Enhance HPLC Selectivity with Ion Exchange Techniques
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ion Exchange HPLC Technology Background and Objectives
Ion exchange chromatography has evolved significantly since its inception in the 1970s as a specialized technique within High-Performance Liquid Chromatography (HPLC). The technology leverages differences in ionic interactions between analytes and stationary phases to achieve separation, making it particularly valuable for charged or ionizable compounds that traditional reversed-phase HPLC struggles to differentiate effectively.
The historical trajectory of ion exchange HPLC shows remarkable advancement from rudimentary ion exchange resins to today's highly engineered stationary phases with controlled surface chemistry and particle morphology. Early applications were primarily limited to inorganic ion analysis, but technological refinements have expanded its utility across pharmaceutical, environmental, food safety, and biopharmaceutical sectors.
Recent technological innovations have focused on enhancing selectivity through mixed-mode stationary phases that combine ion exchange with other retention mechanisms such as hydrophobic interactions. This hybrid approach addresses the growing complexity of sample matrices in modern analytical challenges, particularly in biological and pharmaceutical applications where traditional single-mode separations often prove inadequate.
The current market demand for improved selectivity stems from increasingly stringent regulatory requirements for impurity profiling and the analytical challenges presented by complex biological matrices. Conventional HPLC methods frequently encounter limitations when analyzing compounds with similar physicochemical properties but different charge characteristics.
The primary objective of enhancing HPLC selectivity through ion exchange techniques is to develop more discriminating analytical methods capable of resolving structurally similar compounds, particularly those that differ subtly in their ionic properties. This includes the separation of positional isomers, stereoisomers, and closely related impurities that conventional reversed-phase techniques struggle to differentiate.
Secondary objectives include improving method robustness across varying sample matrices, reducing analysis time through more efficient separations, and developing environmentally sustainable methodologies with reduced solvent consumption. The ultimate goal is to establish analytical platforms that can be readily adapted to diverse applications while maintaining high separation efficiency and reproducibility.
The technology evolution trend points toward miniaturization, automation, and integration with advanced detection systems such as mass spectrometry. Additionally, there is growing interest in developing predictive models for ion exchange selectivity to facilitate method development and optimization, reducing the empirical trial-and-error approach that has historically dominated chromatographic method development.
The historical trajectory of ion exchange HPLC shows remarkable advancement from rudimentary ion exchange resins to today's highly engineered stationary phases with controlled surface chemistry and particle morphology. Early applications were primarily limited to inorganic ion analysis, but technological refinements have expanded its utility across pharmaceutical, environmental, food safety, and biopharmaceutical sectors.
Recent technological innovations have focused on enhancing selectivity through mixed-mode stationary phases that combine ion exchange with other retention mechanisms such as hydrophobic interactions. This hybrid approach addresses the growing complexity of sample matrices in modern analytical challenges, particularly in biological and pharmaceutical applications where traditional single-mode separations often prove inadequate.
The current market demand for improved selectivity stems from increasingly stringent regulatory requirements for impurity profiling and the analytical challenges presented by complex biological matrices. Conventional HPLC methods frequently encounter limitations when analyzing compounds with similar physicochemical properties but different charge characteristics.
The primary objective of enhancing HPLC selectivity through ion exchange techniques is to develop more discriminating analytical methods capable of resolving structurally similar compounds, particularly those that differ subtly in their ionic properties. This includes the separation of positional isomers, stereoisomers, and closely related impurities that conventional reversed-phase techniques struggle to differentiate.
Secondary objectives include improving method robustness across varying sample matrices, reducing analysis time through more efficient separations, and developing environmentally sustainable methodologies with reduced solvent consumption. The ultimate goal is to establish analytical platforms that can be readily adapted to diverse applications while maintaining high separation efficiency and reproducibility.
The technology evolution trend points toward miniaturization, automation, and integration with advanced detection systems such as mass spectrometry. Additionally, there is growing interest in developing predictive models for ion exchange selectivity to facilitate method development and optimization, reducing the empirical trial-and-error approach that has historically dominated chromatographic method development.
Market Analysis for Enhanced HPLC Selectivity Solutions
The global market for HPLC selectivity enhancement solutions has been experiencing robust growth, driven by increasing demands for more precise analytical methods across pharmaceutical, biotechnology, and environmental testing sectors. The current market size for ion exchange chromatography techniques within HPLC applications is estimated at $1.2 billion, with a compound annual growth rate of 7.3% projected through 2028.
Pharmaceutical and biopharmaceutical industries represent the largest market segment, accounting for approximately 45% of the total market share. This dominance stems from stringent regulatory requirements for drug purity analysis and the growing complexity of biological therapeutics requiring advanced separation techniques. The ion exchange HPLC market in this sector alone is expected to reach $650 million by 2025.
Academic and research institutions constitute the second-largest market segment at 25%, where ion exchange techniques are increasingly utilized for proteomics, metabolomics, and other complex biological sample analyses. Environmental testing laboratories represent a rapidly growing segment with 15% market share, driven by heightened regulatory scrutiny of water quality and environmental contaminants.
Geographically, North America leads the market with 38% share, followed by Europe (30%) and Asia-Pacific (24%). The Asia-Pacific region, particularly China and India, demonstrates the fastest growth rate at 9.5% annually, attributed to expanding pharmaceutical manufacturing capabilities and increasing R&D investments.
Customer demand trends indicate a strong preference for integrated HPLC systems that offer enhanced selectivity through specialized ion exchange columns and optimized buffer systems. End-users increasingly seek solutions that reduce analysis time while maintaining or improving separation efficiency, particularly for complex biological samples containing multiple charged species.
Market challenges include price sensitivity among academic and smaller industrial laboratories, technical complexity requiring specialized training, and competition from alternative separation technologies such as affinity chromatography and size exclusion methods. However, the superior resolution capabilities of ion exchange techniques for charged molecules continue to drive market expansion.
Leading suppliers report that consumables, particularly specialized ion exchange columns and buffer solutions, generate approximately 65% of revenue in this market segment, with instrumentation accounting for the remainder. This indicates a significant recurring revenue opportunity for companies offering comprehensive ion exchange HPLC solutions with proprietary column technologies and optimized buffer systems.
Pharmaceutical and biopharmaceutical industries represent the largest market segment, accounting for approximately 45% of the total market share. This dominance stems from stringent regulatory requirements for drug purity analysis and the growing complexity of biological therapeutics requiring advanced separation techniques. The ion exchange HPLC market in this sector alone is expected to reach $650 million by 2025.
Academic and research institutions constitute the second-largest market segment at 25%, where ion exchange techniques are increasingly utilized for proteomics, metabolomics, and other complex biological sample analyses. Environmental testing laboratories represent a rapidly growing segment with 15% market share, driven by heightened regulatory scrutiny of water quality and environmental contaminants.
Geographically, North America leads the market with 38% share, followed by Europe (30%) and Asia-Pacific (24%). The Asia-Pacific region, particularly China and India, demonstrates the fastest growth rate at 9.5% annually, attributed to expanding pharmaceutical manufacturing capabilities and increasing R&D investments.
Customer demand trends indicate a strong preference for integrated HPLC systems that offer enhanced selectivity through specialized ion exchange columns and optimized buffer systems. End-users increasingly seek solutions that reduce analysis time while maintaining or improving separation efficiency, particularly for complex biological samples containing multiple charged species.
Market challenges include price sensitivity among academic and smaller industrial laboratories, technical complexity requiring specialized training, and competition from alternative separation technologies such as affinity chromatography and size exclusion methods. However, the superior resolution capabilities of ion exchange techniques for charged molecules continue to drive market expansion.
Leading suppliers report that consumables, particularly specialized ion exchange columns and buffer solutions, generate approximately 65% of revenue in this market segment, with instrumentation accounting for the remainder. This indicates a significant recurring revenue opportunity for companies offering comprehensive ion exchange HPLC solutions with proprietary column technologies and optimized buffer systems.
Current Challenges in Ion Exchange Chromatography
Despite significant advancements in ion exchange chromatography (IEC) techniques, several persistent challenges continue to limit the full potential of these methods for enhancing HPLC selectivity. One of the most significant obstacles remains the complex interplay between pH, buffer concentration, and ionic strength, which can dramatically affect separation efficiency. Researchers frequently encounter difficulties in predicting how slight modifications to these parameters will influence selectivity, often necessitating extensive empirical optimization.
The phenomenon of non-specific interactions presents another substantial challenge. Even with carefully selected stationary phases, analytes may interact with the column through multiple mechanisms simultaneously—including hydrophobic interactions, hydrogen bonding, and π-π interactions—complicating the interpretation of chromatographic behavior and reducing separation predictability.
Resolution of structurally similar compounds with minimal differences in charge properties continues to be problematic, particularly for biomolecules like proteins with subtle post-translational modifications or oligonucleotides differing by only a single nucleotide. Current ion exchange materials often lack the selectivity required to distinguish these minor variations efficiently.
Column capacity limitations represent a significant bottleneck, especially when analyzing complex biological samples. As sample complexity increases, the finite number of ion exchange sites can become saturated, leading to peak broadening, reduced resolution, and compromised quantitative accuracy. This is particularly evident in proteomics applications where dynamic concentration ranges can span several orders of magnitude.
The stability and reproducibility of ion exchange materials under varying conditions remain inconsistent. Many commercially available columns exhibit performance degradation after repeated use or exposure to extreme pH conditions, necessitating frequent replacement and recalibration. This instability introduces variability into analytical methods and complicates method transfer between laboratories.
Compatibility issues between ion exchange techniques and mass spectrometry detection persist due to the non-volatile nature of many commonly used buffers. While progress has been made with MS-compatible volatile buffers, these often provide suboptimal separation compared to traditional phosphate or citrate buffers, forcing analysts to compromise between separation quality and detection sensitivity.
Finally, the time-consuming nature of method development in ion exchange chromatography remains a significant challenge. The multidimensional parameter space that must be explored to optimize separations—including stationary phase chemistry, mobile phase composition, gradient profiles, and temperature—requires substantial resources and expertise, limiting the broader adoption of these powerful techniques in routine analytical workflows.
The phenomenon of non-specific interactions presents another substantial challenge. Even with carefully selected stationary phases, analytes may interact with the column through multiple mechanisms simultaneously—including hydrophobic interactions, hydrogen bonding, and π-π interactions—complicating the interpretation of chromatographic behavior and reducing separation predictability.
Resolution of structurally similar compounds with minimal differences in charge properties continues to be problematic, particularly for biomolecules like proteins with subtle post-translational modifications or oligonucleotides differing by only a single nucleotide. Current ion exchange materials often lack the selectivity required to distinguish these minor variations efficiently.
Column capacity limitations represent a significant bottleneck, especially when analyzing complex biological samples. As sample complexity increases, the finite number of ion exchange sites can become saturated, leading to peak broadening, reduced resolution, and compromised quantitative accuracy. This is particularly evident in proteomics applications where dynamic concentration ranges can span several orders of magnitude.
The stability and reproducibility of ion exchange materials under varying conditions remain inconsistent. Many commercially available columns exhibit performance degradation after repeated use or exposure to extreme pH conditions, necessitating frequent replacement and recalibration. This instability introduces variability into analytical methods and complicates method transfer between laboratories.
Compatibility issues between ion exchange techniques and mass spectrometry detection persist due to the non-volatile nature of many commonly used buffers. While progress has been made with MS-compatible volatile buffers, these often provide suboptimal separation compared to traditional phosphate or citrate buffers, forcing analysts to compromise between separation quality and detection sensitivity.
Finally, the time-consuming nature of method development in ion exchange chromatography remains a significant challenge. The multidimensional parameter space that must be explored to optimize separations—including stationary phase chemistry, mobile phase composition, gradient profiles, and temperature—requires substantial resources and expertise, limiting the broader adoption of these powerful techniques in routine analytical workflows.
Current Ion Exchange Stationary Phase Solutions
01 Ion exchange stationary phases for HPLC selectivity enhancement
Specialized ion exchange stationary phases can significantly improve HPLC selectivity. These phases contain functional groups that interact with analytes through ionic interactions, allowing for separation based on charge differences. By selecting appropriate ion exchange materials with specific functional groups, analysts can achieve enhanced selectivity for target compounds, particularly for charged or ionizable molecules. These stationary phases can be modified to provide different levels of ion exchange capacity and selectivity.- Ion exchange stationary phases for HPLC selectivity: Various ion exchange stationary phases can be used in HPLC to enhance selectivity. These include modified silica, polymeric resins, and hybrid materials with different functional groups that provide specific interactions with analytes. The choice of stationary phase affects retention behavior, resolution, and separation efficiency for different types of compounds, particularly charged molecules and biomolecules.
- Mobile phase modifiers for ion exchange selectivity: The composition of the mobile phase significantly impacts ion exchange selectivity in HPLC. By adjusting parameters such as pH, buffer concentration, ionic strength, and organic modifier content, chromatographers can fine-tune separations. Counter-ions in the mobile phase compete with analytes for ion exchange sites, allowing for controlled elution and improved resolution of complex mixtures.
- Mixed-mode ion exchange techniques: Mixed-mode ion exchange techniques combine multiple separation mechanisms (such as ion exchange with hydrophobic interactions or size exclusion) to achieve enhanced selectivity. These approaches utilize stationary phases with dual or multiple functionalities, allowing for unique retention patterns and improved resolution of complex samples containing compounds with diverse chemical properties.
- Temperature and pH control for ion exchange selectivity: Temperature and pH are critical parameters affecting ion exchange selectivity in HPLC. Precise control of these variables allows for manipulation of analyte charge state, ionization, and interaction with the stationary phase. Temperature affects the kinetics of ion exchange processes, while pH determines the ionization state of both analytes and stationary phase functional groups, enabling fine-tuning of separations.
- Ion exchange techniques for specific applications: Ion exchange HPLC techniques have been optimized for specific applications such as protein purification, oligonucleotide analysis, and pharmaceutical compound separation. These specialized methods involve tailored stationary phases, gradient elution programs, and detection systems designed to address the unique challenges of particular sample types, enhancing selectivity for target analytes in complex matrices.
02 Mobile phase modification techniques for ion exchange HPLC
The composition of the mobile phase significantly affects ion exchange selectivity in HPLC. By adjusting parameters such as pH, buffer concentration, ionic strength, and organic modifier content, analysts can fine-tune the separation of complex mixtures. Counter-ions in the mobile phase compete with analytes for interaction with the stationary phase, providing a mechanism to control retention and selectivity. Gradient elution techniques using varying salt concentrations or pH can further enhance separation power for complex samples.Expand Specific Solutions03 Mixed-mode ion exchange chromatography approaches
Mixed-mode chromatography combines ion exchange with other retention mechanisms such as hydrophobic interactions, providing multiple separation mechanisms simultaneously. These hybrid approaches offer unique selectivity patterns not achievable with single-mode techniques. By incorporating both ionic and non-ionic interactions, mixed-mode techniques can separate compounds with similar charge but different hydrophobicity, or vice versa. This approach is particularly valuable for complex biological samples containing diverse analytes with varying physicochemical properties.Expand Specific Solutions04 Temperature and pH control for optimizing ion exchange selectivity
Temperature and pH are critical parameters affecting ion exchange selectivity in HPLC. Temperature changes can alter the ionization state of both analytes and stationary phase, modifying retention behavior and separation selectivity. Precise pH control is essential as it determines the charge state of ionizable compounds, directly affecting their interaction with the ion exchange stationary phase. By systematically optimizing these parameters, analysts can develop methods with improved selectivity for challenging separations.Expand Specific Solutions05 Novel ion exchange materials and surface modifications
Recent advances in ion exchange materials include novel polymeric supports, surface-modified silica, and hybrid organic-inorganic materials with enhanced selectivity properties. These materials often feature controlled pore structures, uniform particle size distribution, and specialized functional groups designed for specific separation challenges. Surface modifications can introduce unique selectivity characteristics while maintaining good mechanical stability and chromatographic efficiency. Some innovations include grafted polymeric layers, zwitterionic phases, and materials with controlled charge density for optimized ion exchange interactions.Expand Specific Solutions
Leading Manufacturers and Research Institutions in HPLC Technology
The ion exchange chromatography market is currently in a growth phase, with increasing adoption across pharmaceutical, biotechnology, and environmental sectors. The global market size is estimated to exceed $2 billion, driven by rising demand for purification technologies in biopharmaceutical manufacturing and water treatment applications. Technologically, the field shows moderate maturity with continuous innovations enhancing selectivity and efficiency. Leading players include Dionex Corp. (pioneering advanced ion exchange systems), Agilent Technologies (offering comprehensive analytical solutions), Shimadzu Corp. (providing high-performance instrumentation), and Sunresin New Materials (specializing in adsorptive separation materials). Emerging competitors like QIAGEN Sciences and Sielc Technologies are introducing novel approaches to enhance HPLC selectivity through specialized ion exchange techniques, while established pharmaceutical companies such as Genentech and Merck are developing proprietary applications.
Dionex Corp.
Technical Solution: Dionex has developed advanced ion exchange chromatography systems specifically designed to enhance HPLC selectivity. Their IonPac line of columns features unique polymer-based substrates with carefully controlled surface chemistry that allows for exceptional separation of complex ionic mixtures. The company's patented high-capacity, pellicular anion-exchange technology enables superior peak resolution and sensitivity for challenging analytical applications. Dionex's systems incorporate eluent generation technology that automatically produces precise gradient compositions, eliminating the need for manual preparation of mobile phases and reducing run-to-run variability. Their integrated suppression systems effectively neutralize the eluent conductivity while enhancing analyte detection, resulting in significantly improved signal-to-noise ratios and detection limits in the low parts-per-trillion range for many ionic compounds.
Strengths: Superior ion selectivity through specialized polymer-based substrates; automated eluent generation for precise gradient control; integrated suppression systems for enhanced sensitivity. Weaknesses: Higher initial investment compared to conventional HPLC systems; requires specialized training for optimal operation; limited application scope outside of ionic compound analysis.
QIAGEN Sciences LLC
Technical Solution: QIAGEN has developed a sophisticated approach to enhancing HPLC selectivity through specialized ion exchange techniques focused primarily on biomolecular applications. Their patented QIAGEN Anion Exchange (AEX) technology utilizes a unique quaternary ammonium functional group configuration that provides exceptional selectivity for nucleic acids and proteins. The company's proprietary membrane-based ion exchangers offer significantly reduced diffusion paths compared to traditional bead-based resins, resulting in faster mass transfer and improved resolution of closely related biomolecules. QIAGEN's integrated workflow solutions combine optimized buffers, calibrated columns, and application-specific protocols to simplify method development while maximizing reproducibility. Their automated systems incorporate inline pH and conductivity monitoring to ensure consistent separation conditions, with feedback control mechanisms that make real-time adjustments to maintain optimal selectivity throughout the analytical run.
Strengths: Exceptional selectivity for biomolecules, particularly nucleic acids; integrated workflow solutions reduce method development time; excellent reproducibility across different sample types. Weaknesses: More specialized focus limits application range outside of biomolecular analysis; higher consumable costs compared to generic alternatives; requires dedicated instrumentation for optimal performance.
Key Patents and Innovations in Ion Exchange Selectivity
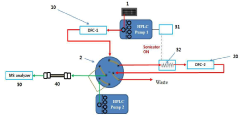
High-performance liquid chromatography with a controllable transverse flow inducer
PatentActiveEP3322978A1
Innovation
- The use of a controllable transverse flow inducer, which generates micro-scale vortices through alternating current electrokinetics, allowing for orthogonal flow induction independent of axial velocity, reducing dispersion by combining pressure and electro-osmotic flow, and enabling retention modulation without permanent surface charges.
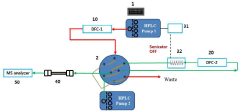
High performance liquid chromatography apparatus and method for screening substance using thereof
PatentActiveKR1020180009057A
Innovation
- A high-performance liquid chromatography (HPLC) apparatus and method that includes a first column for separating target materials, a dissociation device, a second column for further separation, and an analyzer, allowing for rapid screening of candidate substances without separate sample pretreatment.
Environmental Impact and Green Chemistry Considerations
The integration of ion exchange techniques with HPLC systems presents significant environmental considerations that align with modern green chemistry principles. Traditional HPLC methods often rely on large volumes of organic solvents, particularly acetonitrile and methanol, which pose environmental hazards through their production, use, and disposal. Ion exchange chromatography offers a more environmentally friendly alternative by utilizing primarily aqueous mobile phases, substantially reducing organic solvent consumption by up to 70-80% compared to conventional reversed-phase HPLC methods.
The environmental benefits extend to waste reduction as well. Ion exchange columns typically demonstrate longer operational lifespans than traditional silica-based columns, reducing the frequency of column replacement and associated waste. Additionally, the regeneration capabilities of ion exchange resins allow for multiple reuse cycles, further minimizing material consumption and waste generation in analytical laboratories.
Energy efficiency represents another critical environmental advantage. Ion exchange HPLC methods often operate at lower pressures than traditional HPLC techniques, resulting in reduced energy requirements for system operation. Studies indicate potential energy savings of 15-25% when implementing optimized ion exchange methods compared to conventional high-pressure techniques.
Water consumption remains a concern for ion exchange techniques, as they typically require significant volumes for mobile phases and column regeneration. However, recent innovations have introduced recycling systems that can recover and purify buffer solutions, reducing water usage by up to 40% in continuous operations. These closed-loop systems represent an important advancement in sustainable laboratory practices.
The manufacturing of ion exchange resins has also evolved to incorporate green chemistry principles. Modern production methods increasingly utilize renewable raw materials and water-based synthesis processes instead of organic solvent-dependent approaches. Several manufacturers have developed bio-based resins derived from modified polysaccharides and other renewable polymers, reducing the carbon footprint associated with resin production by approximately 30% compared to traditional petroleum-based alternatives.
Regulatory frameworks worldwide are increasingly recognizing these environmental benefits. The EPA's Green Chemistry Program and the European Union's REACH regulations have specifically highlighted ion exchange chromatography as a preferred analytical technique that aligns with sustainability goals. Laboratories adopting these methods can often qualify for green certification programs and demonstrate compliance with increasingly stringent environmental regulations governing chemical waste disposal and management.
The environmental benefits extend to waste reduction as well. Ion exchange columns typically demonstrate longer operational lifespans than traditional silica-based columns, reducing the frequency of column replacement and associated waste. Additionally, the regeneration capabilities of ion exchange resins allow for multiple reuse cycles, further minimizing material consumption and waste generation in analytical laboratories.
Energy efficiency represents another critical environmental advantage. Ion exchange HPLC methods often operate at lower pressures than traditional HPLC techniques, resulting in reduced energy requirements for system operation. Studies indicate potential energy savings of 15-25% when implementing optimized ion exchange methods compared to conventional high-pressure techniques.
Water consumption remains a concern for ion exchange techniques, as they typically require significant volumes for mobile phases and column regeneration. However, recent innovations have introduced recycling systems that can recover and purify buffer solutions, reducing water usage by up to 40% in continuous operations. These closed-loop systems represent an important advancement in sustainable laboratory practices.
The manufacturing of ion exchange resins has also evolved to incorporate green chemistry principles. Modern production methods increasingly utilize renewable raw materials and water-based synthesis processes instead of organic solvent-dependent approaches. Several manufacturers have developed bio-based resins derived from modified polysaccharides and other renewable polymers, reducing the carbon footprint associated with resin production by approximately 30% compared to traditional petroleum-based alternatives.
Regulatory frameworks worldwide are increasingly recognizing these environmental benefits. The EPA's Green Chemistry Program and the European Union's REACH regulations have specifically highlighted ion exchange chromatography as a preferred analytical technique that aligns with sustainability goals. Laboratories adopting these methods can often qualify for green certification programs and demonstrate compliance with increasingly stringent environmental regulations governing chemical waste disposal and management.
Validation and Quality Control Standards for Ion Exchange HPLC
Validation and quality control are critical components in ion exchange HPLC methodologies, ensuring reliable, reproducible, and accurate analytical results. The implementation of robust validation protocols is essential for maintaining the enhanced selectivity that ion exchange techniques offer in HPLC applications.
System suitability tests represent the cornerstone of daily operational quality control in ion exchange HPLC. These tests typically include assessments of retention time reproducibility, peak resolution, column efficiency, and peak symmetry. For ion exchange applications specifically, monitoring of ion capacity and exchange efficiency becomes particularly important as these parameters directly influence selectivity and separation performance.
Method validation for ion exchange HPLC follows internationally recognized guidelines such as ICH Q2(R1), USP <1225>, and FDA validation protocols. These frameworks require thorough evaluation of specificity, linearity, accuracy, precision, detection limit, quantitation limit, range, and robustness. For ion exchange separations, additional validation parameters include buffer concentration effects, pH stability, and ionic strength influences on selectivity.
Quality control charts and statistical process control tools provide valuable mechanisms for tracking system performance over time. Establishing control limits for critical parameters such as resolution between critical pairs, retention time shifts, and theoretical plate counts enables early detection of system drift or column degradation. This proactive monitoring approach is particularly valuable for ion exchange columns, which may experience gradual capacity loss or selectivity changes.
Reference standards play a vital role in validation and ongoing quality control. Certified reference materials with well-characterized ionic properties should be employed to verify system performance and method transferability. For specialized ion exchange applications, custom reference standards may be necessary to accurately represent the target analytes' ionic behavior under the specific separation conditions.
Interlaboratory comparison studies provide an external quality assurance mechanism, confirming method robustness across different laboratory environments. These collaborative studies are particularly important when implementing novel ion exchange selectivity enhancements, as they verify that the selectivity advantages are maintainable across different instruments and operator conditions.
Documentation requirements for validated ion exchange HPLC methods include comprehensive standard operating procedures, method validation reports, and ongoing quality control records. These documents should specifically address the unique aspects of ion exchange selectivity, including buffer preparation protocols, column conditioning procedures, and system equilibration requirements that directly impact separation performance.
System suitability tests represent the cornerstone of daily operational quality control in ion exchange HPLC. These tests typically include assessments of retention time reproducibility, peak resolution, column efficiency, and peak symmetry. For ion exchange applications specifically, monitoring of ion capacity and exchange efficiency becomes particularly important as these parameters directly influence selectivity and separation performance.
Method validation for ion exchange HPLC follows internationally recognized guidelines such as ICH Q2(R1), USP <1225>, and FDA validation protocols. These frameworks require thorough evaluation of specificity, linearity, accuracy, precision, detection limit, quantitation limit, range, and robustness. For ion exchange separations, additional validation parameters include buffer concentration effects, pH stability, and ionic strength influences on selectivity.
Quality control charts and statistical process control tools provide valuable mechanisms for tracking system performance over time. Establishing control limits for critical parameters such as resolution between critical pairs, retention time shifts, and theoretical plate counts enables early detection of system drift or column degradation. This proactive monitoring approach is particularly valuable for ion exchange columns, which may experience gradual capacity loss or selectivity changes.
Reference standards play a vital role in validation and ongoing quality control. Certified reference materials with well-characterized ionic properties should be employed to verify system performance and method transferability. For specialized ion exchange applications, custom reference standards may be necessary to accurately represent the target analytes' ionic behavior under the specific separation conditions.
Interlaboratory comparison studies provide an external quality assurance mechanism, confirming method robustness across different laboratory environments. These collaborative studies are particularly important when implementing novel ion exchange selectivity enhancements, as they verify that the selectivity advantages are maintainable across different instruments and operator conditions.
Documentation requirements for validated ion exchange HPLC methods include comprehensive standard operating procedures, method validation reports, and ongoing quality control records. These documents should specifically address the unique aspects of ion exchange selectivity, including buffer preparation protocols, column conditioning procedures, and system equilibration requirements that directly impact separation performance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!