all peaks, eliminating the need for calibration curves in some applications. Normalization techniques help compensate for variations in injection volume and improve the comparability of results across different analytical runs.
- HPLC peak area optimization and validation: Optimization and validation of HPLC peak area measurements involve adjusting chromatographic parameters to improve peak resolution, symmetry, and reproducibility. This includes optimizing mobile phase composition, flow rate, column temperature, and detection parameters. Validation procedures ensure the reliability of peak area measurements through assessments of linearity, precision, accuracy, specificity, and robustness according to regulatory guidelines.
- HPLC peak area in pharmaceutical analysis: In pharmaceutical analysis, HPLC peak area is critical for drug quality control, stability testing, and impurity profiling. The technique enables precise quantification of active pharmaceutical ingredients and their impurities at trace levels. Advanced HPLC methods with optimized peak area detection are used for bioequivalence studies, formulation development, and ensuring compliance with pharmacopoeial standards for drug products.
- Automated HPLC peak area analysis systems: Automated systems for HPLC peak area analysis incorporate advanced software algorithms for peak detection, integration, and data processing. These systems can automatically identify peaks, calculate areas, and perform statistical analysis with minimal human intervention. Machine learning approaches are increasingly being applied to improve peak identification and integration in complex chromatograms, enhancing throughput and reducing operator-dependent variability in analytical results.
02 HPLC peak area normalization techniques
Peak area normalization is a critical technique in HPLC analysis where the area of each peak is expressed as a percentage of the total area of all peaks. This approach is particularly useful when absolute concentrations are not required or when analyzing complex mixtures. Normalization techniques help compensate for variations in injection volume and detector response, improving the reproducibility and reliability of analytical results. Various mathematical models are applied to normalize peak areas based on internal standards or reference compounds.Expand Specific Solutions03 HPLC peak area validation and quality control
Validation of HPLC peak area measurements is essential for ensuring analytical method reliability. This involves establishing parameters such as linearity, precision, accuracy, and limits of detection and quantification. Quality control procedures include system suitability tests, regular calibration, and the use of control samples. Statistical methods are applied to evaluate the robustness of peak area measurements across different analytical conditions, equipment, and operators to ensure consistent and reliable results.Expand Specific Solutions04 Advanced algorithms for HPLC peak area integration
Modern HPLC analysis employs sophisticated algorithms for peak area integration to improve accuracy and reproducibility. These algorithms can automatically detect peak boundaries, correct baseline drift, and resolve overlapping peaks. Machine learning approaches are increasingly used to optimize peak integration parameters based on the specific characteristics of different sample types and chromatographic conditions. These advanced integration methods significantly enhance the reliability of quantitative analysis, especially for complex samples with multiple analytes.Expand Specific Solutions05 Applications of HPLC peak area in pharmaceutical analysis
HPLC peak area analysis is widely applied in pharmaceutical research and quality control. It is used for drug content uniformity testing, impurity profiling, stability studies, and bioanalytical method development. The technique enables precise quantification of active pharmaceutical ingredients and their degradation products in various formulations. Regulatory guidelines provide specific requirements for HPLC peak area analysis in pharmaceutical applications, including system suitability criteria, validation parameters, and acceptance limits for analytical results.Expand Specific Solutions
Leading Manufacturers and Software Providers
The HPLC peak area measurement market for quantitative analysis is in a mature growth phase, with an estimated global market size exceeding $5 billion. The competitive landscape is dominated by established analytical instrument manufacturers like Shimadzu, JEOL, and Tosoh, who offer comprehensive HPLC systems with advanced peak integration algorithms. Technological maturity is high, with recent innovations focusing on automation and AI-assisted peak identification. Leading pharmaceutical companies such as Roche, F. Hoffmann-La Roche, and Daiichi Sankyo are driving demand through stringent quality control requirements, while research institutions like Politecnico di Milano and KIST contribute to methodological advancements. The market shows increasing integration of HPLC systems with mass spectrometry and other analytical techniques, with companies like Revvity Health Sciences pioneering hybrid solutions.
Tosoh Corp.
Technical Solution: Tosoh Corporation has pioneered specialized HPLC systems with their EcoSEC® technology specifically designed for high-precision peak area measurement in polymer analysis and biopharmaceutical applications. Their approach integrates advanced refractive index detection with multi-angle light scattering for absolute molecular weight determination alongside quantitative analysis. Tosoh's proprietary TS-UP software employs adaptive baseline algorithms that can handle complex gradient separations with minimal manual intervention. Their systems feature temperature-controlled detection cells with precision of ±0.01°C to eliminate baseline drift that can compromise peak area measurements[4]. For biopharmaceutical applications, Tosoh has developed specialized columns and detection methods that enable accurate glycan profiling through precise peak area quantification, with validated methods showing CVs below 1% for replicate injections. Their latest innovation includes automated system suitability testing that verifies peak area measurement accuracy before sample analysis begins.
Strengths: Exceptional stability for long analytical runs; specialized expertise in biopharmaceutical and polymer applications; highly reproducible peak area measurements with minimal drift. Weaknesses: More limited application range compared to general-purpose HPLC vendors; higher specialization requirements for operators; less extensive third-party support network.
Revvity Health Sciences, Inc.
Technical Solution: Revvity Health Sciences (formerly PerkinElmer) has developed the Chromera CDS platform specifically optimized for peak area measurement in quantitative HPLC analysis. Their system employs a multi-point calibration approach with advanced statistical tools for calibration curve evaluation, including automatic outlier detection and weighted regression analysis. The company's PeakSimple™ technology uses dynamic baseline projection algorithms that adapt to chromatographic drift during long analytical runs, maintaining quantification accuracy. Their QsightTM LC/MS/MS systems incorporate Time-of-Flight technology with high-resolution mass detection for peak confirmation alongside area measurement, providing orthogonal verification of analyte identity during quantification[2]. Revvity's software includes compliance-ready audit trails for all peak integration adjustments, critical for regulated environments, and their patented AutoPeak integration algorithm can process thousands of samples with consistent integration parameters.
Strengths: Exceptional reproducibility in peak area measurements across different operators; comprehensive statistical tools for method validation; strong regulatory compliance features for pharmaceutical applications. Weaknesses: Less intuitive user interface compared to market leaders; more complex implementation in multi-vendor laboratory environments; higher computational requirements for advanced algorithms.
Critical Algorithms for Accurate Peak Integration
Method for measuring ambrisentan content by high performance liquid chromatography
PatentWO2019046997A1
Innovation
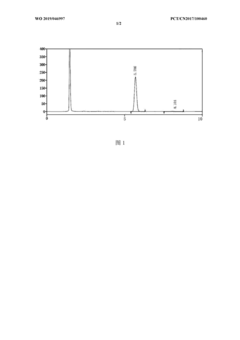
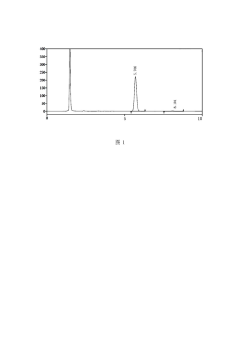
- High performance liquid chromatography was used. The specific steps included using octadecylsilane bonded silica gel as the filler, column length 250mm, detection wavelength 220nm, column temperature 35°C, flow rate 1.0ml/min, and reversed phase high performance liquid chromatography. Combined with a UV detector, phosphate buffer solution and polar organic solvents are used for elution to determine the content of ambrisentan.
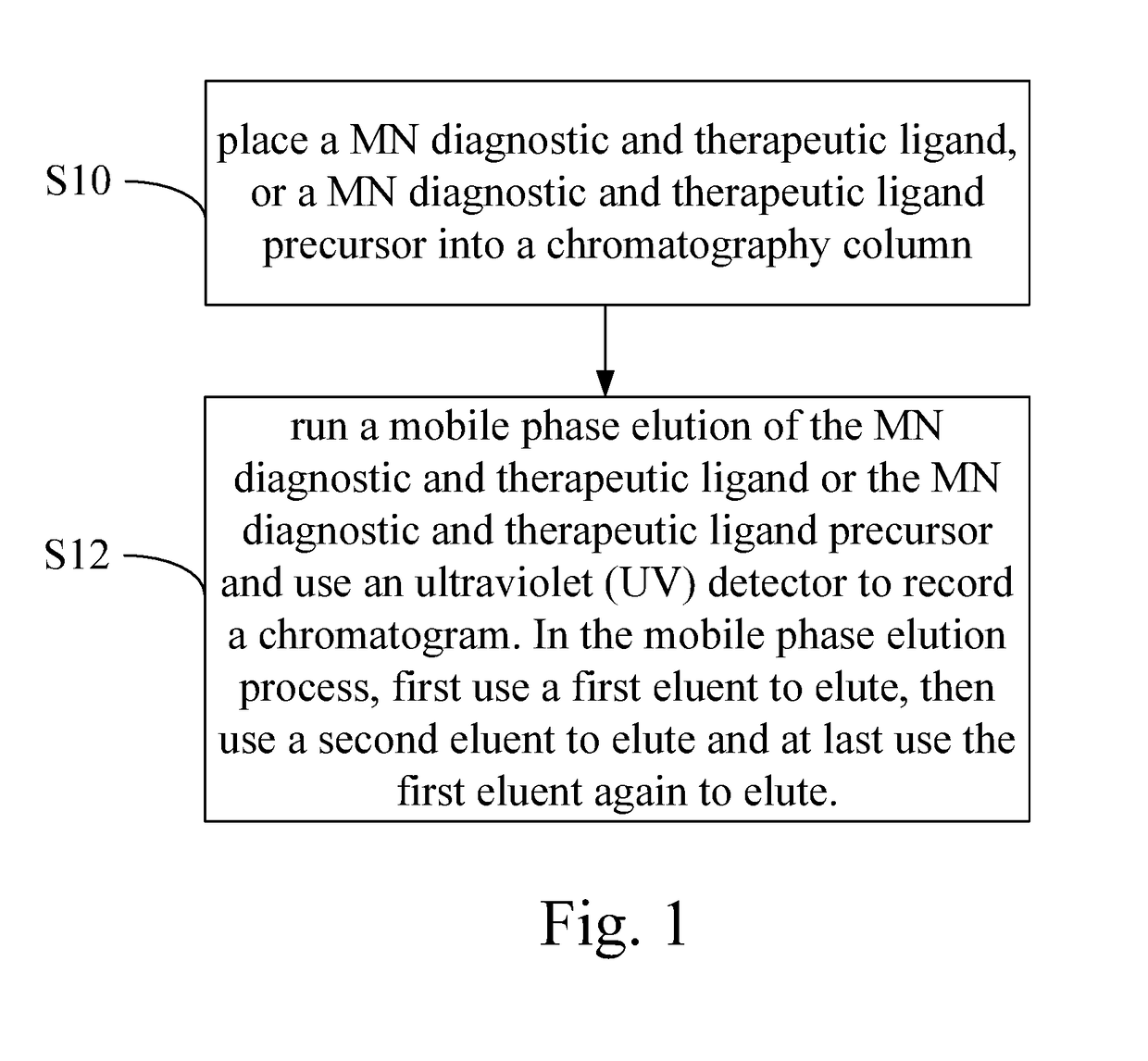
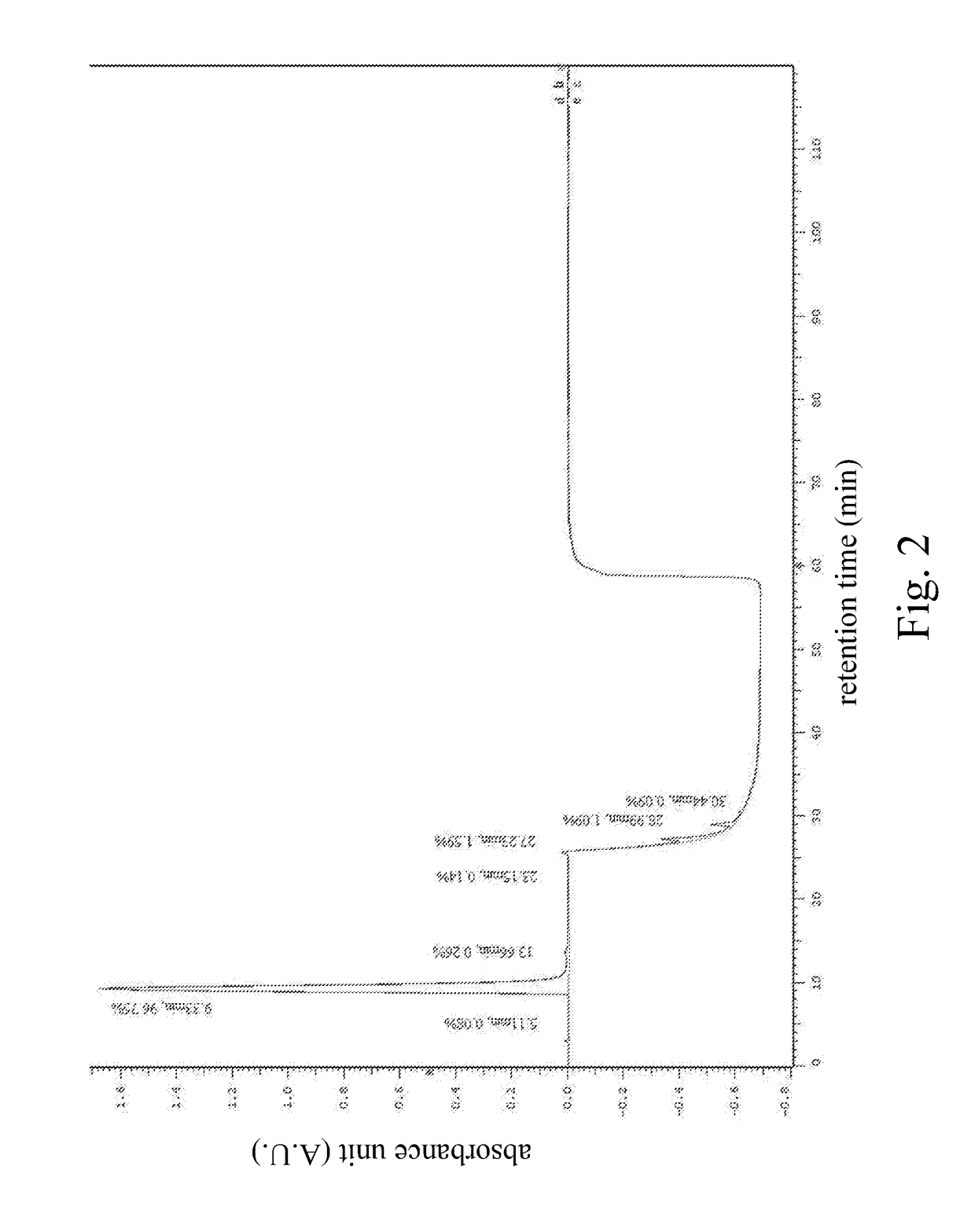
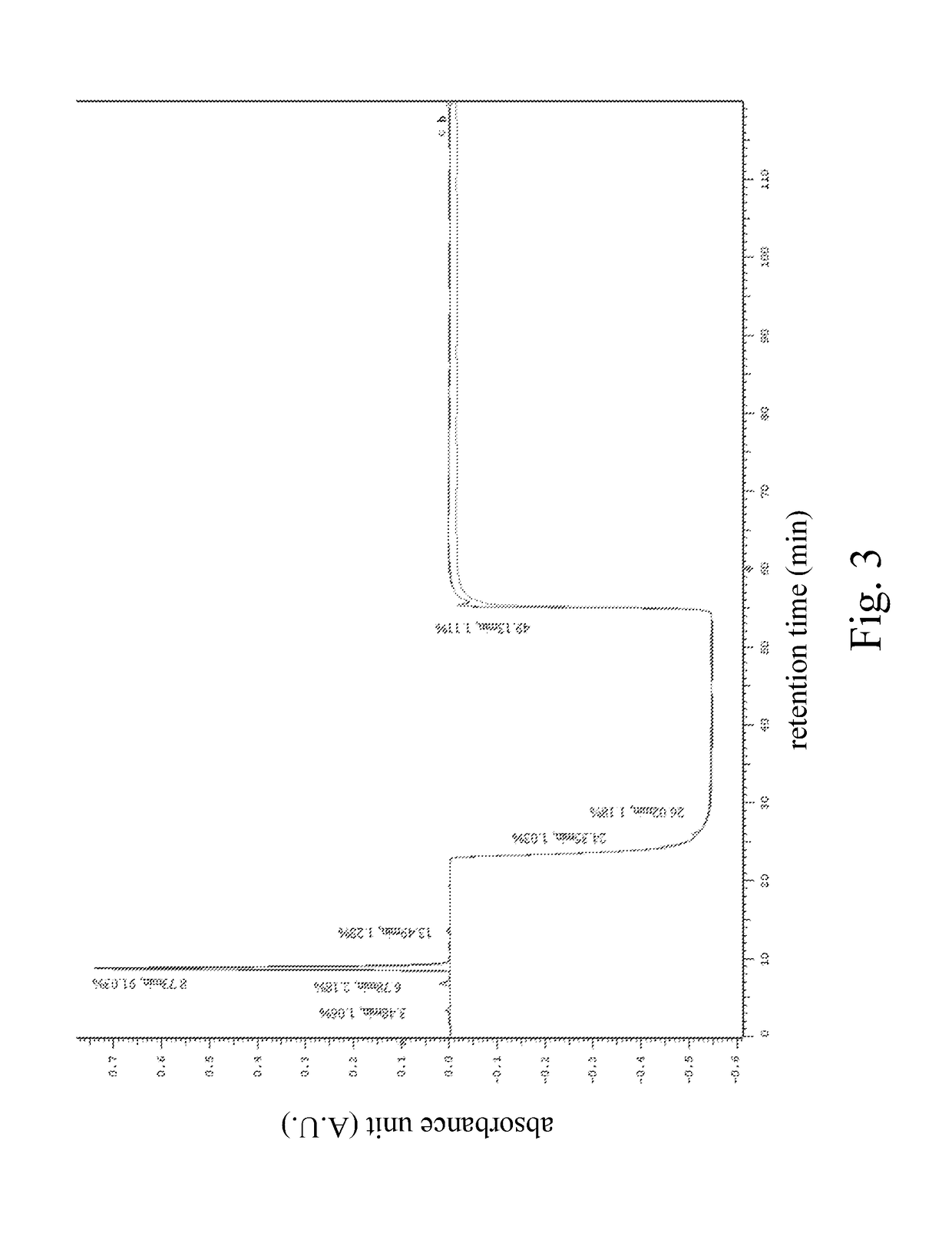
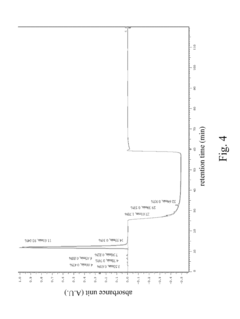
High performance liquid chromatography method for analysis of MN diagnostic and therapeutic ligand and precursor
PatentInactiveUS20180059072A1
Innovation
- A HPLC method utilizing a high ratio of acetonitrile in the mobile phase to wash out low-polarity impurities and employing gradient elution with a UV detector at 210 nm to improve detection accuracy, ensuring accurate analysis and reducing residual impurities in the column.
Validation and Calibration Protocols
Validation and calibration protocols form the cornerstone of reliable quantitative analysis in HPLC peak area measurements. These protocols ensure that analytical methods consistently produce accurate, precise, and reproducible results across different laboratories, instruments, and analysts.
The validation process for HPLC peak area measurement typically follows international guidelines such as ICH Q2(R1), USP <1225>, or FDA guidance documents. These frameworks require evaluation of several critical parameters: specificity, linearity, range, accuracy, precision, detection limit, quantitation limit, and robustness. For peak area measurements specifically, system suitability tests must verify adequate resolution, peak symmetry, and reproducibility before analytical runs.
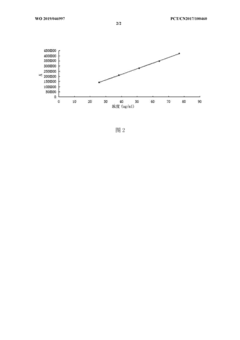
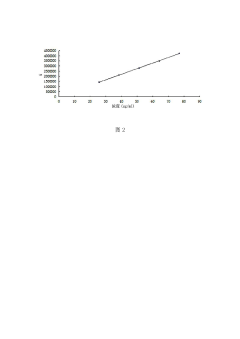
Calibration protocols establish the relationship between analyte concentration and detector response. The standard calibration approach utilizes external standards at multiple concentration levels (typically 5-8 points) spanning 50-150% of the target analyte concentration. The resulting calibration curve must demonstrate acceptable linearity with r² values exceeding 0.999 for most pharmaceutical applications. Weighted regression models (1/x or 1/x²) are increasingly employed to address heteroscedasticity when working across wide concentration ranges.
Quality control samples at low, medium, and high concentrations within the calibration range must be analyzed alongside unknown samples to continuously verify method performance. Acceptance criteria typically require QC samples to fall within ±15% of their nominal values (±20% at the lower limit of quantification). System suitability parameters including retention time variability (<2% RSD), peak area reproducibility (<2% RSD), and tailing factor (<2.0) must be established and monitored.
Method transfer protocols deserve special attention when peak area measurements will be conducted across multiple laboratories or instruments. These protocols should include detailed procedures for instrument qualification, analyst training, and comparative testing to ensure equivalent results regardless of location or equipment.
Automated integration parameters require thorough validation to ensure consistent peak area determination. This includes validation of baseline algorithms, peak detection thresholds, and integration boundaries. Modern HPLC data systems offer sophisticated integration tools, but their parameters must be locked and documented within validated methods to prevent manipulation that could compromise quantitative accuracy.
Periodic revalidation schedules must be established based on risk assessment, with full or partial revalidation triggered by significant changes to methods, instruments, or sample matrices. This ongoing verification ensures the continued reliability of peak area measurements throughout the analytical method's lifecycle.
The validation process for HPLC peak area measurement typically follows international guidelines such as ICH Q2(R1), USP <1225>, or FDA guidance documents. These frameworks require evaluation of several critical parameters: specificity, linearity, range, accuracy, precision, detection limit, quantitation limit, and robustness. For peak area measurements specifically, system suitability tests must verify adequate resolution, peak symmetry, and reproducibility before analytical runs.
Calibration protocols establish the relationship between analyte concentration and detector response. The standard calibration approach utilizes external standards at multiple concentration levels (typically 5-8 points) spanning 50-150% of the target analyte concentration. The resulting calibration curve must demonstrate acceptable linearity with r² values exceeding 0.999 for most pharmaceutical applications. Weighted regression models (1/x or 1/x²) are increasingly employed to address heteroscedasticity when working across wide concentration ranges.
Quality control samples at low, medium, and high concentrations within the calibration range must be analyzed alongside unknown samples to continuously verify method performance. Acceptance criteria typically require QC samples to fall within ±15% of their nominal values (±20% at the lower limit of quantification). System suitability parameters including retention time variability (<2% RSD), peak area reproducibility (<2% RSD), and tailing factor (<2.0) must be established and monitored.
Method transfer protocols deserve special attention when peak area measurements will be conducted across multiple laboratories or instruments. These protocols should include detailed procedures for instrument qualification, analyst training, and comparative testing to ensure equivalent results regardless of location or equipment.
Automated integration parameters require thorough validation to ensure consistent peak area determination. This includes validation of baseline algorithms, peak detection thresholds, and integration boundaries. Modern HPLC data systems offer sophisticated integration tools, but their parameters must be locked and documented within validated methods to prevent manipulation that could compromise quantitative accuracy.
Periodic revalidation schedules must be established based on risk assessment, with full or partial revalidation triggered by significant changes to methods, instruments, or sample matrices. This ongoing verification ensures the continued reliability of peak area measurements throughout the analytical method's lifecycle.
Regulatory Compliance in Analytical Chemistry
Regulatory compliance in analytical chemistry forms a critical framework governing the implementation of HPLC peak area measurement for quantitative analysis. Organizations must navigate complex regulatory landscapes established by authorities such as the FDA, EMA, and ICH, which mandate strict adherence to Good Laboratory Practices (GLP) and Good Manufacturing Practices (GMP) when conducting analytical measurements.
The FDA's 21 CFR Part 11 specifically addresses electronic records and signatures, requiring validated systems, audit trails, and secure data handling for HPLC analysis data. This regulation ensures data integrity throughout the analytical workflow, from sample preparation to final quantitative results based on peak area measurements.
Similarly, the ICH Q2(R1) guideline on Validation of Analytical Procedures provides essential parameters for validating HPLC methods used in peak area quantification. These include specificity, linearity, range, accuracy, precision, detection limit, and robustness - all critical for ensuring reliable peak area measurements that can withstand regulatory scrutiny.
Pharmaceutical and clinical laboratories must also comply with USP <621> Chromatography standards, which establish system suitability requirements for HPLC methods. These requirements ensure that peak area measurements remain consistent and accurate across different analytical runs and between different analysts.
ISO/IEC 17025 accreditation represents another important compliance consideration for laboratories performing quantitative HPLC analysis. This standard focuses on technical competence and quality management systems, requiring documented procedures for peak area measurement, regular instrument calibration, and proficiency testing.
Data integrity requirements have become increasingly stringent, with regulatory bodies emphasizing ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available). These principles must be applied to all peak area measurements and subsequent quantitative calculations.
Method transfer protocols present additional compliance challenges when HPLC methods for peak area measurement must be transferred between laboratories or sites. Regulatory bodies expect comprehensive documentation demonstrating equivalent performance at the receiving site, with statistical evaluation of peak area measurements between locations.
Compliance with these regulations necessitates robust documentation practices, including Standard Operating Procedures (SOPs) for peak area integration, analyst training records, instrument qualification documentation, and complete audit trails for any manual integration of chromatographic peaks.
The FDA's 21 CFR Part 11 specifically addresses electronic records and signatures, requiring validated systems, audit trails, and secure data handling for HPLC analysis data. This regulation ensures data integrity throughout the analytical workflow, from sample preparation to final quantitative results based on peak area measurements.
Similarly, the ICH Q2(R1) guideline on Validation of Analytical Procedures provides essential parameters for validating HPLC methods used in peak area quantification. These include specificity, linearity, range, accuracy, precision, detection limit, and robustness - all critical for ensuring reliable peak area measurements that can withstand regulatory scrutiny.
Pharmaceutical and clinical laboratories must also comply with USP <621> Chromatography standards, which establish system suitability requirements for HPLC methods. These requirements ensure that peak area measurements remain consistent and accurate across different analytical runs and between different analysts.
ISO/IEC 17025 accreditation represents another important compliance consideration for laboratories performing quantitative HPLC analysis. This standard focuses on technical competence and quality management systems, requiring documented procedures for peak area measurement, regular instrument calibration, and proficiency testing.
Data integrity requirements have become increasingly stringent, with regulatory bodies emphasizing ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available). These principles must be applied to all peak area measurements and subsequent quantitative calculations.
Method transfer protocols present additional compliance challenges when HPLC methods for peak area measurement must be transferred between laboratories or sites. Regulatory bodies expect comprehensive documentation demonstrating equivalent performance at the receiving site, with statistical evaluation of peak area measurements between locations.
Compliance with these regulations necessitates robust documentation practices, including Standard Operating Procedures (SOPs) for peak area integration, analyst training records, instrument qualification documentation, and complete audit trails for any manual integration of chromatographic peaks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!