How to Troubleshoot HPLC Baseline Drift Issues
SEP 19, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC Baseline Drift Background and Objectives
High-Performance Liquid Chromatography (HPLC) has evolved significantly since its inception in the 1960s, becoming an indispensable analytical technique in pharmaceutical, environmental, food safety, and clinical research sectors. Baseline drift, characterized by a gradual, unintended change in the detector signal over time, represents one of the most persistent challenges in HPLC analysis, affecting measurement accuracy and reproducibility.
The evolution of HPLC technology has seen remarkable improvements in column technology, detector sensitivity, and system integration. However, baseline stability issues have remained a consistent challenge throughout this development trajectory. Historical data indicates that approximately 30-40% of troubleshooting time in analytical laboratories is dedicated to resolving baseline-related problems, highlighting the significance of this technical challenge.
Baseline drift manifests through various patterns including positive drift, negative drift, cyclic fluctuations, and random noise. Each pattern provides diagnostic clues about underlying causes, which may include temperature fluctuations, mobile phase composition changes, column equilibration issues, detector instability, or electronic interference. Understanding these patterns is crucial for effective troubleshooting.
The primary objective of addressing HPLC baseline drift is to enhance analytical precision and reliability. Specifically, this involves achieving signal-to-noise ratios that consistently exceed 3:1 for limit of detection and 10:1 for limit of quantification across diverse sample matrices and analytical conditions. Additionally, maintaining baseline stability within ±2-3 mAU over extended analytical runs represents an industry benchmark for high-quality chromatographic performance.
Recent technological trends indicate a shift toward integrated system approaches that combine hardware solutions with advanced software algorithms for real-time drift compensation. The development of temperature-controlled compartments, improved degassing systems, and pressure-resistant connections has contributed significantly to baseline stability improvements in modern HPLC systems.
Looking forward, the field is moving toward predictive maintenance systems that can anticipate baseline drift before it affects analytical results. Machine learning algorithms are increasingly being employed to recognize patterns in system performance data that precede drift issues, allowing for preemptive intervention.
This technical investigation aims to comprehensively examine the root causes of HPLC baseline drift, evaluate current troubleshooting methodologies, and propose systematic approaches for both reactive problem-solving and proactive prevention strategies. The ultimate goal is to establish a framework that minimizes baseline-related analytical variability while maximizing instrument uptime and data reliability.
The evolution of HPLC technology has seen remarkable improvements in column technology, detector sensitivity, and system integration. However, baseline stability issues have remained a consistent challenge throughout this development trajectory. Historical data indicates that approximately 30-40% of troubleshooting time in analytical laboratories is dedicated to resolving baseline-related problems, highlighting the significance of this technical challenge.
Baseline drift manifests through various patterns including positive drift, negative drift, cyclic fluctuations, and random noise. Each pattern provides diagnostic clues about underlying causes, which may include temperature fluctuations, mobile phase composition changes, column equilibration issues, detector instability, or electronic interference. Understanding these patterns is crucial for effective troubleshooting.
The primary objective of addressing HPLC baseline drift is to enhance analytical precision and reliability. Specifically, this involves achieving signal-to-noise ratios that consistently exceed 3:1 for limit of detection and 10:1 for limit of quantification across diverse sample matrices and analytical conditions. Additionally, maintaining baseline stability within ±2-3 mAU over extended analytical runs represents an industry benchmark for high-quality chromatographic performance.
Recent technological trends indicate a shift toward integrated system approaches that combine hardware solutions with advanced software algorithms for real-time drift compensation. The development of temperature-controlled compartments, improved degassing systems, and pressure-resistant connections has contributed significantly to baseline stability improvements in modern HPLC systems.
Looking forward, the field is moving toward predictive maintenance systems that can anticipate baseline drift before it affects analytical results. Machine learning algorithms are increasingly being employed to recognize patterns in system performance data that precede drift issues, allowing for preemptive intervention.
This technical investigation aims to comprehensively examine the root causes of HPLC baseline drift, evaluate current troubleshooting methodologies, and propose systematic approaches for both reactive problem-solving and proactive prevention strategies. The ultimate goal is to establish a framework that minimizes baseline-related analytical variability while maximizing instrument uptime and data reliability.
Market Demand for Stable HPLC Analysis
The High-Performance Liquid Chromatography (HPLC) market has witnessed substantial growth over the past decade, with a particularly strong demand for stable analytical performance. Laboratory professionals across pharmaceutical, biotechnology, food safety, environmental monitoring, and clinical diagnostics sectors consistently emphasize the critical importance of baseline stability in their analytical workflows.
Market research indicates that laboratories are increasingly seeking HPLC systems and solutions that minimize baseline drift issues, as these problems directly impact data quality, method validation, and regulatory compliance. The pharmaceutical industry, representing the largest market segment for HPLC technologies, faces stringent regulatory requirements that necessitate highly reliable and reproducible chromatographic results.
Contract Research Organizations (CROs) have emerged as significant consumers of advanced HPLC technologies, driven by their need to deliver consistent, high-quality analytical services to clients. These organizations frequently cite baseline stability as a key factor in equipment purchasing decisions, with many willing to invest in premium solutions that guarantee superior performance.
The growing trend toward automated, high-throughput analysis has further amplified the demand for stable HPLC systems. As laboratories process larger sample volumes with minimal operator intervention, the tolerance for baseline issues has decreased substantially. Market surveys reveal that laboratory managers rank baseline stability among their top three considerations when evaluating new HPLC equipment.
Academic and government research institutions, while often more budget-conscious than industrial laboratories, similarly prioritize baseline stability in their HPLC applications, particularly for research involving trace analysis and complex biological matrices where signal-to-noise ratios are critical.
The market for HPLC troubleshooting services, specialized training, and consulting has expanded in parallel with the primary instrument market. Laboratories are allocating significant resources to address baseline drift issues, creating opportunities for service providers offering specialized expertise in this area.
Geographically, mature markets in North America and Europe demonstrate the highest sensitivity to baseline performance issues, while rapidly growing markets in Asia-Pacific regions are increasingly adopting similar quality standards as their laboratory infrastructure develops. This global convergence of expectations has prompted HPLC manufacturers to emphasize baseline stability in their product development and marketing strategies.
The economic impact of baseline drift extends beyond immediate analytical concerns, affecting laboratory productivity, sample throughput, and ultimately business performance. Market analysis suggests that laboratories experiencing frequent baseline issues report significantly higher operational costs and reduced analytical capacity, creating a compelling economic case for investing in solutions that address these challenges.
Market research indicates that laboratories are increasingly seeking HPLC systems and solutions that minimize baseline drift issues, as these problems directly impact data quality, method validation, and regulatory compliance. The pharmaceutical industry, representing the largest market segment for HPLC technologies, faces stringent regulatory requirements that necessitate highly reliable and reproducible chromatographic results.
Contract Research Organizations (CROs) have emerged as significant consumers of advanced HPLC technologies, driven by their need to deliver consistent, high-quality analytical services to clients. These organizations frequently cite baseline stability as a key factor in equipment purchasing decisions, with many willing to invest in premium solutions that guarantee superior performance.
The growing trend toward automated, high-throughput analysis has further amplified the demand for stable HPLC systems. As laboratories process larger sample volumes with minimal operator intervention, the tolerance for baseline issues has decreased substantially. Market surveys reveal that laboratory managers rank baseline stability among their top three considerations when evaluating new HPLC equipment.
Academic and government research institutions, while often more budget-conscious than industrial laboratories, similarly prioritize baseline stability in their HPLC applications, particularly for research involving trace analysis and complex biological matrices where signal-to-noise ratios are critical.
The market for HPLC troubleshooting services, specialized training, and consulting has expanded in parallel with the primary instrument market. Laboratories are allocating significant resources to address baseline drift issues, creating opportunities for service providers offering specialized expertise in this area.
Geographically, mature markets in North America and Europe demonstrate the highest sensitivity to baseline performance issues, while rapidly growing markets in Asia-Pacific regions are increasingly adopting similar quality standards as their laboratory infrastructure develops. This global convergence of expectations has prompted HPLC manufacturers to emphasize baseline stability in their product development and marketing strategies.
The economic impact of baseline drift extends beyond immediate analytical concerns, affecting laboratory productivity, sample throughput, and ultimately business performance. Market analysis suggests that laboratories experiencing frequent baseline issues report significantly higher operational costs and reduced analytical capacity, creating a compelling economic case for investing in solutions that address these challenges.
Current Challenges in HPLC Baseline Stability
High-performance liquid chromatography (HPLC) baseline stability remains a critical challenge in analytical chemistry laboratories worldwide. Despite significant technological advancements in HPLC instrumentation, baseline drift continues to plague analysts, compromising data integrity and analytical precision. The primary manifestation of this issue is a gradual, often unpredictable deviation of the chromatographic baseline from horizontal linearity, which can obscure small peaks, complicate integration, and reduce quantitative accuracy.
Baseline drift in HPLC systems stems from multiple interconnected factors that create a complex troubleshooting landscape. Temperature fluctuations represent one of the most persistent challenges, as even minor variations in ambient or column temperature can cause significant detector response changes, particularly in UV-Vis detection systems where optical properties of mobile phases are temperature-dependent.
Mobile phase composition instability presents another major hurdle. Gradual changes in solvent composition due to evaporation, improper mixing, or selective absorption of components can alter refractive indices and UV absorption characteristics. This is especially problematic in gradient elution methods where baseline stability is inherently more challenging to maintain compared to isocratic separations.
Detector-specific issues further complicate baseline stability. UV-Vis detectors suffer from lamp intensity fluctuations and aging effects, while refractive index detectors exhibit extreme sensitivity to pressure and temperature variations. Mass spectrometric detectors face challenges with ion suppression and matrix effects that manifest as baseline irregularities.
System contamination represents a particularly insidious source of baseline instability. Accumulation of particulates, microbial growth, or leached materials from system components can gradually alter detector responses. These contaminants may originate from improperly prepared mobile phases, sample matrices, or degradation of system components such as seals and tubing.
Electronic noise and signal processing limitations constitute another category of challenges. Modern HPLC systems incorporate sophisticated electronics that, while powerful, remain susceptible to electromagnetic interference, grounding issues, and digital signal processing artifacts. These electronic vulnerabilities can introduce periodic baseline fluctuations that are difficult to distinguish from genuine chromatographic phenomena.
The increasing complexity of sample matrices in emerging application areas such as biologics analysis, environmental monitoring, and metabolomics has introduced new baseline stability challenges. Complex matrices often contain components that interact unpredictably with stationary phases, causing gradual column equilibration shifts that manifest as baseline drift during analytical runs.
Addressing these multifaceted challenges requires a systematic approach that integrates hardware improvements, method optimization, and operational best practices. The complexity of modern HPLC applications demands increasingly sophisticated solutions to baseline stability issues, making this an active area of both academic research and instrument development.
Baseline drift in HPLC systems stems from multiple interconnected factors that create a complex troubleshooting landscape. Temperature fluctuations represent one of the most persistent challenges, as even minor variations in ambient or column temperature can cause significant detector response changes, particularly in UV-Vis detection systems where optical properties of mobile phases are temperature-dependent.
Mobile phase composition instability presents another major hurdle. Gradual changes in solvent composition due to evaporation, improper mixing, or selective absorption of components can alter refractive indices and UV absorption characteristics. This is especially problematic in gradient elution methods where baseline stability is inherently more challenging to maintain compared to isocratic separations.
Detector-specific issues further complicate baseline stability. UV-Vis detectors suffer from lamp intensity fluctuations and aging effects, while refractive index detectors exhibit extreme sensitivity to pressure and temperature variations. Mass spectrometric detectors face challenges with ion suppression and matrix effects that manifest as baseline irregularities.
System contamination represents a particularly insidious source of baseline instability. Accumulation of particulates, microbial growth, or leached materials from system components can gradually alter detector responses. These contaminants may originate from improperly prepared mobile phases, sample matrices, or degradation of system components such as seals and tubing.
Electronic noise and signal processing limitations constitute another category of challenges. Modern HPLC systems incorporate sophisticated electronics that, while powerful, remain susceptible to electromagnetic interference, grounding issues, and digital signal processing artifacts. These electronic vulnerabilities can introduce periodic baseline fluctuations that are difficult to distinguish from genuine chromatographic phenomena.
The increasing complexity of sample matrices in emerging application areas such as biologics analysis, environmental monitoring, and metabolomics has introduced new baseline stability challenges. Complex matrices often contain components that interact unpredictably with stationary phases, causing gradual column equilibration shifts that manifest as baseline drift during analytical runs.
Addressing these multifaceted challenges requires a systematic approach that integrates hardware improvements, method optimization, and operational best practices. The complexity of modern HPLC applications demands increasingly sophisticated solutions to baseline stability issues, making this an active area of both academic research and instrument development.
Established Methodologies for Baseline Drift Resolution
01 Temperature control methods to reduce baseline drift
Temperature fluctuations can significantly affect HPLC baseline stability. Implementing precise temperature control systems for both the column and mobile phase can minimize thermal gradients that cause baseline drift. This includes using column ovens with accurate temperature regulation, pre-heating mobile phases, and maintaining stable ambient conditions in the laboratory environment.- Temperature control methods to reduce baseline drift: Temperature fluctuations can significantly affect HPLC baseline stability. Various temperature control methods are employed to maintain consistent column and mobile phase temperatures, including column ovens, pre-heaters for mobile phase, and temperature-controlled environments for the entire HPLC system. These approaches help minimize thermal gradients that can cause baseline drift during analysis.
- Mobile phase composition optimization: The composition of the mobile phase plays a crucial role in baseline stability. Techniques include proper degassing to remove dissolved gases, gradient optimization to reduce solvent mixing effects, buffer selection with appropriate pH and concentration, and use of high-purity solvents. These optimizations help minimize refractive index changes and other physical-chemical variations that contribute to baseline drift.
- Hardware modifications and improvements: Specialized hardware components can be implemented to reduce baseline drift, including advanced pump designs with reduced pulsation, improved detector cells with better thermal stability, specialized connection tubing to minimize dead volumes, and electronic dampening systems. These hardware solutions help maintain consistent flow rates and detection conditions throughout the analysis.
- Signal processing and data correction algorithms: Software-based approaches can compensate for baseline drift through mathematical algorithms. These include baseline subtraction techniques, drift correction algorithms, signal filtering methods, and automated baseline recognition systems. These computational methods can effectively process raw chromatographic data to remove baseline irregularities and improve quantitative analysis accuracy.
- System equilibration and conditioning procedures: Proper system preparation before analysis is essential for baseline stability. This includes thorough system purging to remove contaminants, extended equilibration periods to stabilize the column and detector, mobile phase pre-mixing to ensure homogeneity, and regular system maintenance routines. These preparatory steps help establish stable baseline conditions before sample injection and throughout the analytical run.
02 Mobile phase composition and preparation techniques
The composition and preparation of mobile phases directly impact baseline stability in HPLC. Proper degassing to remove dissolved gases, filtration to eliminate particulates, and careful selection of high-purity solvents can significantly reduce baseline drift. Gradual rather than abrupt changes in mobile phase composition during gradient elution and ensuring thorough mixing of solvents also contribute to baseline stability.Expand Specific Solutions03 Hardware modifications and system optimization
Specialized hardware components and system configurations can be implemented to minimize baseline drift. This includes using low-volume mixing chambers, pulse dampeners to reduce pump pulsation, optimized detector flow cells, and improved fluidic connections. Regular maintenance of system components such as pump seals, check valves, and detector lamps also helps maintain baseline stability during HPLC analysis.Expand Specific Solutions04 Signal processing and data correction algorithms
Advanced signal processing techniques and mathematical algorithms can be applied to correct baseline drift in HPLC chromatograms. These include digital filtering, baseline subtraction, polynomial fitting, and wavelet transformation methods. Real-time signal correction during data acquisition or post-processing of chromatographic data can effectively compensate for various types of baseline drift patterns.Expand Specific Solutions05 Equilibration and conditioning procedures
Proper system equilibration and conditioning before analysis is crucial for baseline stability. This involves sufficient column conditioning with mobile phase, extended system equilibration when changing mobile phase compositions, and implementing blank gradient runs between analyses. These procedures help stabilize the chromatographic system and reduce baseline drift caused by column or system disequilibrium.Expand Specific Solutions
Major Manufacturers and Service Providers in HPLC Industry
The HPLC baseline drift troubleshooting market is in a mature growth phase, with an estimated global analytical instrumentation market size exceeding $5 billion. Major players include established analytical equipment manufacturers like Agilent Technologies, Waters Corporation, and Shimadzu, alongside specialized companies from the list such as IBM, Air Products & Chemicals, and Merlin Biomedical. Technical maturity is high, with solutions ranging from basic mechanical fixes to advanced AI-driven diagnostic tools developed by companies like IBM. The competitive landscape features both large corporations offering comprehensive chromatography systems and specialized firms providing targeted solutions for specific drift issues, creating a fragmented yet sophisticated market with ongoing innovation in automated troubleshooting technologies.
International Business Machines Corp.
Technical Solution: IBM has developed an AI-powered HPLC baseline drift correction system that represents a significant departure from traditional hardware-focused approaches. Their solution employs machine learning algorithms that analyze historical chromatographic data to identify patterns in baseline drift specific to individual instrument configurations. The system continuously monitors real-time chromatographic output and applies predictive corrections before drift becomes problematic. IBM's technology incorporates digital signal processing techniques that can distinguish between actual analyte signals and various types of baseline noise or drift. The platform includes a comprehensive database of common drift patterns associated with specific instrument models, mobile phase compositions, and environmental conditions. This allows the system to suggest targeted interventions based on recognized drift signatures. Additionally, IBM has implemented cloud-based processing capabilities that enable cross-laboratory standardization and drift compensation across multiple instruments.
Strengths: The AI-driven approach allows for predictive rather than reactive drift management, potentially eliminating drift issues before they impact results. The system can continuously improve through machine learning from additional data inputs. Weaknesses: Requires significant computational resources and initial training period with instrument-specific data. May struggle with novel drift patterns not represented in training datasets until sufficient examples are processed.
Air Products & Chemicals, Inc.
Technical Solution: Air Products has developed a specialized HPLC baseline drift solution focused on mobile phase chemistry and gas management. Their system centers on advanced gas purification technology that removes trace contaminants from carrier gases used in HPLC systems. The technology employs proprietary adsorbent materials that selectively trap oxygen, water vapor, and hydrocarbon impurities down to parts-per-billion levels. Air Products' approach includes specialized mobile phase additives that stabilize pH and ionic strength throughout analytical runs, preventing drift caused by buffer depletion or modification. Their comprehensive solution also features specialized solvent blending systems that ensure consistent mobile phase composition through precise metering and mixing technologies. Additionally, they've developed specialized degassing modules using advanced membrane technology with optimized flow patterns to maximize gas removal efficiency while minimizing system dead volume.
Strengths: Exceptional expertise in gas purification and management provides superior control over dissolved gas-related baseline issues. The specialized mobile phase additives address chemical sources of drift often overlooked by hardware-focused solutions. Weaknesses: May require specialized consumables and additives that increase operational costs. Some components may have limited compatibility with certain analytical methods or detection techniques.
Critical Technical Innovations in HPLC Stability Control
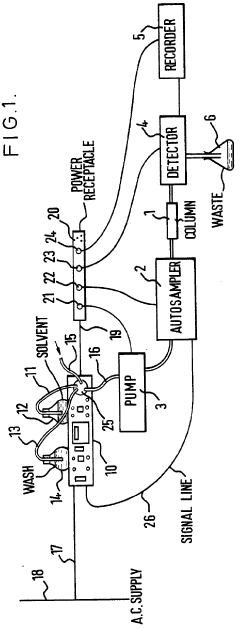
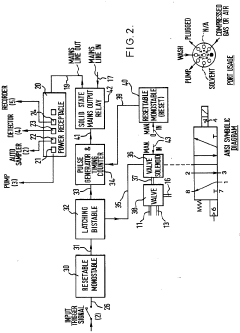
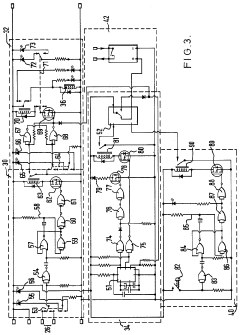
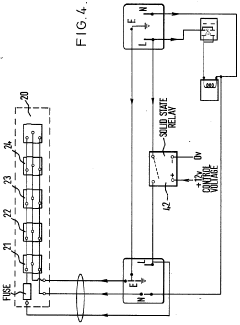
Improvements in or relating to high performance liquid chromatography systems
PatentInactiveGB2174016A
Innovation
- An ancillary apparatus that includes a valve system for selecting between mobile phase and wash liquid sources, a timing mechanism initiated by a trigger pulse from the HPLC system, and an electric switch to disconnect power to the HPLC system units after a predetermined wash liquid supply, ensuring a controlled shutdown and washout process.
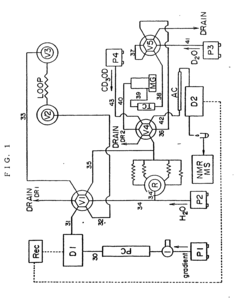
HPLC apparatus for fractioning and preparing sample for NMR spectrometry and method of changing mobile phase
PatentInactiveEP1001263B1
Innovation
- A process and apparatus for HPLC that includes a separation/sampling section, a trapping section, a deuterium oxide replacement section, and a deuterated solvent supplying section, allowing for on-line separation, desalting, concentration, and deuterium replacement using a deuterated solvent other than deuterium oxide, reducing solvent usage and enabling efficient sample preparation for NMR analysis.
Quality Control Standards for Chromatographic Analysis
Quality control standards are essential for ensuring the reliability and reproducibility of chromatographic analyses, particularly when troubleshooting HPLC baseline drift issues. These standards establish systematic approaches to maintain analytical integrity throughout the chromatographic process. The implementation of robust quality control measures begins with the establishment of system suitability tests (SSTs) that verify the chromatographic system's performance before sample analysis.
Standard operating procedures (SOPs) for HPLC systems should include detailed protocols for baseline monitoring and drift evaluation. These protocols typically specify acceptable limits for baseline drift, measured in milliabsorption units per hour (mAU/h), with industry standards generally requiring drift below 0.05 mAU/h for high-sensitivity applications. Regular calibration of detector response using certified reference materials ensures that baseline measurements remain accurate and traceable to recognized standards.
Quality control charts represent a critical tool for monitoring baseline performance over time. These charts track parameters such as noise levels, drift rates, and retention time stability, enabling analysts to identify trends before they develop into significant problems. Statistical process control methods applied to these charts help distinguish between random variations and systematic issues requiring intervention.
Method validation protocols must include specific tests for baseline stability under various operating conditions. These tests should evaluate the robustness of the method against factors known to influence baseline drift, such as temperature fluctuations, mobile phase composition changes, and gradient profile variations. The ICH Q2(R1) guidelines provide a framework for these validation procedures, emphasizing the importance of establishing detection and quantification limits that account for baseline variability.
Proficiency testing and interlaboratory comparisons serve as external quality assurance measures that validate a laboratory's ability to control baseline drift effectively. Participation in these programs allows laboratories to benchmark their performance against peers and identify opportunities for improvement. Documentation requirements for quality control include comprehensive records of system maintenance, calibration history, and corrective actions taken in response to baseline drift incidents.
Modern quality control approaches increasingly incorporate automated system monitoring with predefined alert thresholds for baseline parameters. These systems can detect subtle changes in chromatographic performance before they manifest as visible baseline drift, enabling proactive maintenance rather than reactive troubleshooting. The integration of quality control data with laboratory information management systems (LIMS) further enhances traceability and facilitates trend analysis across multiple instruments and methods.
Standard operating procedures (SOPs) for HPLC systems should include detailed protocols for baseline monitoring and drift evaluation. These protocols typically specify acceptable limits for baseline drift, measured in milliabsorption units per hour (mAU/h), with industry standards generally requiring drift below 0.05 mAU/h for high-sensitivity applications. Regular calibration of detector response using certified reference materials ensures that baseline measurements remain accurate and traceable to recognized standards.
Quality control charts represent a critical tool for monitoring baseline performance over time. These charts track parameters such as noise levels, drift rates, and retention time stability, enabling analysts to identify trends before they develop into significant problems. Statistical process control methods applied to these charts help distinguish between random variations and systematic issues requiring intervention.
Method validation protocols must include specific tests for baseline stability under various operating conditions. These tests should evaluate the robustness of the method against factors known to influence baseline drift, such as temperature fluctuations, mobile phase composition changes, and gradient profile variations. The ICH Q2(R1) guidelines provide a framework for these validation procedures, emphasizing the importance of establishing detection and quantification limits that account for baseline variability.
Proficiency testing and interlaboratory comparisons serve as external quality assurance measures that validate a laboratory's ability to control baseline drift effectively. Participation in these programs allows laboratories to benchmark their performance against peers and identify opportunities for improvement. Documentation requirements for quality control include comprehensive records of system maintenance, calibration history, and corrective actions taken in response to baseline drift incidents.
Modern quality control approaches increasingly incorporate automated system monitoring with predefined alert thresholds for baseline parameters. These systems can detect subtle changes in chromatographic performance before they manifest as visible baseline drift, enabling proactive maintenance rather than reactive troubleshooting. The integration of quality control data with laboratory information management systems (LIMS) further enhances traceability and facilitates trend analysis across multiple instruments and methods.
Environmental Factors Affecting HPLC Performance
High-performance liquid chromatography (HPLC) systems are highly sensitive analytical instruments that can be significantly affected by various environmental factors. Temperature fluctuations represent one of the most critical environmental variables impacting HPLC performance. Even minor temperature changes can alter retention times, modify selectivity, and cause baseline drift. Modern laboratories should maintain temperature stability within ±2°C, with dedicated HPLC rooms ideally regulated at 20-25°C to ensure consistent chromatographic results.
Humidity levels also play a substantial role in HPLC system stability. Excessive humidity can lead to electrical component malfunctions, while insufficient humidity may generate static electricity that interferes with sensitive detector electronics. The recommended relative humidity range for optimal HPLC operation is 40-60%. Laboratories in regions with extreme seasonal variations should consider implementing humidity control systems to maintain this optimal range.
Airborne particulates and chemical contaminants present another significant environmental challenge. Dust particles can infiltrate pumps and valves, causing mechanical wear and irregular flow patterns that manifest as baseline instability. Volatile organic compounds (VOCs) from solvents, cleaning agents, or laboratory processes can be absorbed by mobile phases, leading to detector noise and baseline perturbations. HEPA filtration systems and proper laboratory ventilation are essential preventive measures.
Electrical supply quality directly impacts HPLC performance, particularly for sensitive detectors like UV-Vis and mass spectrometers. Voltage fluctuations, spikes, and electromagnetic interference can introduce noise into detector signals, resulting in erratic baselines. Uninterruptible power supplies (UPS) with power conditioning capabilities are recommended for all HPLC systems, especially in facilities with unstable power infrastructure.
Vibration from nearby equipment, building HVAC systems, or laboratory foot traffic can physically disturb HPLC components, causing flow irregularities and detector noise. Anti-vibration tables or platforms are effective solutions for isolating HPLC systems from environmental vibrations. Additionally, placing HPLC instruments away from high-traffic areas, centrifuges, and mechanical equipment can significantly reduce vibration-induced baseline problems.
Light exposure affects certain HPLC analyses, particularly those involving photosensitive compounds or using UV detection. Ambient light fluctuations can create baseline drift in UV detectors. Proper shielding of sample preparation areas and HPLC components, along with consistent laboratory lighting conditions, helps minimize these effects. For highly sensitive applications, amber glassware and light-protective covers for sample trays are recommended.
Humidity levels also play a substantial role in HPLC system stability. Excessive humidity can lead to electrical component malfunctions, while insufficient humidity may generate static electricity that interferes with sensitive detector electronics. The recommended relative humidity range for optimal HPLC operation is 40-60%. Laboratories in regions with extreme seasonal variations should consider implementing humidity control systems to maintain this optimal range.
Airborne particulates and chemical contaminants present another significant environmental challenge. Dust particles can infiltrate pumps and valves, causing mechanical wear and irregular flow patterns that manifest as baseline instability. Volatile organic compounds (VOCs) from solvents, cleaning agents, or laboratory processes can be absorbed by mobile phases, leading to detector noise and baseline perturbations. HEPA filtration systems and proper laboratory ventilation are essential preventive measures.
Electrical supply quality directly impacts HPLC performance, particularly for sensitive detectors like UV-Vis and mass spectrometers. Voltage fluctuations, spikes, and electromagnetic interference can introduce noise into detector signals, resulting in erratic baselines. Uninterruptible power supplies (UPS) with power conditioning capabilities are recommended for all HPLC systems, especially in facilities with unstable power infrastructure.
Vibration from nearby equipment, building HVAC systems, or laboratory foot traffic can physically disturb HPLC components, causing flow irregularities and detector noise. Anti-vibration tables or platforms are effective solutions for isolating HPLC systems from environmental vibrations. Additionally, placing HPLC instruments away from high-traffic areas, centrifuges, and mechanical equipment can significantly reduce vibration-induced baseline problems.
Light exposure affects certain HPLC analyses, particularly those involving photosensitive compounds or using UV detection. Ambient light fluctuations can create baseline drift in UV detectors. Proper shielding of sample preparation areas and HPLC components, along with consistent laboratory lighting conditions, helps minimize these effects. For highly sensitive applications, amber glassware and light-protective covers for sample trays are recommended.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!