Quantify Analyte Recovery in HPLC—Validation Methods
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC Recovery Validation Background and Objectives
High-performance liquid chromatography (HPLC) has evolved significantly since its inception in the 1960s, becoming an indispensable analytical technique in pharmaceutical, environmental, food safety, and clinical laboratories. The quantification of analyte recovery represents a critical aspect of HPLC method validation, directly impacting the reliability and accuracy of analytical results. Recovery assessment evaluates the efficiency of sample preparation procedures and determines whether the analytical method can accurately quantify the target analyte within complex matrices.
The historical development of recovery validation in HPLC has paralleled the evolution of regulatory frameworks in analytical chemistry. Early approaches were often inconsistent and lacked standardization, leading to significant variability in reported results across laboratories. The introduction of guidelines by organizations such as the International Conference on Harmonisation (ICH), the United States Pharmacopeia (USP), and the Food and Drug Administration (FDA) has progressively established more rigorous and harmonized approaches to recovery validation.
Current technological advancements in HPLC instrumentation, including ultra-high-performance liquid chromatography (UHPLC) and hyphenated techniques like LC-MS/MS, have enabled more sensitive and specific analyte detection. However, these advancements have simultaneously introduced new challenges in recovery validation, particularly for complex biological matrices and trace-level analytes. The increasing complexity of sample matrices and the demand for lower detection limits necessitate more sophisticated recovery validation methodologies.
The primary objective of this technical research is to comprehensively evaluate current methodologies for quantifying analyte recovery in HPLC validation. This includes examining established protocols, identifying limitations in existing approaches, and exploring innovative strategies that address contemporary analytical challenges. The research aims to provide a systematic framework for selecting appropriate recovery validation methods based on specific analytical contexts, sample types, and regulatory requirements.
Additionally, this investigation seeks to bridge the gap between theoretical principles and practical implementation of recovery validation in routine laboratory settings. By synthesizing best practices from regulatory guidelines, scientific literature, and industry experience, we aim to develop actionable recommendations that enhance the reliability and consistency of recovery assessments across different analytical applications.
The technological trajectory suggests a movement toward automated, high-throughput recovery validation methods that integrate seamlessly with existing analytical workflows. This research will explore these emerging trends and evaluate their potential impact on the future of HPLC method validation, particularly in regulated environments where analytical rigor is paramount.
The historical development of recovery validation in HPLC has paralleled the evolution of regulatory frameworks in analytical chemistry. Early approaches were often inconsistent and lacked standardization, leading to significant variability in reported results across laboratories. The introduction of guidelines by organizations such as the International Conference on Harmonisation (ICH), the United States Pharmacopeia (USP), and the Food and Drug Administration (FDA) has progressively established more rigorous and harmonized approaches to recovery validation.
Current technological advancements in HPLC instrumentation, including ultra-high-performance liquid chromatography (UHPLC) and hyphenated techniques like LC-MS/MS, have enabled more sensitive and specific analyte detection. However, these advancements have simultaneously introduced new challenges in recovery validation, particularly for complex biological matrices and trace-level analytes. The increasing complexity of sample matrices and the demand for lower detection limits necessitate more sophisticated recovery validation methodologies.
The primary objective of this technical research is to comprehensively evaluate current methodologies for quantifying analyte recovery in HPLC validation. This includes examining established protocols, identifying limitations in existing approaches, and exploring innovative strategies that address contemporary analytical challenges. The research aims to provide a systematic framework for selecting appropriate recovery validation methods based on specific analytical contexts, sample types, and regulatory requirements.
Additionally, this investigation seeks to bridge the gap between theoretical principles and practical implementation of recovery validation in routine laboratory settings. By synthesizing best practices from regulatory guidelines, scientific literature, and industry experience, we aim to develop actionable recommendations that enhance the reliability and consistency of recovery assessments across different analytical applications.
The technological trajectory suggests a movement toward automated, high-throughput recovery validation methods that integrate seamlessly with existing analytical workflows. This research will explore these emerging trends and evaluate their potential impact on the future of HPLC method validation, particularly in regulated environments where analytical rigor is paramount.
Market Demand for Accurate Analyte Recovery Analysis
The global market for High-Performance Liquid Chromatography (HPLC) analytical techniques continues to expand rapidly, driven by increasing demands for accurate quantification and validation methods across multiple industries. Current market valuations place the HPLC market at over 4 billion USD, with a compound annual growth rate exceeding 5% through 2028, significantly influenced by the need for precise analyte recovery analysis.
Pharmaceutical and biopharmaceutical sectors represent the largest market segment demanding accurate analyte recovery methods, accounting for approximately 60% of the total market share. These industries face stringent regulatory requirements from bodies such as the FDA, EMA, and ICH, which mandate thorough validation of analytical methods including recovery assessments. The implementation of Quality by Design (QbD) principles has further intensified the need for robust recovery quantification methods.
Contract Research Organizations (CROs) have emerged as significant consumers of advanced HPLC validation technologies, reporting annual growth rates of 7-9% in their analytical services divisions. This growth directly correlates with increasing outsourcing of analytical testing by pharmaceutical companies seeking specialized expertise in method validation and recovery analysis.
Environmental monitoring represents another rapidly expanding market segment, growing at nearly 8% annually, as regulatory bodies worldwide implement stricter guidelines for detecting trace contaminants in water, soil, and air samples. Accurate recovery data has become essential for environmental impact assessments and regulatory compliance reporting.
Food safety testing laboratories have reported a 30% increase in demand for validated HPLC methods with comprehensive recovery data over the past five years. This trend reflects growing consumer awareness regarding food contaminants and the implementation of more rigorous food safety standards globally.
Clinical diagnostics laboratories are increasingly adopting HPLC methods for therapeutic drug monitoring and biomarker analysis, creating a market segment valued at approximately 800 million USD with particular emphasis on recovery validation for complex biological matrices.
Market research indicates that end-users are willing to pay premium prices for analytical systems and services that offer comprehensive validation packages, including detailed recovery studies. Vendors who can demonstrate superior recovery rates and reproducibility across diverse sample matrices command price premiums of 15-20% compared to competitors offering less robust validation support.
The geographical distribution of market demand shows North America leading with approximately 40% market share, followed by Europe (30%) and Asia-Pacific (25%), with the latter showing the fastest growth rate as regulatory frameworks in these regions mature and align with international standards.
Pharmaceutical and biopharmaceutical sectors represent the largest market segment demanding accurate analyte recovery methods, accounting for approximately 60% of the total market share. These industries face stringent regulatory requirements from bodies such as the FDA, EMA, and ICH, which mandate thorough validation of analytical methods including recovery assessments. The implementation of Quality by Design (QbD) principles has further intensified the need for robust recovery quantification methods.
Contract Research Organizations (CROs) have emerged as significant consumers of advanced HPLC validation technologies, reporting annual growth rates of 7-9% in their analytical services divisions. This growth directly correlates with increasing outsourcing of analytical testing by pharmaceutical companies seeking specialized expertise in method validation and recovery analysis.
Environmental monitoring represents another rapidly expanding market segment, growing at nearly 8% annually, as regulatory bodies worldwide implement stricter guidelines for detecting trace contaminants in water, soil, and air samples. Accurate recovery data has become essential for environmental impact assessments and regulatory compliance reporting.
Food safety testing laboratories have reported a 30% increase in demand for validated HPLC methods with comprehensive recovery data over the past five years. This trend reflects growing consumer awareness regarding food contaminants and the implementation of more rigorous food safety standards globally.
Clinical diagnostics laboratories are increasingly adopting HPLC methods for therapeutic drug monitoring and biomarker analysis, creating a market segment valued at approximately 800 million USD with particular emphasis on recovery validation for complex biological matrices.
Market research indicates that end-users are willing to pay premium prices for analytical systems and services that offer comprehensive validation packages, including detailed recovery studies. Vendors who can demonstrate superior recovery rates and reproducibility across diverse sample matrices command price premiums of 15-20% compared to competitors offering less robust validation support.
The geographical distribution of market demand shows North America leading with approximately 40% market share, followed by Europe (30%) and Asia-Pacific (25%), with the latter showing the fastest growth rate as regulatory frameworks in these regions mature and align with international standards.
Current Challenges in HPLC Recovery Quantification
Despite significant advancements in High-Performance Liquid Chromatography (HPLC) technology, quantifying analyte recovery remains one of the most challenging aspects of method validation. The primary difficulty lies in distinguishing between true recovery losses and matrix effects, which often manifest similarly in analytical results. Current methodologies frequently fail to adequately differentiate between these phenomena, leading to potential misinterpretation of data and compromised analytical integrity.
Matrix complexity presents another substantial challenge, particularly when analyzing biological samples, environmental specimens, or complex pharmaceutical formulations. These matrices contain numerous interfering compounds that can compete for binding sites, alter retention times, or cause ion suppression in LC-MS applications. The variability of these interactions across different sample types necessitates customized recovery assessment protocols, making standardization difficult.
Instrument sensitivity limitations further complicate recovery quantification, especially when dealing with trace analytes. At low concentrations, the signal-to-noise ratio deteriorates, making accurate recovery determination increasingly uncertain. This challenge is particularly pronounced in regulatory environments where precise recovery values are required for method validation.
The lack of universally accepted recovery acceptance criteria represents another significant hurdle. While regulatory bodies provide general guidelines, specific thresholds vary considerably across different application domains and analyte classes. This inconsistency creates confusion among analysts and can lead to different interpretations of the same recovery data depending on the applied standard.
Reproducibility issues also plague recovery quantification efforts. Even well-established methods often show significant day-to-day variability in recovery values, raising questions about the reliability of single-point recovery determinations. This variability stems from multiple factors including minor fluctuations in mobile phase composition, column performance degradation, and sample preparation inconsistencies.
Statistical approaches for recovery assessment present their own challenges. Traditional methods often assume normal distribution of errors, which may not hold true for complex analytical systems. Additionally, the appropriate number of replicates and concentration levels required for robust recovery determination remains contentious, with practices varying widely across laboratories.
Automation of recovery assessment workflows, while promising, introduces new challenges related to method transfer and validation. Automated systems may handle sample preparation steps differently than manual procedures, potentially altering recovery profiles and necessitating comprehensive revalidation efforts.
Matrix complexity presents another substantial challenge, particularly when analyzing biological samples, environmental specimens, or complex pharmaceutical formulations. These matrices contain numerous interfering compounds that can compete for binding sites, alter retention times, or cause ion suppression in LC-MS applications. The variability of these interactions across different sample types necessitates customized recovery assessment protocols, making standardization difficult.
Instrument sensitivity limitations further complicate recovery quantification, especially when dealing with trace analytes. At low concentrations, the signal-to-noise ratio deteriorates, making accurate recovery determination increasingly uncertain. This challenge is particularly pronounced in regulatory environments where precise recovery values are required for method validation.
The lack of universally accepted recovery acceptance criteria represents another significant hurdle. While regulatory bodies provide general guidelines, specific thresholds vary considerably across different application domains and analyte classes. This inconsistency creates confusion among analysts and can lead to different interpretations of the same recovery data depending on the applied standard.
Reproducibility issues also plague recovery quantification efforts. Even well-established methods often show significant day-to-day variability in recovery values, raising questions about the reliability of single-point recovery determinations. This variability stems from multiple factors including minor fluctuations in mobile phase composition, column performance degradation, and sample preparation inconsistencies.
Statistical approaches for recovery assessment present their own challenges. Traditional methods often assume normal distribution of errors, which may not hold true for complex analytical systems. Additionally, the appropriate number of replicates and concentration levels required for robust recovery determination remains contentious, with practices varying widely across laboratories.
Automation of recovery assessment workflows, while promising, introduces new challenges related to method transfer and validation. Automated systems may handle sample preparation steps differently than manual procedures, potentially altering recovery profiles and necessitating comprehensive revalidation efforts.
Established HPLC Recovery Validation Protocols
01 Standard recovery methods for HPLC validation
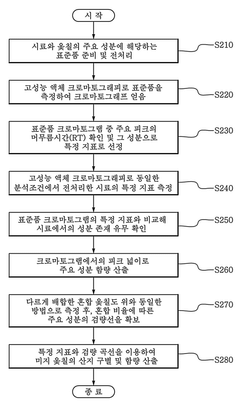
Standard recovery methods are essential for validating HPLC analytical procedures. These methods typically involve spiking known amounts of analyte into sample matrices at different concentration levels, followed by extraction and analysis. The recovery is calculated by comparing the measured concentration to the known added amount, expressed as a percentage. Acceptable recovery ranges are typically 80-120% for most pharmaceutical applications, with tighter ranges for trace analysis.- Recovery assessment methods in HPLC validation: Recovery assessment is a critical component of HPLC validation protocols, measuring the efficiency of analyte extraction from complex matrices. Methods typically involve comparing the response of analyte added to and extracted from a biological matrix to the response of pure analyte at equivalent concentrations. Recovery studies should be performed at multiple concentration levels across the analytical range to ensure consistency. Statistical analysis of recovery data helps establish acceptable recovery ranges and correction factors if needed.
- Matrix effect evaluation in analyte recovery: Matrix effects can significantly impact analyte recovery in HPLC methods, requiring specific validation approaches. These effects occur when components in the sample matrix interfere with analyte detection, leading to ion suppression or enhancement. Validation protocols should include matrix-matched calibration standards and post-extraction spike experiments to quantify matrix effects. Techniques such as standard addition, internal standardization, and matrix dilution can be employed to minimize matrix interference and improve recovery consistency across different sample types.
- Optimization of sample preparation for improved recovery: Sample preparation techniques significantly influence analyte recovery in HPLC validation. Methods such as liquid-liquid extraction, solid-phase extraction, protein precipitation, and filtration can be optimized to maximize recovery while minimizing matrix interference. Validation protocols should evaluate parameters including extraction solvent composition, pH, temperature, and extraction time. Multiple extraction cycles may be necessary for compounds with challenging recovery profiles. The stability of analytes during sample preparation must also be assessed to ensure accurate recovery determination.
- Statistical approaches for recovery data analysis: Statistical methods are essential for evaluating analyte recovery data in HPLC validation. These include calculation of mean recovery percentages, relative standard deviation, confidence intervals, and uncertainty measurements. Acceptance criteria typically require recovery percentages within 85-115% with RSD values below 15%. Statistical tools such as ANOVA can be used to assess recovery consistency across concentration levels and matrices. Outlier tests may be applied to identify and address anomalous recovery results. Statistical evaluation should consider the intended application of the method when establishing acceptance criteria.
- Automated systems for recovery validation: Automated systems and technologies enhance the efficiency and reliability of recovery validation in HPLC methods. These include automated sample preparation platforms, robotic liquid handling systems, and integrated HPLC-MS/MS workflows. Automation reduces human error, improves reproducibility, and enables higher throughput in recovery studies. Specialized software solutions facilitate data processing, statistical analysis, and reporting of recovery results. Validation of automated systems themselves is necessary to ensure they provide accurate and consistent recovery assessments across different analytes and matrices.
02 Matrix effect evaluation in analyte recovery
Matrix effects can significantly impact analyte recovery in HPLC validation. These effects occur when components in the sample matrix interfere with the detection or extraction of the target analyte. Evaluation methods include post-extraction spike analysis, matrix-matched calibration curves, and standard addition techniques. Proper assessment of matrix effects is crucial for ensuring accurate quantification and reliable analytical results, particularly in complex biological samples or formulations with multiple ingredients.Expand Specific Solutions03 Optimization of extraction procedures for improved recovery
Optimization of extraction procedures is critical for achieving high analyte recovery rates in HPLC validation. This involves systematic evaluation of extraction solvents, pH conditions, temperature, and extraction time. Techniques such as liquid-liquid extraction, solid-phase extraction, and protein precipitation are commonly employed depending on the sample type and analyte properties. The optimization process typically follows a design of experiments approach to identify the most efficient extraction parameters that maximize recovery while minimizing interference.Expand Specific Solutions04 Internal standard techniques for recovery correction
Internal standard techniques are widely used to correct for variations in analyte recovery during HPLC validation. This approach involves adding a known amount of a compound (internal standard) with similar chemical properties to the analyte but distinguishable by the detection system. The internal standard undergoes the same extraction and analysis processes as the target analyte, allowing for compensation of recovery losses. Isotopically labeled analogs are particularly effective as internal standards due to their nearly identical chemical behavior to the target compounds.Expand Specific Solutions05 Statistical approaches for recovery data analysis
Statistical approaches are essential for properly analyzing recovery data in HPLC validation. These include calculation of mean recovery percentages, standard deviation, relative standard deviation, and confidence intervals. Statistical tests are applied to evaluate the significance of recovery variations across concentration levels and to detect outliers. Acceptance criteria typically require that recovery be consistent, precise, and reproducible across the analytical range, with appropriate statistical limits established based on the intended application of the method.Expand Specific Solutions
Key Industry Players in HPLC Technology
The HPLC analyte recovery quantification market is currently in a growth phase, characterized by increasing demand for precise validation methods across pharmaceutical and diagnostic sectors. The global HPLC market, valued at approximately $4.5 billion, is expected to expand at 5-6% CAGR through 2027. Leading pharmaceutical companies like F. Hoffmann-La Roche, Bristol Myers Squibb, and Novartis are driving technological advancements in this field, while specialized analytical instrument manufacturers including Waters Technology, Bio-Rad Laboratories, and Beckman Coulter provide cutting-edge solutions. The technology has reached moderate maturity with established validation protocols, but continuous innovation is occurring in automated systems, improved detection sensitivity, and integration with data analytics platforms. Research institutions such as University of Zurich and University of Chicago contribute significantly to method development and standardization efforts.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed a comprehensive approach to analyte recovery quantification in HPLC validation specifically tailored for pharmaceutical applications. Their methodology employs a risk-based strategy that focuses validation efforts on critical quality attributes of the analytical method. Roche's approach incorporates a systematic assessment of recovery across the entire analytical process, from sample preparation through chromatographic separation and detection. Their protocol includes evaluation of recovery at multiple concentration levels spanning the method's range, with particular emphasis on the lower limit of quantification where recovery issues are most pronounced. Roche has pioneered the use of Design of Experiments (DoE) approaches to identify critical factors affecting recovery and establish robust operating ranges for method parameters. Their validation strategy includes assessment of recovery consistency across different sample matrices relevant to the drug development process, from raw materials to finished products. The company's integrated data management system provides comprehensive documentation of recovery studies with statistical analysis to support regulatory submissions.
Strengths: Regulatory-focused approach with extensive documentation suitable for submission to health authorities; sophisticated statistical analysis of recovery data. Weaknesses: Highly specialized for pharmaceutical applications; resource-intensive implementation requiring significant analytical expertise.
Bio-Rad Laboratories, Inc.
Technical Solution: Bio-Rad Laboratories has pioneered a matrix-matched recovery assessment protocol for HPLC validation that addresses the challenges of complex biological samples. Their approach utilizes a dual-pathway validation strategy where analyte recovery is determined through parallel processing of spiked and unspiked samples across multiple concentration ranges. The company's proprietary ChromAccuracy™ technology incorporates automated internal standard addition with precise volumetric control to minimize procedural errors during sample preparation. Bio-Rad's validation methodology emphasizes statistical rigor through minimum sextuplicates at each concentration level, with outlier identification algorithms to enhance data reliability. Their protocol includes stability assessment of analytes during the extraction process, accounting for potential degradation that could affect recovery calculations. The company has developed specialized reference materials with certified recovery characteristics for method validation across pharmaceutical, clinical, and food safety applications, ensuring traceability to recognized standards.
Strengths: Exceptional performance with complex biological matrices; comprehensive statistical approach to validation with robust outlier detection. Weaknesses: More time-consuming than simplified approaches; higher reagent consumption due to multiple replicate requirements.
Critical Innovations in Recovery Quantification Techniques
Method of identification and quantitative analysis of lacquers from different regions using high performance liquid chromatography
PatentActiveKR1020220022238A
Innovation
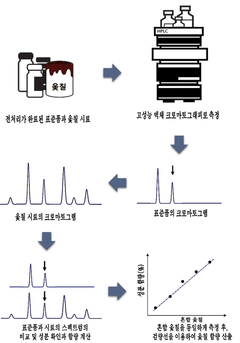
- A method using High Performance Liquid Chromatography (HPLC) to select a specific indicator (standard product) for each lacquer origin, measuring the main peak retention time, and generating calibration curves to determine the content of lacquer samples, enabling quick and accurate identification and quantification of lacquer origins.
Systems, materials, and methods for reversed-phase high performance liquid chromatography (RP-HPLC) for monitoring formation of multi-specific molecules
PatentWO2021209953A1
Innovation
- The implementation of reversed-phase high-performance liquid chromatography (RP-HPLC) using a polar aqueous mobile phase with an ion-pairing agent and an organic non-polar mobile phase to elute and monitor the formation of multi-specific molecules, allowing for gradient elution and detection of multi-specific antibody formation.
Regulatory Compliance for Analytical Method Validation
Regulatory compliance forms the cornerstone of analytical method validation in pharmaceutical and biotechnology industries, particularly for HPLC-based analyte recovery quantification. The validation process must adhere to guidelines established by major regulatory bodies including the FDA (Food and Drug Administration), EMA (European Medicines Agency), and ICH (International Council for Harmonisation). These organizations have developed comprehensive frameworks such as ICH Q2(R1) "Validation of Analytical Procedures: Text and Methodology," which specifically addresses recovery assessment requirements.
For HPLC analyte recovery validation, compliance necessitates adherence to 21 CFR Part 11 for electronic records and signatures when using computerized systems for data acquisition and processing. Additionally, USP <1225> Validation of Compendial Procedures and USP <621> Chromatography provide specific parameters that must be evaluated during method validation, including recovery studies across the analytical range.
The regulatory landscape requires documented evidence that recovery methods consistently meet predefined acceptance criteria. This documentation must include standard operating procedures (SOPs), validation protocols, and comprehensive reports detailing experimental design, statistical analysis, and conclusions. Regulatory bodies typically expect recovery values between 80-120% with relative standard deviation (RSD) not exceeding 15%, though these parameters may vary depending on analyte concentration and matrix complexity.
Quality by Design (QbD) principles have been increasingly incorporated into regulatory expectations, requiring systematic evaluation of method variables that might impact recovery. This approach necessitates risk assessment documentation and establishment of a method operable design region (MODR) where consistent recovery can be achieved despite minor variations in chromatographic conditions.
Regulatory inspections frequently focus on data integrity aspects of recovery validation. Companies must implement appropriate controls to prevent data manipulation, maintain audit trails, and ensure traceability of all recovery determinations. The ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available) must be applied to all recovery data.
For global pharmaceutical companies, harmonizing recovery validation approaches across different regulatory jurisdictions presents significant challenges. Strategic planning is essential to develop validation protocols that simultaneously satisfy FDA, EMA, PMDA (Japan), and other regional requirements, avoiding redundant testing while maintaining compliance with each authority's specific expectations for HPLC recovery validation.
For HPLC analyte recovery validation, compliance necessitates adherence to 21 CFR Part 11 for electronic records and signatures when using computerized systems for data acquisition and processing. Additionally, USP <1225> Validation of Compendial Procedures and USP <621> Chromatography provide specific parameters that must be evaluated during method validation, including recovery studies across the analytical range.
The regulatory landscape requires documented evidence that recovery methods consistently meet predefined acceptance criteria. This documentation must include standard operating procedures (SOPs), validation protocols, and comprehensive reports detailing experimental design, statistical analysis, and conclusions. Regulatory bodies typically expect recovery values between 80-120% with relative standard deviation (RSD) not exceeding 15%, though these parameters may vary depending on analyte concentration and matrix complexity.
Quality by Design (QbD) principles have been increasingly incorporated into regulatory expectations, requiring systematic evaluation of method variables that might impact recovery. This approach necessitates risk assessment documentation and establishment of a method operable design region (MODR) where consistent recovery can be achieved despite minor variations in chromatographic conditions.
Regulatory inspections frequently focus on data integrity aspects of recovery validation. Companies must implement appropriate controls to prevent data manipulation, maintain audit trails, and ensure traceability of all recovery determinations. The ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available) must be applied to all recovery data.
For global pharmaceutical companies, harmonizing recovery validation approaches across different regulatory jurisdictions presents significant challenges. Strategic planning is essential to develop validation protocols that simultaneously satisfy FDA, EMA, PMDA (Japan), and other regional requirements, avoiding redundant testing while maintaining compliance with each authority's specific expectations for HPLC recovery validation.
Quality Control Standards for HPLC Recovery Methods
Quality control standards for HPLC recovery methods have evolved significantly over the past decade, establishing rigorous frameworks to ensure analytical reliability. These standards typically encompass acceptance criteria for recovery rates, which generally fall between 80-120% for most pharmaceutical applications, though may vary depending on analyte concentration and regulatory context.
The ICH (International Council for Harmonisation) guidelines, particularly Q2(R1), provide foundational requirements for validation parameters including recovery studies, emphasizing the need for consistent methodology across different concentration levels. These standards mandate that recovery experiments be conducted at a minimum of three concentration levels covering the expected range.
Statistical considerations form a critical component of quality control standards, with requirements for replicate analyses (typically n≥3) to establish confidence intervals and relative standard deviations (RSDs). Most regulatory bodies specify that RSDs should not exceed 15% for high concentration samples and 20% for samples near the limit of quantification.
System suitability tests (SSTs) have become integral to recovery method validation, requiring periodic verification of chromatographic parameters such as resolution, tailing factor, and theoretical plate count. These parameters must meet predefined criteria before recovery studies can be considered valid, ensuring the analytical system is performing optimally.
Matrix-specific standards have emerged as recovery rates can be significantly influenced by sample matrices. Regulatory guidelines now often require matrix-matched calibration and recovery studies that account for potential interferences and matrix effects. This approach has become particularly important in complex biological samples and environmental analyses.
Documentation requirements have become increasingly stringent, with standards mandating detailed records of recovery calculation methodologies, raw data preservation, and traceability of standards used. Modern quality control frameworks emphasize the implementation of electronic data integrity measures that comply with 21 CFR Part 11 or equivalent regulations.
Proficiency testing and interlaboratory comparisons represent the newest evolution in quality control standards, with many regulatory bodies now recommending participation in collaborative studies to verify method transferability and establish reproducibility across different laboratory environments. These collaborative approaches help establish robust recovery methods that can be reliably implemented across multiple testing facilities.
The ICH (International Council for Harmonisation) guidelines, particularly Q2(R1), provide foundational requirements for validation parameters including recovery studies, emphasizing the need for consistent methodology across different concentration levels. These standards mandate that recovery experiments be conducted at a minimum of three concentration levels covering the expected range.
Statistical considerations form a critical component of quality control standards, with requirements for replicate analyses (typically n≥3) to establish confidence intervals and relative standard deviations (RSDs). Most regulatory bodies specify that RSDs should not exceed 15% for high concentration samples and 20% for samples near the limit of quantification.
System suitability tests (SSTs) have become integral to recovery method validation, requiring periodic verification of chromatographic parameters such as resolution, tailing factor, and theoretical plate count. These parameters must meet predefined criteria before recovery studies can be considered valid, ensuring the analytical system is performing optimally.
Matrix-specific standards have emerged as recovery rates can be significantly influenced by sample matrices. Regulatory guidelines now often require matrix-matched calibration and recovery studies that account for potential interferences and matrix effects. This approach has become particularly important in complex biological samples and environmental analyses.
Documentation requirements have become increasingly stringent, with standards mandating detailed records of recovery calculation methodologies, raw data preservation, and traceability of standards used. Modern quality control frameworks emphasize the implementation of electronic data integrity measures that comply with 21 CFR Part 11 or equivalent regulations.
Proficiency testing and interlaboratory comparisons represent the newest evolution in quality control standards, with many regulatory bodies now recommending participation in collaborative studies to verify method transferability and establish reproducibility across different laboratory environments. These collaborative approaches help establish robust recovery methods that can be reliably implemented across multiple testing facilities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!