HPLC vs MS: Sensitivity and Selectivity Comparison
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC-MS Technology Evolution and Objectives
High-performance liquid chromatography (HPLC) and mass spectrometry (MS) have evolved significantly since their inception in the mid-20th century. HPLC emerged in the 1960s as an advancement over traditional column chromatography, offering improved resolution and quantification capabilities. The technology progressed from normal-phase to reverse-phase chromatography, enabling analysis of a broader range of compounds with enhanced efficiency.
Mass spectrometry's journey began earlier, with the first mass spectrometer developed by J.J. Thomson in 1913. However, the technology remained primarily in physics laboratories until the 1950s when it began finding applications in analytical chemistry. The development of various ionization techniques—from electron impact (EI) to electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI)—revolutionized MS capabilities, particularly for analyzing large biomolecules.
The integration of these technologies into HPLC-MS systems represents a watershed moment in analytical chemistry. Early coupling attempts in the 1970s faced significant interface challenges due to the incompatibility between HPLC's liquid phase and MS's vacuum requirements. The breakthrough came with atmospheric pressure ionization techniques in the 1980s, particularly electrospray ionization, which effectively bridged this gap.
The evolution of HPLC-MS technology has been driven by increasing demands for sensitivity and selectivity across various fields. In pharmaceutical research, the need to detect increasingly potent drugs at lower concentrations pushed technological boundaries. Environmental monitoring required the ability to identify trace contaminants in complex matrices, while proteomics research demanded tools capable of analyzing intricate biological samples.
Current technological objectives focus on several key areas: improving detection limits to reach femtogram or even attogram levels; enhancing selectivity to distinguish between structurally similar compounds; increasing throughput for high-volume screening applications; and developing more user-friendly systems that require less specialized expertise to operate effectively.
The miniaturization trend represents another significant direction, with efforts to develop portable HPLC-MS systems for field applications. Simultaneously, there is a push toward greater integration with other analytical techniques and computational tools, creating comprehensive analytical platforms rather than standalone instruments.
The ultimate goal of modern HPLC-MS development is to achieve what might be termed "universal detection"—the ability to reliably identify and quantify any compound in any matrix at any concentration relevant to the application. While this remains aspirational, each technological advancement brings the field closer to this ideal, expanding the boundaries of what can be detected and measured in complex samples.
Mass spectrometry's journey began earlier, with the first mass spectrometer developed by J.J. Thomson in 1913. However, the technology remained primarily in physics laboratories until the 1950s when it began finding applications in analytical chemistry. The development of various ionization techniques—from electron impact (EI) to electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI)—revolutionized MS capabilities, particularly for analyzing large biomolecules.
The integration of these technologies into HPLC-MS systems represents a watershed moment in analytical chemistry. Early coupling attempts in the 1970s faced significant interface challenges due to the incompatibility between HPLC's liquid phase and MS's vacuum requirements. The breakthrough came with atmospheric pressure ionization techniques in the 1980s, particularly electrospray ionization, which effectively bridged this gap.
The evolution of HPLC-MS technology has been driven by increasing demands for sensitivity and selectivity across various fields. In pharmaceutical research, the need to detect increasingly potent drugs at lower concentrations pushed technological boundaries. Environmental monitoring required the ability to identify trace contaminants in complex matrices, while proteomics research demanded tools capable of analyzing intricate biological samples.
Current technological objectives focus on several key areas: improving detection limits to reach femtogram or even attogram levels; enhancing selectivity to distinguish between structurally similar compounds; increasing throughput for high-volume screening applications; and developing more user-friendly systems that require less specialized expertise to operate effectively.
The miniaturization trend represents another significant direction, with efforts to develop portable HPLC-MS systems for field applications. Simultaneously, there is a push toward greater integration with other analytical techniques and computational tools, creating comprehensive analytical platforms rather than standalone instruments.
The ultimate goal of modern HPLC-MS development is to achieve what might be termed "universal detection"—the ability to reliably identify and quantify any compound in any matrix at any concentration relevant to the application. While this remains aspirational, each technological advancement brings the field closer to this ideal, expanding the boundaries of what can be detected and measured in complex samples.
Market Applications and Analytical Demand
The analytical instrumentation market has witnessed substantial growth driven by increasing demand for precise chemical analysis across various industries. High Performance Liquid Chromatography (HPLC) and Mass Spectrometry (MS) represent two cornerstone technologies in this sector, with the global analytical instrumentation market valued at approximately $85 billion in 2022 and projected to reach $110 billion by 2027, growing at a CAGR of 5.3%.
Pharmaceutical and biotechnology sectors remain the largest consumers of both HPLC and MS technologies, accounting for nearly 40% of the market share. These industries require exceptional sensitivity and selectivity for drug discovery, development, and quality control processes. The ability to detect impurities at parts-per-billion levels has become standard for regulatory compliance, with MS offering superior capabilities in this regard.
Environmental monitoring represents another significant application area, where the detection of emerging contaminants such as PFAS (per- and polyfluoroalkyl substances) at ultra-trace levels has created strong demand for advanced MS systems. Government regulations worldwide have tightened detection limits for environmental pollutants, necessitating instrumentation with enhanced sensitivity.
The food and beverage industry has similarly experienced increased analytical demands, particularly for pesticide residue testing, allergen detection, and authentication of premium products. Here, both technologies find complementary applications, with HPLC offering cost-effective routine analysis and MS providing definitive identification capabilities for complex matrices.
Clinical diagnostics represents the fastest-growing application segment, with a CAGR exceeding 7%. The rise of precision medicine has driven demand for highly selective analytical methods capable of quantifying biomarkers, therapeutic drugs, and metabolites in biological fluids. LC-MS/MS has emerged as the gold standard for many clinical applications due to its unmatched selectivity.
Regional market analysis reveals that North America holds the largest market share (approximately 35%), followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is experiencing the fastest growth rate due to expanding pharmaceutical manufacturing, environmental concerns, and food safety initiatives in countries like China and India.
The analytical demand trends clearly indicate a shift toward integrated systems that combine the separation power of HPLC with the identification capabilities of MS. End-users increasingly prioritize analytical solutions that offer improved sensitivity, selectivity, and throughput while reducing sample preparation requirements and analysis time. This market dynamic has accelerated the development of hybrid technologies and miniaturized systems suitable for point-of-need testing.
Pharmaceutical and biotechnology sectors remain the largest consumers of both HPLC and MS technologies, accounting for nearly 40% of the market share. These industries require exceptional sensitivity and selectivity for drug discovery, development, and quality control processes. The ability to detect impurities at parts-per-billion levels has become standard for regulatory compliance, with MS offering superior capabilities in this regard.
Environmental monitoring represents another significant application area, where the detection of emerging contaminants such as PFAS (per- and polyfluoroalkyl substances) at ultra-trace levels has created strong demand for advanced MS systems. Government regulations worldwide have tightened detection limits for environmental pollutants, necessitating instrumentation with enhanced sensitivity.
The food and beverage industry has similarly experienced increased analytical demands, particularly for pesticide residue testing, allergen detection, and authentication of premium products. Here, both technologies find complementary applications, with HPLC offering cost-effective routine analysis and MS providing definitive identification capabilities for complex matrices.
Clinical diagnostics represents the fastest-growing application segment, with a CAGR exceeding 7%. The rise of precision medicine has driven demand for highly selective analytical methods capable of quantifying biomarkers, therapeutic drugs, and metabolites in biological fluids. LC-MS/MS has emerged as the gold standard for many clinical applications due to its unmatched selectivity.
Regional market analysis reveals that North America holds the largest market share (approximately 35%), followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is experiencing the fastest growth rate due to expanding pharmaceutical manufacturing, environmental concerns, and food safety initiatives in countries like China and India.
The analytical demand trends clearly indicate a shift toward integrated systems that combine the separation power of HPLC with the identification capabilities of MS. End-users increasingly prioritize analytical solutions that offer improved sensitivity, selectivity, and throughput while reducing sample preparation requirements and analysis time. This market dynamic has accelerated the development of hybrid technologies and miniaturized systems suitable for point-of-need testing.
Current Capabilities and Technical Limitations
High-Performance Liquid Chromatography (HPLC) and Mass Spectrometry (MS) represent two cornerstone analytical technologies with distinct capabilities and limitations in terms of sensitivity and selectivity. Current HPLC systems typically offer detection limits in the nanogram to picogram range, with modern ultra-high-performance liquid chromatography (UHPLC) pushing these boundaries further. However, HPLC fundamentally relies on the detector technology employed, with UV-Vis detectors being most common but limited in their ability to distinguish between co-eluting compounds with similar absorption profiles.
MS systems demonstrate significantly superior sensitivity, routinely achieving detection limits in the picogram to femtogram range, with high-end instruments capable of attogram detection under optimal conditions. This represents a 1000-fold or greater sensitivity advantage over standard HPLC configurations. The exceptional sensitivity of MS stems from its ability to detect specific mass-to-charge ratios rather than bulk properties like UV absorption.
Regarding selectivity, HPLC's discrimination capability depends heavily on column chemistry and mobile phase composition. Even with optimized conditions, HPLC struggles to differentiate compounds with similar physicochemical properties. The selectivity is primarily determined by differences in partition coefficients between the mobile and stationary phases, which can be insufficient for complex matrices.
MS offers unparalleled selectivity through its ability to distinguish compounds based on their unique molecular masses and fragmentation patterns. Modern MS instruments with high-resolution capabilities can differentiate compounds differing by less than 0.001 atomic mass units. This exceptional mass accuracy, combined with MS/MS capabilities, enables the identification of compounds even in highly complex matrices where conventional HPLC would fail.
A significant technical limitation for HPLC is its dependence on reference standards for accurate quantification and identification. Without authentic standards, HPLC analysis remains tentative at best. Additionally, HPLC faces challenges with compounds lacking chromophores when using optical detection methods.
MS systems, while superior in many aspects, face limitations in quantitative reproducibility due to matrix effects and ion suppression phenomena. These effects can significantly impact quantification accuracy without proper internal standardization. Furthermore, MS systems require more extensive maintenance, specialized operator training, and considerably higher capital investment, with high-end systems costing 5-10 times more than comparable HPLC setups.
The integration of these technologies as LC-MS combines the separation power of HPLC with the detection capabilities of MS, addressing many individual limitations. However, this hyphenated approach introduces new challenges in method development, data interpretation, and system optimization, requiring specialized expertise to fully leverage its capabilities.
MS systems demonstrate significantly superior sensitivity, routinely achieving detection limits in the picogram to femtogram range, with high-end instruments capable of attogram detection under optimal conditions. This represents a 1000-fold or greater sensitivity advantage over standard HPLC configurations. The exceptional sensitivity of MS stems from its ability to detect specific mass-to-charge ratios rather than bulk properties like UV absorption.
Regarding selectivity, HPLC's discrimination capability depends heavily on column chemistry and mobile phase composition. Even with optimized conditions, HPLC struggles to differentiate compounds with similar physicochemical properties. The selectivity is primarily determined by differences in partition coefficients between the mobile and stationary phases, which can be insufficient for complex matrices.
MS offers unparalleled selectivity through its ability to distinguish compounds based on their unique molecular masses and fragmentation patterns. Modern MS instruments with high-resolution capabilities can differentiate compounds differing by less than 0.001 atomic mass units. This exceptional mass accuracy, combined with MS/MS capabilities, enables the identification of compounds even in highly complex matrices where conventional HPLC would fail.
A significant technical limitation for HPLC is its dependence on reference standards for accurate quantification and identification. Without authentic standards, HPLC analysis remains tentative at best. Additionally, HPLC faces challenges with compounds lacking chromophores when using optical detection methods.
MS systems, while superior in many aspects, face limitations in quantitative reproducibility due to matrix effects and ion suppression phenomena. These effects can significantly impact quantification accuracy without proper internal standardization. Furthermore, MS systems require more extensive maintenance, specialized operator training, and considerably higher capital investment, with high-end systems costing 5-10 times more than comparable HPLC setups.
The integration of these technologies as LC-MS combines the separation power of HPLC with the detection capabilities of MS, addressing many individual limitations. However, this hyphenated approach introduces new challenges in method development, data interpretation, and system optimization, requiring specialized expertise to fully leverage its capabilities.
Comparative Analysis of HPLC and MS Methodologies
01 Enhanced sensitivity techniques for HPLC-MS analysis
Various techniques can be employed to enhance the sensitivity of HPLC-MS analysis, including optimized sample preparation methods, concentration techniques, and specialized detection parameters. These approaches allow for the detection of trace amounts of analytes in complex matrices. Improved sensitivity enables the quantification of compounds at lower concentrations, which is particularly important in pharmaceutical, environmental, and clinical applications where target analytes may be present at very low levels.- Enhanced sensitivity techniques for HPLC-MS analysis: Various techniques can be employed to enhance the sensitivity of HPLC-MS analysis, including optimized sample preparation methods, concentration techniques, and specialized detection parameters. These approaches allow for the detection of trace amounts of analytes in complex matrices. Improvements in ionization efficiency and reduction of matrix effects contribute significantly to achieving lower detection limits and better quantification of target compounds.
- Advanced selectivity methods in HPLC-MS systems: Selectivity in HPLC-MS can be improved through various chromatographic separation techniques and mass spectrometric detection modes. Multiple reaction monitoring (MRM), high-resolution mass spectrometry, and specialized column chemistries enable highly selective analysis of target compounds in complex samples. These methods allow for discrimination between structurally similar compounds and reduce interference from matrix components.
- Novel column technologies for improved HPLC separation: Innovative stationary phase materials and column designs significantly enhance the separation efficiency of HPLC systems. These include sub-2-micron particles, core-shell particles, monolithic columns, and specialized surface chemistries that provide improved resolution, faster analysis times, and better peak shapes. When coupled with mass spectrometry, these advanced column technologies contribute to both enhanced sensitivity and selectivity in analytical methods.
- MS interface optimization for improved analytical performance: Optimization of the interface between HPLC and MS systems is crucial for maximizing sensitivity and selectivity. This includes advancements in ionization sources (such as electrospray ionization, atmospheric pressure chemical ionization, and photoionization), ion transfer technologies, and vacuum system designs. Proper tuning of interface parameters significantly reduces ion suppression effects and enhances the overall performance of the analytical system.
- Data processing and method validation strategies: Advanced data processing algorithms and comprehensive method validation strategies are essential for ensuring reliable HPLC-MS analysis. These include automated peak detection, integration techniques, calibration approaches, and statistical evaluation of method performance characteristics. Proper validation of sensitivity and selectivity parameters according to regulatory guidelines ensures the robustness and reproducibility of analytical methods across different laboratories and applications.
02 Selectivity improvements through column technology
Advanced column technologies significantly improve the selectivity of HPLC-MS systems. Specialized stationary phases, such as chiral columns, mixed-mode columns, and columns with unique functional groups, enable better separation of structurally similar compounds. Column parameters including particle size, pore size, and surface modifications can be optimized to achieve higher resolution and more effective separation of target analytes from interfering substances in complex samples.Expand Specific Solutions03 Mass spectrometry detection modes for improved selectivity
Different mass spectrometry detection modes can be utilized to enhance the selectivity of HPLC-MS analysis. Multiple reaction monitoring (MRM), selected ion monitoring (SIM), and tandem mass spectrometry (MS/MS) techniques allow for the specific detection of target compounds based on their unique fragmentation patterns. These advanced detection modes significantly reduce background noise and interference from matrix components, resulting in improved selectivity and more accurate quantification of analytes.Expand Specific Solutions04 Mobile phase optimization for enhanced performance
Optimization of mobile phase composition plays a crucial role in improving both sensitivity and selectivity in HPLC-MS analysis. Parameters such as pH, buffer concentration, organic modifier type and percentage, and gradient profiles can be adjusted to enhance chromatographic separation and ionization efficiency. The addition of specific modifiers like formic acid or ammonium acetate can improve ionization in the mass spectrometer, leading to better sensitivity, while careful selection of mobile phase components can enhance selectivity by manipulating analyte retention behavior.Expand Specific Solutions05 Sample preparation techniques for complex matrices
Advanced sample preparation techniques are essential for achieving high sensitivity and selectivity in HPLC-MS analysis of complex matrices. Methods such as solid-phase extraction (SPE), liquid-liquid extraction (LLE), QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe), and immunoaffinity purification can effectively remove interfering compounds and concentrate target analytes. These techniques reduce matrix effects that can suppress ionization in the mass spectrometer, thereby improving both the sensitivity and selectivity of the analysis, particularly for challenging samples like biological fluids, environmental samples, and food products.Expand Specific Solutions
Leading Manufacturers and Research Institutions
HPLC vs MS technology comparison is currently in a mature market phase, with a global analytical instruments market valued at approximately $80 billion. High-Performance Liquid Chromatography (HPLC) offers excellent reproducibility and ease of use, while Mass Spectrometry (MS) provides superior sensitivity and selectivity for complex analytes. Leading players like Agilent Technologies, Thermo Fisher Scientific, and Shimadzu dominate with comprehensive product portfolios. Pharmaceutical companies such as Roche, Merck, and Bayer utilize both technologies extensively for drug development and quality control. The integration of HPLC-MS systems represents the current technological frontier, with companies like Waters Corporation and PureHoney Technologies developing innovative hybrid solutions that combine the separation power of HPLC with the identification capabilities of MS.
Agilent Technologies, Inc.
Technical Solution: Agilent has developed integrated HPLC-MS systems that combine the separation power of HPLC with the sensitivity and selectivity of mass spectrometry. Their InfinityLab LC/MSD systems feature optimized interfaces that minimize analyte loss during transfer from LC to MS, achieving detection limits in the picogram range. Agilent's Jet Stream Technology enhances MS sensitivity through improved ion generation and focusing, resulting in up to 5-10x sensitivity improvement for many compounds. Their systems incorporate intelligent software algorithms that automatically optimize MS parameters based on compound characteristics, enhancing both sensitivity and selectivity. Agilent has also pioneered the development of triple quadrupole MS systems that can achieve femtogram-level detection limits when coupled with their UHPLC technology, making them suitable for trace analysis in complex matrices.
Strengths: Industry-leading integration between HPLC and MS platforms; exceptional sensitivity through innovative ion source technologies; comprehensive software solutions for method development and data analysis. Weaknesses: Higher initial investment compared to standalone HPLC systems; requires specialized training for optimal operation; more complex maintenance requirements.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed proprietary HPLC-MS technologies specifically optimized for pharmaceutical and clinical applications. Their integrated systems feature specialized sample preparation modules that enhance both sensitivity and selectivity by removing matrix interferences prior to analysis. Roche's HPLC-MS platforms incorporate novel ionization techniques that improve sensitivity for traditionally difficult-to-ionize compounds, achieving detection limits in the low picogram range for many pharmaceutical compounds and biomarkers. Their systems utilize intelligent peak detection algorithms that can distinguish target analytes from background noise, enhancing selectivity in complex biological matrices. Roche has pioneered automated calibration and quality control procedures that ensure consistent sensitivity and selectivity across multiple instruments and laboratories, critical for clinical applications. Their HPLC-MS technology includes specialized interfaces designed to minimize carryover, a common challenge affecting both sensitivity and selectivity in bioanalytical applications.
Strengths: Exceptional performance in clinical and pharmaceutical applications; robust automation features for high-throughput environments; specialized software for regulated environments with compliance features. Weaknesses: Systems often optimized for specific applications rather than general-purpose analysis; proprietary consumables may increase operating costs; less flexibility for method customization compared to some research-focused platforms.
Key Innovations in Detection Sensitivity
Device for detecting neurotoxins and process for manufacture thereof
PatentActiveEP3394618A1
Innovation
- A lateral flow test device utilizing immobilized ion-channel-linked receptors or voltage-gated ion-channels, coupled with enzymes or tagged ligands, allows for rapid detection of neurotoxins by visualizing toxin-receptor interactions on a test surface, enabling direct and immediate assessment of toxin presence in samples.
Coupling electrochemistry to mass spectrometry and high performance liquid chromatography
PatentInactiveUS7028537B2
Innovation
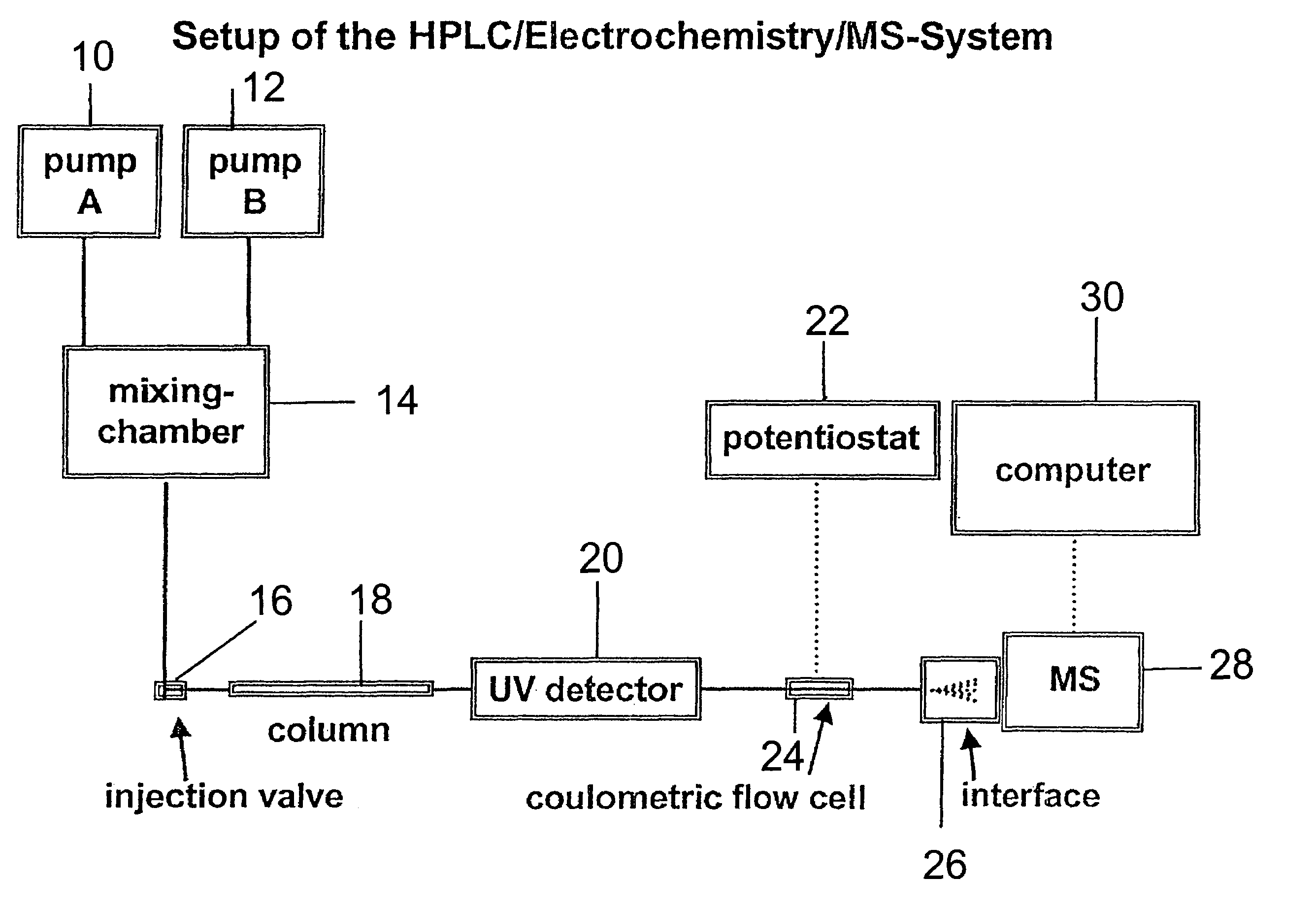
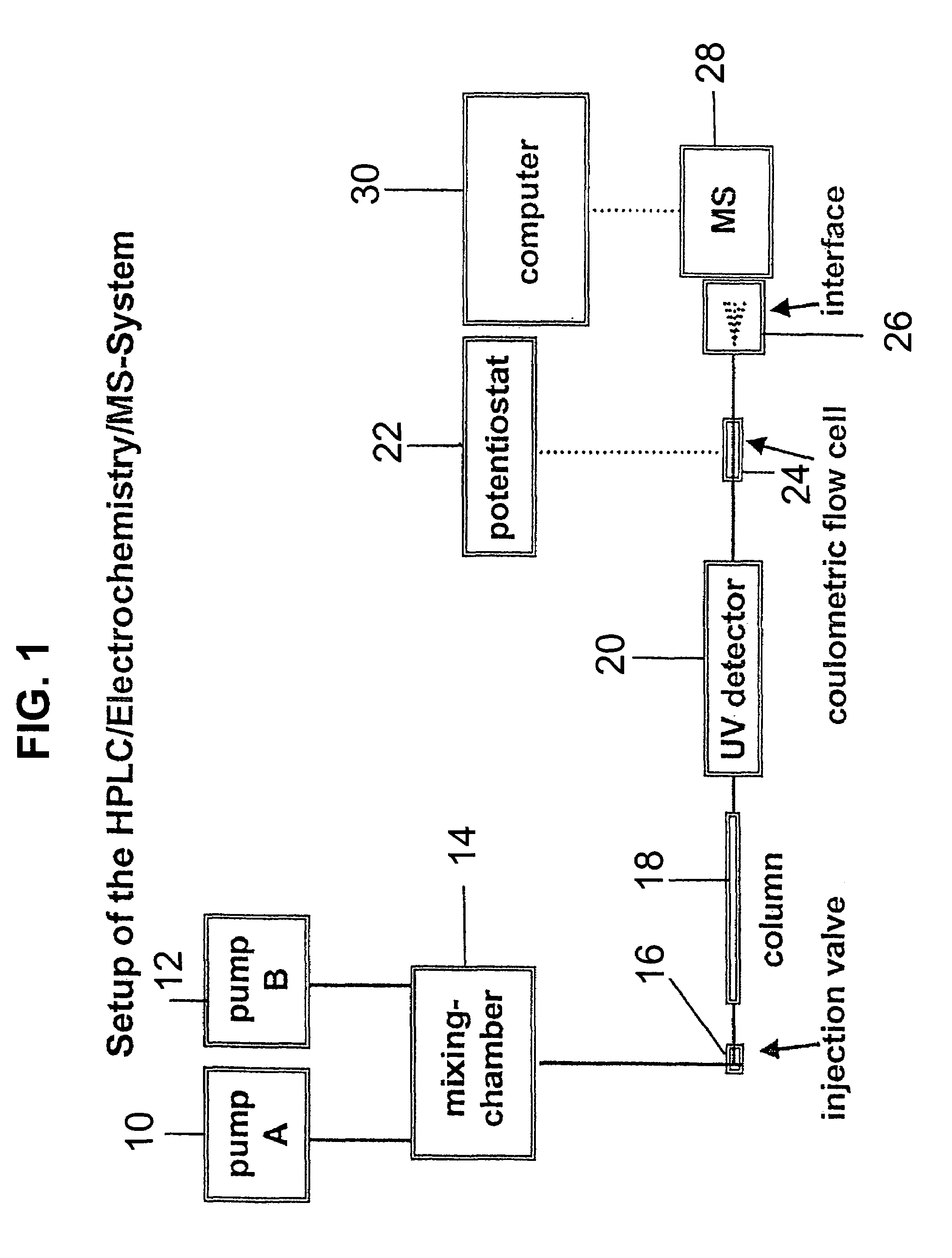
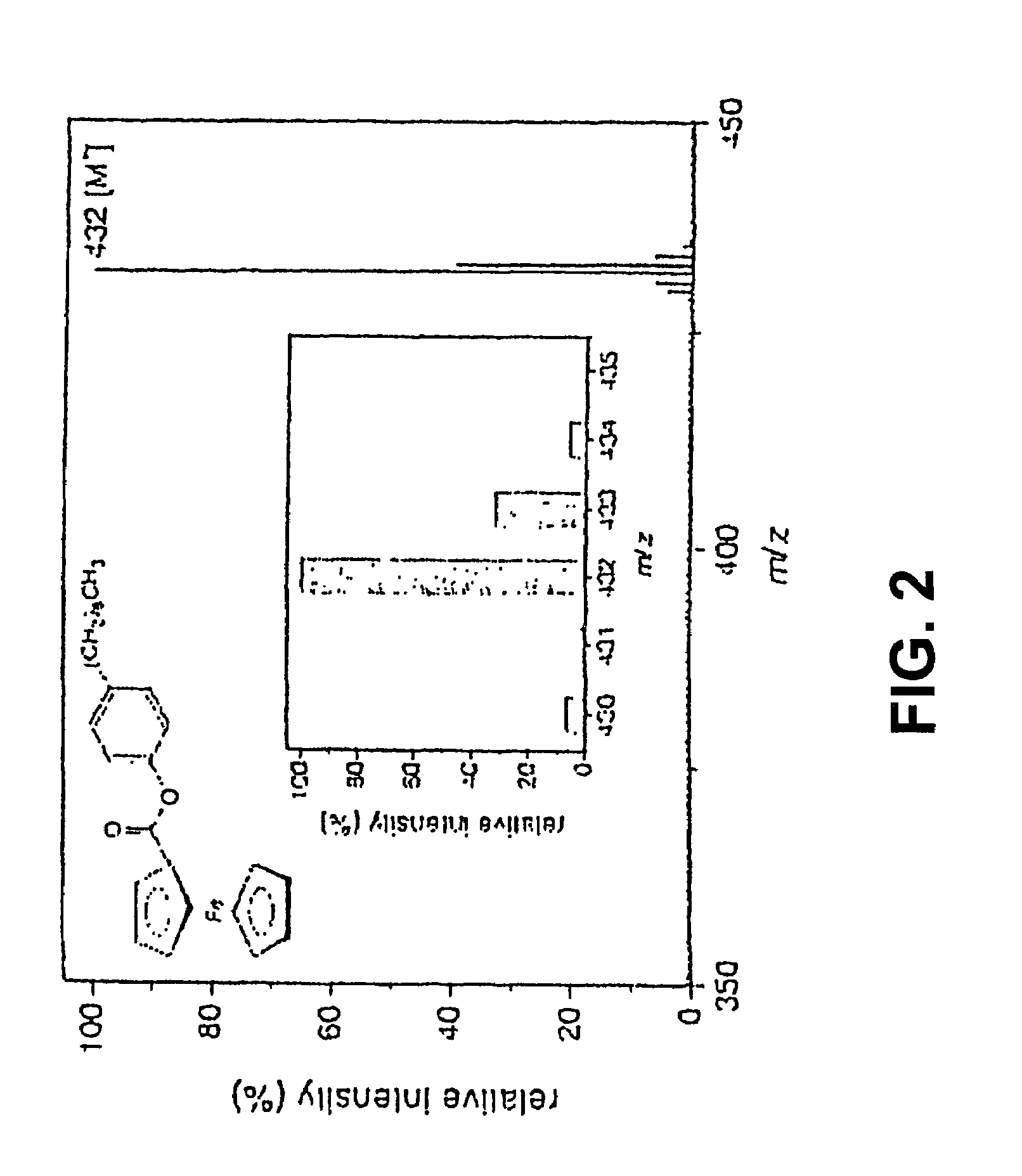
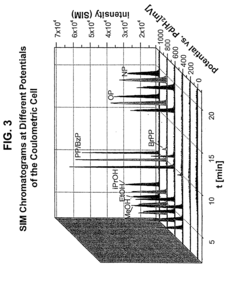
- A new HPLC-electrochemistry-MS technique is developed, where a coulometric three-electrode electrochemical cell is inserted between the HPLC column and mass spectrometer, enabling post-column electrochemical oxidation or reduction of analytes, forming charged or strongly polar products compatible with ESI or APCI mass spectrometry, thereby improving ionization efficiency.
Method Validation and Standardization Protocols
Method validation is a critical component in analytical chemistry that ensures the reliability and reproducibility of results obtained from both HPLC and MS techniques. For HPLC validation, protocols typically focus on parameters such as linearity, accuracy, precision, limit of detection (LOD), limit of quantification (LOQ), and robustness. These parameters are established through standardized procedures outlined by regulatory bodies like ICH, FDA, and USP.
MS validation protocols incorporate additional parameters specific to mass spectrometry, including ion suppression/enhancement effects, matrix effects assessment, and isotope ratio precision. The validation of MS methods often requires more complex reference standards and internal standardization approaches due to the technique's higher sensitivity and susceptibility to matrix interference.
Interlaboratory comparison studies play a crucial role in standardizing both HPLC and MS methods. These collaborative trials establish method transferability and reproducibility across different laboratory environments and instrument configurations. For HPLC methods, such studies typically focus on retention time reproducibility and peak resolution, while MS method comparisons emphasize mass accuracy, fragmentation patterns, and quantitative response factors.
Quality control procedures differ significantly between the two techniques. HPLC quality control typically involves system suitability tests that monitor parameters like column efficiency, peak symmetry, and resolution. In contrast, MS quality control procedures include additional checks for mass calibration, detector response linearity across concentration ranges, and vacuum system integrity.
Method robustness testing for HPLC examines the effects of minor variations in mobile phase composition, pH, temperature, and flow rate. For MS methods, robustness testing extends to source parameters such as ionization voltage, desolvation temperature, and collision energy optimization. These parameters must be carefully controlled to maintain consistent sensitivity and selectivity.
Regulatory acceptance criteria for HPLC and MS methods reflect their different performance characteristics. MS methods generally have more stringent requirements for specificity and sensitivity validation, often requiring demonstration of selectivity against potential isobaric interferences. HPLC methods, while less sensitive, must demonstrate consistent chromatographic performance across a wider range of operating conditions.
Documentation requirements for both techniques include comprehensive standard operating procedures (SOPs), method validation reports, and ongoing quality control records. However, MS method documentation typically requires additional details regarding instrument tuning parameters, ion source configurations, and mass calibration procedures that are not relevant to HPLC methods.
MS validation protocols incorporate additional parameters specific to mass spectrometry, including ion suppression/enhancement effects, matrix effects assessment, and isotope ratio precision. The validation of MS methods often requires more complex reference standards and internal standardization approaches due to the technique's higher sensitivity and susceptibility to matrix interference.
Interlaboratory comparison studies play a crucial role in standardizing both HPLC and MS methods. These collaborative trials establish method transferability and reproducibility across different laboratory environments and instrument configurations. For HPLC methods, such studies typically focus on retention time reproducibility and peak resolution, while MS method comparisons emphasize mass accuracy, fragmentation patterns, and quantitative response factors.
Quality control procedures differ significantly between the two techniques. HPLC quality control typically involves system suitability tests that monitor parameters like column efficiency, peak symmetry, and resolution. In contrast, MS quality control procedures include additional checks for mass calibration, detector response linearity across concentration ranges, and vacuum system integrity.
Method robustness testing for HPLC examines the effects of minor variations in mobile phase composition, pH, temperature, and flow rate. For MS methods, robustness testing extends to source parameters such as ionization voltage, desolvation temperature, and collision energy optimization. These parameters must be carefully controlled to maintain consistent sensitivity and selectivity.
Regulatory acceptance criteria for HPLC and MS methods reflect their different performance characteristics. MS methods generally have more stringent requirements for specificity and sensitivity validation, often requiring demonstration of selectivity against potential isobaric interferences. HPLC methods, while less sensitive, must demonstrate consistent chromatographic performance across a wider range of operating conditions.
Documentation requirements for both techniques include comprehensive standard operating procedures (SOPs), method validation reports, and ongoing quality control records. However, MS method documentation typically requires additional details regarding instrument tuning parameters, ion source configurations, and mass calibration procedures that are not relevant to HPLC methods.
Cost-Benefit Analysis of Analytical Platforms
When comparing HPLC and MS technologies, cost-benefit analysis becomes a critical factor in decision-making for laboratories and research institutions. The initial investment for HPLC systems typically ranges from $30,000 to $100,000, while MS platforms demand significantly higher capital expenditure, often between $200,000 and $1,000,000 depending on the configuration and capabilities.
Operational costs present another dimension of comparison. HPLC systems generally require lower maintenance expenses, with annual costs averaging 10-15% of the initial investment. Consumables such as columns and mobile phases are relatively affordable, contributing to lower per-sample analysis costs. Conversely, MS systems incur higher operational expenses, including specialized maintenance contracts, vacuum system components, and costly calibration standards, potentially reaching 15-25% of the initial investment annually.
Personnel requirements differ substantially between these platforms. HPLC operation can be mastered with moderate training, typically requiring 1-2 weeks for basic proficiency. MS systems demand more specialized expertise, often necessitating dedicated operators with advanced degrees and extensive training periods of 1-3 months, translating to higher labor costs.
Sample throughput capabilities significantly impact the cost-benefit equation. Modern HPLC systems can process 20-100 samples daily depending on analysis complexity, while MS platforms may handle fewer samples but provide substantially more data points per sample. This creates a complex efficiency calculation based on the specific analytical needs.
Return on investment timelines vary considerably. HPLC systems typically achieve ROI within 1-3 years in routine analytical settings. MS platforms, despite higher initial costs, may deliver superior ROI for specialized applications requiring exceptional sensitivity or specificity, particularly in pharmaceutical development, clinical diagnostics, or advanced research environments where detection of trace compounds delivers substantial value.
Infrastructure requirements present additional considerations. HPLC systems operate with standard laboratory utilities, while MS platforms often require specialized facilities with controlled environments, dedicated power supplies, and specialized gas delivery systems, adding to the total cost of ownership.
The optimal choice ultimately depends on specific analytical requirements, sample volumes, and the economic value of the enhanced data quality that MS provides. Organizations must carefully weigh these factors against their analytical needs and financial constraints when selecting between these complementary technologies.
Operational costs present another dimension of comparison. HPLC systems generally require lower maintenance expenses, with annual costs averaging 10-15% of the initial investment. Consumables such as columns and mobile phases are relatively affordable, contributing to lower per-sample analysis costs. Conversely, MS systems incur higher operational expenses, including specialized maintenance contracts, vacuum system components, and costly calibration standards, potentially reaching 15-25% of the initial investment annually.
Personnel requirements differ substantially between these platforms. HPLC operation can be mastered with moderate training, typically requiring 1-2 weeks for basic proficiency. MS systems demand more specialized expertise, often necessitating dedicated operators with advanced degrees and extensive training periods of 1-3 months, translating to higher labor costs.
Sample throughput capabilities significantly impact the cost-benefit equation. Modern HPLC systems can process 20-100 samples daily depending on analysis complexity, while MS platforms may handle fewer samples but provide substantially more data points per sample. This creates a complex efficiency calculation based on the specific analytical needs.
Return on investment timelines vary considerably. HPLC systems typically achieve ROI within 1-3 years in routine analytical settings. MS platforms, despite higher initial costs, may deliver superior ROI for specialized applications requiring exceptional sensitivity or specificity, particularly in pharmaceutical development, clinical diagnostics, or advanced research environments where detection of trace compounds delivers substantial value.
Infrastructure requirements present additional considerations. HPLC systems operate with standard laboratory utilities, while MS platforms often require specialized facilities with controlled environments, dedicated power supplies, and specialized gas delivery systems, adding to the total cost of ownership.
The optimal choice ultimately depends on specific analytical requirements, sample volumes, and the economic value of the enhanced data quality that MS provides. Organizations must carefully weigh these factors against their analytical needs and financial constraints when selecting between these complementary technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!