How to Achieve Low LOD in HPLC—Key Methods
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC LOD Reduction Background and Objectives
High-performance liquid chromatography (HPLC) has evolved significantly since its inception in the 1960s, becoming an indispensable analytical technique across pharmaceutical, environmental, food safety, and clinical diagnostics industries. The ability to detect increasingly lower concentrations of analytes has been a consistent driving force in HPLC development, with limits of detection (LOD) improving from parts per million (ppm) to parts per trillion (ppt) over decades of technological advancement.
The pursuit of lower LODs in HPLC stems from regulatory requirements, scientific necessity, and commercial demands. Regulatory bodies worldwide have progressively lowered acceptable limits for contaminants, residues, and impurities, necessitating more sensitive analytical methods. The FDA, EMA, and other regulatory authorities continually update their guidelines, pushing laboratories to enhance detection capabilities for trace compounds.
Technological evolution in HPLC has followed a clear trajectory, from conventional systems with UV detection to modern ultra-high-performance liquid chromatography (UHPLC) coupled with mass spectrometry. Each advancement has contributed to lowering detection limits, with significant breakthroughs occurring in detector technology, column chemistry, and sample preparation techniques.
Current challenges in achieving lower LODs include matrix interference effects, instrumental limitations, and the physical constraints of signal-to-noise ratios. As detection limits approach femtogram levels, distinguishing true analyte signals from background noise becomes increasingly complex, requiring sophisticated signal processing algorithms and enhanced detection systems.
The objective of this technical research is to comprehensively evaluate key methodologies for achieving lower LODs in HPLC applications. This includes examining innovations in sample preparation techniques, column technology advancements, detector sensitivity enhancements, and signal processing strategies. The research aims to identify the most effective approaches for specific analytical challenges across different industries.
Additionally, this investigation seeks to establish a framework for method development that systematically addresses the factors influencing detection limits. By understanding the interplay between chromatographic parameters, sample characteristics, and detection systems, analysts can make informed decisions to optimize their methods for maximum sensitivity.
Future trends indicate continued progress toward even lower detection limits through emerging technologies such as nano-LC systems, novel stationary phases, and advanced hybrid detection techniques. The integration of artificial intelligence for automated method development and optimization represents a promising frontier in pushing the boundaries of HPLC sensitivity.
The pursuit of lower LODs in HPLC stems from regulatory requirements, scientific necessity, and commercial demands. Regulatory bodies worldwide have progressively lowered acceptable limits for contaminants, residues, and impurities, necessitating more sensitive analytical methods. The FDA, EMA, and other regulatory authorities continually update their guidelines, pushing laboratories to enhance detection capabilities for trace compounds.
Technological evolution in HPLC has followed a clear trajectory, from conventional systems with UV detection to modern ultra-high-performance liquid chromatography (UHPLC) coupled with mass spectrometry. Each advancement has contributed to lowering detection limits, with significant breakthroughs occurring in detector technology, column chemistry, and sample preparation techniques.
Current challenges in achieving lower LODs include matrix interference effects, instrumental limitations, and the physical constraints of signal-to-noise ratios. As detection limits approach femtogram levels, distinguishing true analyte signals from background noise becomes increasingly complex, requiring sophisticated signal processing algorithms and enhanced detection systems.
The objective of this technical research is to comprehensively evaluate key methodologies for achieving lower LODs in HPLC applications. This includes examining innovations in sample preparation techniques, column technology advancements, detector sensitivity enhancements, and signal processing strategies. The research aims to identify the most effective approaches for specific analytical challenges across different industries.
Additionally, this investigation seeks to establish a framework for method development that systematically addresses the factors influencing detection limits. By understanding the interplay between chromatographic parameters, sample characteristics, and detection systems, analysts can make informed decisions to optimize their methods for maximum sensitivity.
Future trends indicate continued progress toward even lower detection limits through emerging technologies such as nano-LC systems, novel stationary phases, and advanced hybrid detection techniques. The integration of artificial intelligence for automated method development and optimization represents a promising frontier in pushing the boundaries of HPLC sensitivity.
Market Demand for Enhanced HPLC Sensitivity
The global HPLC market has witnessed substantial growth driven by increasing demand for higher sensitivity and lower detection limits across various industries. The pharmaceutical sector represents the largest market segment, where regulatory requirements for impurity detection at trace levels continue to tighten, necessitating HPLC systems with enhanced sensitivity. According to market research, the global HPLC market is projected to grow significantly through 2028, with sensitivity improvements being a primary driver of innovation and purchasing decisions.
Clinical diagnostics represents another critical market segment demanding enhanced HPLC sensitivity. The ability to detect biomarkers at increasingly lower concentrations enables earlier disease detection and more precise therapeutic monitoring. This is particularly evident in areas such as therapeutic drug monitoring, where clinicians require accurate quantification of drugs at sub-nanogram levels to ensure patient safety and treatment efficacy.
Environmental testing laboratories face growing pressure to detect contaminants at ever-decreasing concentrations as regulatory standards become more stringent worldwide. The detection of emerging contaminants such as PFAS (per- and polyfluoroalkyl substances), pharmaceutical residues, and microplastics in water sources requires HPLC methods with exceptionally low detection limits, often in the parts-per-trillion range.
Food safety testing represents another significant market driver, with manufacturers and regulatory bodies requiring more sensitive detection of pesticides, mycotoxins, and other contaminants. Consumer demand for transparency regarding trace contaminants has pushed the industry toward adopting more sensitive analytical methods, with HPLC being the gold standard for many applications.
The academic and research sector continues to push the boundaries of HPLC sensitivity for fundamental research in proteomics, metabolomics, and other -omics fields. The ability to detect and quantify low-abundance biomolecules is critical for understanding complex biological systems and developing new therapeutic approaches.
Market surveys indicate that laboratories are willing to invest in new HPLC technologies that offer demonstrable improvements in sensitivity and detection limits. This willingness is reflected in the premium pricing sustainable for systems that deliver verified performance enhancements in LOD. The return on investment for such systems is justified through improved data quality, reduced sample preparation requirements, and the ability to meet increasingly stringent regulatory standards.
Contract research organizations (CROs) and contract manufacturing organizations (CMOs) represent a growing market segment where enhanced HPLC sensitivity provides competitive advantages in securing contracts for analytical testing services. These organizations frequently highlight their analytical capabilities, including detection limits, as key differentiators in a competitive marketplace.
Clinical diagnostics represents another critical market segment demanding enhanced HPLC sensitivity. The ability to detect biomarkers at increasingly lower concentrations enables earlier disease detection and more precise therapeutic monitoring. This is particularly evident in areas such as therapeutic drug monitoring, where clinicians require accurate quantification of drugs at sub-nanogram levels to ensure patient safety and treatment efficacy.
Environmental testing laboratories face growing pressure to detect contaminants at ever-decreasing concentrations as regulatory standards become more stringent worldwide. The detection of emerging contaminants such as PFAS (per- and polyfluoroalkyl substances), pharmaceutical residues, and microplastics in water sources requires HPLC methods with exceptionally low detection limits, often in the parts-per-trillion range.
Food safety testing represents another significant market driver, with manufacturers and regulatory bodies requiring more sensitive detection of pesticides, mycotoxins, and other contaminants. Consumer demand for transparency regarding trace contaminants has pushed the industry toward adopting more sensitive analytical methods, with HPLC being the gold standard for many applications.
The academic and research sector continues to push the boundaries of HPLC sensitivity for fundamental research in proteomics, metabolomics, and other -omics fields. The ability to detect and quantify low-abundance biomolecules is critical for understanding complex biological systems and developing new therapeutic approaches.
Market surveys indicate that laboratories are willing to invest in new HPLC technologies that offer demonstrable improvements in sensitivity and detection limits. This willingness is reflected in the premium pricing sustainable for systems that deliver verified performance enhancements in LOD. The return on investment for such systems is justified through improved data quality, reduced sample preparation requirements, and the ability to meet increasingly stringent regulatory standards.
Contract research organizations (CROs) and contract manufacturing organizations (CMOs) represent a growing market segment where enhanced HPLC sensitivity provides competitive advantages in securing contracts for analytical testing services. These organizations frequently highlight their analytical capabilities, including detection limits, as key differentiators in a competitive marketplace.
Current HPLC Detection Limits and Technical Barriers
High-performance liquid chromatography (HPLC) has become an indispensable analytical technique across various industries, from pharmaceuticals to environmental monitoring. Despite its widespread adoption, achieving lower limits of detection (LOD) remains a significant challenge. Current HPLC systems typically achieve LOD values in the range of 0.1-10 ng/mL for UV detection and 0.01-1 ng/mL for fluorescence detection, while mass spectrometry can push these limits further to the pg/mL range.
The primary technical barriers limiting LOD improvement in HPLC systems stem from multiple sources. Detector sensitivity represents a fundamental constraint, with conventional UV-Vis detectors struggling to detect analytes at concentrations below 10^-8 M. Even with advanced photodiode array technology, the signal-to-noise ratio deteriorates significantly at ultra-low concentrations, making reliable detection challenging.
Column efficiency and separation performance constitute another critical barrier. Traditional HPLC columns with particle sizes of 3-5 μm offer limited theoretical plates, resulting in peak broadening that diminishes detection capability for trace analytes. While sub-2 μm particles in UHPLC systems have improved this aspect, they introduce challenges related to system pressure limitations and frictional heating.
Sample preparation techniques present additional obstacles. Conventional liquid-liquid extraction and solid-phase extraction methods often result in sample loss and contamination, directly impacting achievable detection limits. The recovery rates typically range from 70-95%, meaning valuable trace analytes may be lost during preparation steps.
Instrumental noise represents a persistent challenge across all HPLC systems. Baseline fluctuations, pump pulsations, and electronic noise from detectors collectively establish a noise floor that masks weak analyte signals. Modern systems employ various noise reduction technologies, but fundamental physical and electronic limitations remain.
Matrix effects pose particularly difficult challenges in complex samples. Co-eluting compounds can cause ion suppression in mass spectrometry detection or interfere with optical detection methods. These matrix effects can reduce sensitivity by orders of magnitude in complex biological or environmental samples.
Carryover between injections, particularly at trace levels, creates false positives and elevates detection baselines. Even with modern autosampler technologies, complete elimination of carryover remains elusive, with typical carryover rates ranging from 0.01-0.1% for standard systems.
The integration of these barriers creates a complex technical landscape where improvements in one area may be negated by limitations in another. Achieving breakthrough advancements in HPLC detection limits requires a holistic approach addressing multiple technical challenges simultaneously.
The primary technical barriers limiting LOD improvement in HPLC systems stem from multiple sources. Detector sensitivity represents a fundamental constraint, with conventional UV-Vis detectors struggling to detect analytes at concentrations below 10^-8 M. Even with advanced photodiode array technology, the signal-to-noise ratio deteriorates significantly at ultra-low concentrations, making reliable detection challenging.
Column efficiency and separation performance constitute another critical barrier. Traditional HPLC columns with particle sizes of 3-5 μm offer limited theoretical plates, resulting in peak broadening that diminishes detection capability for trace analytes. While sub-2 μm particles in UHPLC systems have improved this aspect, they introduce challenges related to system pressure limitations and frictional heating.
Sample preparation techniques present additional obstacles. Conventional liquid-liquid extraction and solid-phase extraction methods often result in sample loss and contamination, directly impacting achievable detection limits. The recovery rates typically range from 70-95%, meaning valuable trace analytes may be lost during preparation steps.
Instrumental noise represents a persistent challenge across all HPLC systems. Baseline fluctuations, pump pulsations, and electronic noise from detectors collectively establish a noise floor that masks weak analyte signals. Modern systems employ various noise reduction technologies, but fundamental physical and electronic limitations remain.
Matrix effects pose particularly difficult challenges in complex samples. Co-eluting compounds can cause ion suppression in mass spectrometry detection or interfere with optical detection methods. These matrix effects can reduce sensitivity by orders of magnitude in complex biological or environmental samples.
Carryover between injections, particularly at trace levels, creates false positives and elevates detection baselines. Even with modern autosampler technologies, complete elimination of carryover remains elusive, with typical carryover rates ranging from 0.01-0.1% for standard systems.
The integration of these barriers creates a complex technical landscape where improvements in one area may be negated by limitations in another. Achieving breakthrough advancements in HPLC detection limits requires a holistic approach addressing multiple technical challenges simultaneously.
State-of-the-Art LOD Reduction Methodologies
01 HPLC method development for low LOD determination
Development of specialized HPLC methods to achieve lower limits of detection for various analytes. These methods involve optimization of mobile phase composition, column selection, and detection parameters to enhance sensitivity. Advanced techniques include gradient elution, temperature control, and specialized sample preparation to minimize interference and maximize signal-to-noise ratio.- HPLC method development for LOD determination: Development of specific HPLC methodologies to determine the limit of detection for various compounds. These methods involve optimizing chromatographic conditions such as mobile phase composition, flow rate, column selection, and detector settings to achieve the lowest possible detection limits. The approaches focus on enhancing sensitivity through parameter optimization and validation protocols to establish reliable LOD values.
- Signal-to-noise ratio enhancement techniques: Techniques to improve signal-to-noise ratio in HPLC systems for better LOD performance. These include advanced signal processing algorithms, baseline noise reduction methods, and detector sensitivity improvements. By enhancing the signal-to-noise ratio, these techniques allow for detection of lower analyte concentrations, effectively lowering the limit of detection in chromatographic analyses.
- Sample preparation and pre-concentration methods: Innovative sample preparation and pre-concentration techniques to improve HPLC detection limits. These methods include solid-phase extraction, liquid-liquid extraction, derivatization reactions, and concentration steps that enhance the detectability of target analytes. By increasing the effective concentration of analytes prior to injection, these approaches significantly lower the practical limits of detection in complex matrices.
- Advanced detector technologies for improved LOD: Implementation of advanced detection technologies in HPLC systems to achieve lower limits of detection. These include mass spectrometry coupling, fluorescence detection, electrochemical detection, and diode array detection with specialized algorithms. The integration of these sensitive detection methods with HPLC allows for significant improvements in detection capability for trace analysis applications.
- Statistical and mathematical approaches for LOD calculation: Statistical and mathematical methodologies for calculating and validating HPLC limits of detection. These approaches include calibration curve methods, standard deviation of blank measurements, signal-to-noise ratio calculations, and regression analysis techniques. The methods provide standardized ways to determine LOD values that are scientifically sound and comply with regulatory requirements for analytical method validation.
02 Novel detection systems for improved LOD in HPLC
Implementation of advanced detection systems in HPLC to lower detection limits. These include mass spectrometry coupling, fluorescence detection, electrochemical detection, and diode array detection with signal enhancement algorithms. These detection systems provide higher sensitivity and selectivity compared to conventional UV detection, enabling quantification of trace amounts of analytes.Expand Specific Solutions03 Sample preparation techniques to enhance HPLC LOD
Advanced sample preparation methods to improve HPLC detection limits by concentrating analytes and removing interfering substances. Techniques include solid-phase extraction, liquid-liquid extraction, derivatization reactions, and pre-concentration steps. These methods effectively increase the signal-to-noise ratio and improve the overall sensitivity of the analytical method.Expand Specific Solutions04 Statistical approaches for LOD calculation in HPLC analysis
Various statistical methods for determining and validating the limit of detection in HPLC analysis. These include signal-to-noise ratio approaches, standard deviation of the response and slope methods, and calibration curve techniques. Advanced statistical tools help in establishing reliable LOD values that account for both systematic and random errors in the analytical procedure.Expand Specific Solutions05 Automated systems for consistent LOD in HPLC applications
Development of automated HPLC systems that ensure consistent and reproducible limits of detection across multiple analyses. These systems incorporate automated sample handling, precise injection volumes, temperature control, and computerized method optimization. Automation reduces human error and improves the reliability of LOD determinations in routine analytical applications.Expand Specific Solutions
Leading Manufacturers and Research Institutions in HPLC
The HPLC low LOD (Limit of Detection) technology landscape is currently in a mature growth phase, with an estimated global market size of $4-5 billion and annual growth of 5-7%. Major pharmaceutical and analytical instrument companies dominate this space, with Agilent Technologies and Thermo Fisher Scientific (parent of Dionex Softron) leading technological innovation through advanced detection systems and column technologies. Companies like IDEX Health & Science and Sunshine Lake Pharma are focusing on specialized applications in life sciences and drug development. Academic-industry partnerships, exemplified by collaborations between CNRS, University of Strasbourg, and commercial entities, are accelerating innovation in detection methodologies. The competitive landscape is characterized by continuous improvements in sensitivity, specificity, and automation, with emerging players from Asia, particularly China, increasingly challenging established Western manufacturers.
IDEX Health & Science LLC
Technical Solution: IDEX Health & Science has pioneered microfluidic and nanofluidic technologies to achieve exceptionally low LODs in HPLC applications. Their approach centers on precision-engineered fluidic components that minimize extra-column band broadening and maximize detection sensitivity. Key elements include: 1) Ultra-low dispersion fluidic connections with their MarvelX UHPLC connection systems that maintain peak integrity by eliminating dead volumes; 2) Advanced optical flow cells with optimized light paths and minimal volumes (as low as 2 nL) that enhance detection sensitivity while reducing noise; 3) Specialized degassing modules that eliminate dissolved gases which can cause baseline instability and detection interference; 4) Nanoliter-scale injection systems that enable precise handling of minimal sample volumes while maintaining concentration integrity; 5) Biocompatible and inert flow path components that prevent sample adsorption and contamination. Their integrated approach to fluidic path optimization has enabled detection improvements of 2-5x compared to conventional systems, particularly beneficial for applications requiring trace analysis of biomolecules, environmental contaminants, and pharmaceutical compounds.
Strengths: Specialized expertise in fluidic path optimization; components designed for seamless integration with various HPLC platforms; solutions particularly effective for challenging biological samples and limited volume applications. Weaknesses: Primarily a component provider rather than complete system manufacturer; requires integration with other vendors' equipment; specialized components may require additional maintenance expertise.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has developed comprehensive solutions for achieving low limits of detection (LOD) in HPLC through their InfinityLab LC series. Their approach combines multiple technological innovations: 1) Advanced pump designs with active damping systems that minimize baseline noise and pulse variations, achieving flow precision <0.07% RSD; 2) High-sensitivity detectors including diode array detectors with programmable slit widths (1-16 nm) and the 1290 Infinity II Fluorescence Detector with xenon flash lamp technology providing detection limits in the pg range; 3) Proprietary MaxLight flow cell technology with optimized light path that increases signal intensity while reducing noise; 4) Column technologies featuring sub-2-μm particles and superficially porous particles (Poroshell) that enhance separation efficiency while maintaining lower backpressure; 5) Integrated sample preparation workflows with online SPE capabilities that concentrate analytes prior to separation. Their InfinityLab LC/MSD systems combine these HPLC innovations with mass spectrometry detection, pushing LODs into the sub-pg/mL range for many applications.
Strengths: Comprehensive ecosystem approach integrating hardware, columns, and software solutions; industry-leading detector sensitivity; robust and reproducible performance across diverse applications. Weaknesses: Premium pricing structure may be prohibitive for some laboratories; proprietary consumables can increase operational costs; some advanced features require significant user training and method development expertise.
Critical Innovations in Sample Preparation and Detection
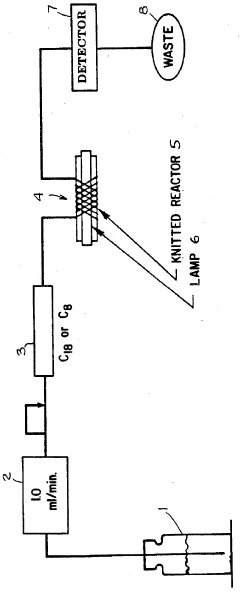
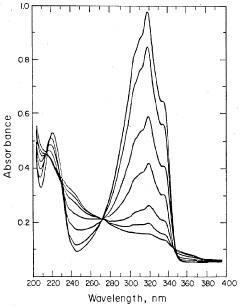
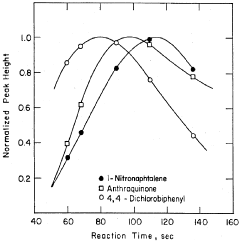
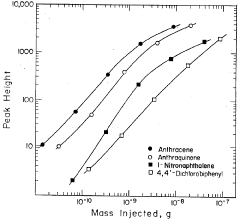
Method of improving the detection limits of UV-VIS absorbing compounds in HPLC by the use of a singlet oxygen trap
PatentInactiveUS4806485A
Innovation
- A post-column photochemical reaction method using a photochemical reactor with a singlet oxygen trap, such as substituted furans, to produce singlet oxygen, which reacts with analytes to enhance detectability without requiring oxygen removal, by promoting analyte molecules to an excited triplet state and transferring energy to oxygen in the mobile phase.
Validation and Standardization of Low LOD Methods
Validation and standardization are critical components in establishing reliable low LOD methods in HPLC analysis. The process begins with method validation protocols that must adhere to regulatory guidelines such as ICH Q2(R1), FDA, and USP standards. These protocols typically include assessments of specificity, linearity, accuracy, precision, robustness, and stability, with particular emphasis on the lower end of the calibration curve.
For low LOD methods, validation requires specialized approaches including determination of the signal-to-noise ratio (typically 3:1 for LOD and 10:1 for LOQ), statistical evaluation of the standard deviation of response and slope, and blank determination methods. The use of multiple blank injections and statistical analysis of background noise provides a more robust foundation for LOD claims.
Standardization efforts must focus on establishing consistent system suitability tests that verify the analytical system's performance before sample analysis. These tests should include parameters specifically relevant to low-level detection, such as baseline noise evaluation, detector sensitivity checks, and carryover assessment. Regular performance verification using certified reference materials at concentrations near the claimed LOD is essential for maintaining method reliability.
Inter-laboratory validation represents another crucial aspect of standardization, where multiple laboratories perform identical analyses to establish reproducibility across different instruments, operators, and environments. This collaborative approach helps identify variables that may affect low-level detection capabilities and establishes realistic performance expectations.
Documentation standards for low LOD methods must be particularly rigorous, including detailed specifications for sample preparation, instrument parameters, data processing algorithms, and integration methods. Signal processing techniques should be standardized, with clear guidelines for baseline correction, smoothing parameters, and peak integration boundaries.
Quality control measures specific to low LOD methods include the regular use of quality control samples at concentrations near the LOD, system suitability tests before each analytical run, and periodic proficiency testing. Trending of system performance data over time allows for early identification of sensitivity drift or other issues that could compromise detection capabilities.
The implementation of automated compliance tools can significantly enhance standardization efforts by ensuring consistent application of method parameters and data processing techniques across different analysts and laboratory locations. These tools should incorporate built-in verification steps that flag deviations from established protocols, particularly for critical parameters affecting low-level detection.
For low LOD methods, validation requires specialized approaches including determination of the signal-to-noise ratio (typically 3:1 for LOD and 10:1 for LOQ), statistical evaluation of the standard deviation of response and slope, and blank determination methods. The use of multiple blank injections and statistical analysis of background noise provides a more robust foundation for LOD claims.
Standardization efforts must focus on establishing consistent system suitability tests that verify the analytical system's performance before sample analysis. These tests should include parameters specifically relevant to low-level detection, such as baseline noise evaluation, detector sensitivity checks, and carryover assessment. Regular performance verification using certified reference materials at concentrations near the claimed LOD is essential for maintaining method reliability.
Inter-laboratory validation represents another crucial aspect of standardization, where multiple laboratories perform identical analyses to establish reproducibility across different instruments, operators, and environments. This collaborative approach helps identify variables that may affect low-level detection capabilities and establishes realistic performance expectations.
Documentation standards for low LOD methods must be particularly rigorous, including detailed specifications for sample preparation, instrument parameters, data processing algorithms, and integration methods. Signal processing techniques should be standardized, with clear guidelines for baseline correction, smoothing parameters, and peak integration boundaries.
Quality control measures specific to low LOD methods include the regular use of quality control samples at concentrations near the LOD, system suitability tests before each analytical run, and periodic proficiency testing. Trending of system performance data over time allows for early identification of sensitivity drift or other issues that could compromise detection capabilities.
The implementation of automated compliance tools can significantly enhance standardization efforts by ensuring consistent application of method parameters and data processing techniques across different analysts and laboratory locations. These tools should incorporate built-in verification steps that flag deviations from established protocols, particularly for critical parameters affecting low-level detection.
Environmental and Regulatory Considerations for Trace Analysis
The increasing focus on trace analysis in HPLC has brought environmental and regulatory considerations to the forefront of analytical method development. Regulatory bodies worldwide have established stringent guidelines for detecting contaminants at increasingly lower concentrations, particularly for pharmaceuticals, food safety, and environmental monitoring. The FDA, EPA, and European Medicines Agency have progressively lowered acceptable limits for impurities, driving the need for enhanced LOD capabilities in HPLC methods.
Environmental considerations play a dual role in trace analysis. First, the detection of environmental contaminants at ultra-low concentrations has become critical for assessing ecological impacts and human health risks. Persistent organic pollutants, pharmaceutical residues in water systems, and microplastics require detection at parts-per-billion or even parts-per-trillion levels, necessitating advanced HPLC techniques with exceptional sensitivity.
Secondly, the environmental impact of analytical methods themselves has gained attention. Green analytical chemistry principles encourage the development of HPLC methods that minimize solvent consumption and waste generation while maintaining low LOD performance. This has led to innovations in microflow HPLC and solvent recycling systems that reduce the ecological footprint of trace analysis while maintaining sensitivity requirements.
Regulatory compliance for trace analysis varies significantly across industries and regions, creating challenges for laboratories developing universally applicable low-LOD methods. For pharmaceutical applications, ICH guidelines (particularly Q3A and Q3B) define thresholds for reporting, identification, and qualification of impurities, directly influencing required LOD specifications. Similarly, food safety regulations such as those from Codex Alimentarius Commission establish maximum residue limits that analytical methods must reliably detect.
The validation of low-LOD methods presents unique regulatory challenges. Demonstrating method robustness, reproducibility, and accuracy at trace levels requires specialized approaches to validation. Regulatory bodies increasingly require uncertainty measurements for analytical results near detection limits, adding complexity to method development and validation protocols.
Future regulatory trends indicate continued pressure toward lower detection requirements as analytical capabilities advance. This regulatory-technology feedback loop drives continuous innovation in HPLC instrumentation and methodology. Laboratories must stay informed about evolving regulatory landscapes while developing flexible analytical platforms capable of adapting to increasingly stringent detection requirements across multiple regulatory frameworks.
Environmental considerations play a dual role in trace analysis. First, the detection of environmental contaminants at ultra-low concentrations has become critical for assessing ecological impacts and human health risks. Persistent organic pollutants, pharmaceutical residues in water systems, and microplastics require detection at parts-per-billion or even parts-per-trillion levels, necessitating advanced HPLC techniques with exceptional sensitivity.
Secondly, the environmental impact of analytical methods themselves has gained attention. Green analytical chemistry principles encourage the development of HPLC methods that minimize solvent consumption and waste generation while maintaining low LOD performance. This has led to innovations in microflow HPLC and solvent recycling systems that reduce the ecological footprint of trace analysis while maintaining sensitivity requirements.
Regulatory compliance for trace analysis varies significantly across industries and regions, creating challenges for laboratories developing universally applicable low-LOD methods. For pharmaceutical applications, ICH guidelines (particularly Q3A and Q3B) define thresholds for reporting, identification, and qualification of impurities, directly influencing required LOD specifications. Similarly, food safety regulations such as those from Codex Alimentarius Commission establish maximum residue limits that analytical methods must reliably detect.
The validation of low-LOD methods presents unique regulatory challenges. Demonstrating method robustness, reproducibility, and accuracy at trace levels requires specialized approaches to validation. Regulatory bodies increasingly require uncertainty measurements for analytical results near detection limits, adding complexity to method development and validation protocols.
Future regulatory trends indicate continued pressure toward lower detection requirements as analytical capabilities advance. This regulatory-technology feedback loop drives continuous innovation in HPLC instrumentation and methodology. Laboratories must stay informed about evolving regulatory landscapes while developing flexible analytical platforms capable of adapting to increasingly stringent detection requirements across multiple regulatory frameworks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!