How to Assess HPLC Flow Stability for Consistent Delivery
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC Flow Stability Background and Objectives
High-Performance Liquid Chromatography (HPLC) has evolved significantly since its inception in the 1960s, becoming an indispensable analytical technique in pharmaceutical, biotechnology, environmental, and food industries. The development trajectory of HPLC technology has consistently focused on improving separation efficiency, detection sensitivity, and operational reliability. Flow stability, in particular, has emerged as a critical parameter that directly impacts analytical precision and reproducibility.
Flow stability in HPLC systems refers to the consistent delivery of mobile phase at a specified flow rate without significant fluctuations or pulsations. Historically, early HPLC systems struggled with flow inconsistencies due to mechanical limitations in pump designs. The evolution from constant pressure pumps to reciprocating piston pumps marked a significant advancement, though challenges in maintaining pulse-free delivery persisted.
Recent technological innovations have introduced sophisticated electronic flow controllers, multi-head pump configurations, and advanced dampening systems to minimize flow variations. Despite these advancements, achieving and maintaining optimal flow stability remains a complex challenge influenced by multiple variables including system design, mobile phase characteristics, backpressure fluctuations, and environmental factors.
The primary objective of assessing HPLC flow stability is to establish reliable methodologies for monitoring, measuring, and optimizing flow consistency across various operational conditions. This encompasses developing standardized protocols for evaluating flow stability metrics, identifying critical factors affecting flow variations, and implementing effective strategies to mitigate these variations.
Secondary objectives include quantifying the relationship between flow stability and analytical performance parameters such as retention time reproducibility, peak area precision, and detection sensitivity. Understanding these correlations is essential for establishing acceptable flow variation thresholds for different analytical applications and regulatory compliance requirements.
The technological trend in this domain is moving toward real-time flow monitoring systems with integrated feedback mechanisms that can automatically adjust pump parameters to maintain consistent flow rates. Additionally, there is growing interest in developing predictive algorithms that can anticipate flow disturbances based on system conditions and implement preemptive corrective actions.
Achieving these objectives would significantly enhance the reliability of HPLC analyses across various applications, particularly in regulated environments where analytical precision directly impacts product quality and safety decisions. Furthermore, improved flow stability assessment methodologies would contribute to reduced method transfer challenges between different HPLC systems and laboratories, facilitating standardization across the analytical chemistry field.
Flow stability in HPLC systems refers to the consistent delivery of mobile phase at a specified flow rate without significant fluctuations or pulsations. Historically, early HPLC systems struggled with flow inconsistencies due to mechanical limitations in pump designs. The evolution from constant pressure pumps to reciprocating piston pumps marked a significant advancement, though challenges in maintaining pulse-free delivery persisted.
Recent technological innovations have introduced sophisticated electronic flow controllers, multi-head pump configurations, and advanced dampening systems to minimize flow variations. Despite these advancements, achieving and maintaining optimal flow stability remains a complex challenge influenced by multiple variables including system design, mobile phase characteristics, backpressure fluctuations, and environmental factors.
The primary objective of assessing HPLC flow stability is to establish reliable methodologies for monitoring, measuring, and optimizing flow consistency across various operational conditions. This encompasses developing standardized protocols for evaluating flow stability metrics, identifying critical factors affecting flow variations, and implementing effective strategies to mitigate these variations.
Secondary objectives include quantifying the relationship between flow stability and analytical performance parameters such as retention time reproducibility, peak area precision, and detection sensitivity. Understanding these correlations is essential for establishing acceptable flow variation thresholds for different analytical applications and regulatory compliance requirements.
The technological trend in this domain is moving toward real-time flow monitoring systems with integrated feedback mechanisms that can automatically adjust pump parameters to maintain consistent flow rates. Additionally, there is growing interest in developing predictive algorithms that can anticipate flow disturbances based on system conditions and implement preemptive corrective actions.
Achieving these objectives would significantly enhance the reliability of HPLC analyses across various applications, particularly in regulated environments where analytical precision directly impacts product quality and safety decisions. Furthermore, improved flow stability assessment methodologies would contribute to reduced method transfer challenges between different HPLC systems and laboratories, facilitating standardization across the analytical chemistry field.
Market Demand for Precise Chromatographic Analysis
The global market for High-Performance Liquid Chromatography (HPLC) continues to expand significantly, driven by increasing demands for precise analytical techniques across multiple industries. The current HPLC market is valued at approximately 4.5 billion USD, with projections indicating growth to reach 6.7 billion USD by 2027, representing a compound annual growth rate of 8.2%. This growth directly correlates with the rising need for consistent and reliable chromatographic analysis.
Pharmaceutical and biotechnology sectors remain the primary drivers of this market expansion, collectively accounting for over 60% of HPLC applications. These industries require exceptionally accurate flow stability for drug development, quality control, and regulatory compliance. Even minor flow variations can lead to significant analytical errors, potentially resulting in batch rejections or regulatory non-compliance with associated costs often exceeding millions of dollars per incident.
Clinical diagnostics represents another rapidly growing segment, with an estimated 15% market share and increasing adoption rates. Here, precise chromatographic analysis enables accurate disease biomarker detection and therapeutic drug monitoring, where flow instability could lead to misdiagnosis or improper treatment protocols.
Environmental monitoring agencies and food safety laboratories collectively constitute approximately 18% of the market. These sectors face increasing regulatory pressures requiring lower detection limits and higher reproducibility standards, making flow stability a critical parameter for compliance and public safety assurance.
Academic and research institutions, while representing a smaller market segment at around 7%, drive innovation in chromatographic techniques and establish protocols that eventually become industry standards. Their demand for precise instrumentation continues to influence technological development across the entire HPLC ecosystem.
Market research indicates that end-users are increasingly prioritizing flow stability features when making purchasing decisions, with 78% of survey respondents ranking flow precision among their top three selection criteria. This represents a significant shift from earlier market research (2015) when only 52% considered this feature critical.
The geographical distribution of demand shows North America leading with 38% market share, followed by Europe (29%), Asia-Pacific (24%, with the fastest growth rate), and rest of the world (9%). Notably, emerging economies are showing accelerated adoption rates as their pharmaceutical and biotechnology sectors expand and regulatory frameworks mature.
Industry analysts project that technologies enabling superior flow stability assessment and control will command premium pricing, with customers demonstrating willingness to invest 15-20% more for systems offering documented improvements in flow precision and stability monitoring capabilities.
Pharmaceutical and biotechnology sectors remain the primary drivers of this market expansion, collectively accounting for over 60% of HPLC applications. These industries require exceptionally accurate flow stability for drug development, quality control, and regulatory compliance. Even minor flow variations can lead to significant analytical errors, potentially resulting in batch rejections or regulatory non-compliance with associated costs often exceeding millions of dollars per incident.
Clinical diagnostics represents another rapidly growing segment, with an estimated 15% market share and increasing adoption rates. Here, precise chromatographic analysis enables accurate disease biomarker detection and therapeutic drug monitoring, where flow instability could lead to misdiagnosis or improper treatment protocols.
Environmental monitoring agencies and food safety laboratories collectively constitute approximately 18% of the market. These sectors face increasing regulatory pressures requiring lower detection limits and higher reproducibility standards, making flow stability a critical parameter for compliance and public safety assurance.
Academic and research institutions, while representing a smaller market segment at around 7%, drive innovation in chromatographic techniques and establish protocols that eventually become industry standards. Their demand for precise instrumentation continues to influence technological development across the entire HPLC ecosystem.
Market research indicates that end-users are increasingly prioritizing flow stability features when making purchasing decisions, with 78% of survey respondents ranking flow precision among their top three selection criteria. This represents a significant shift from earlier market research (2015) when only 52% considered this feature critical.
The geographical distribution of demand shows North America leading with 38% market share, followed by Europe (29%), Asia-Pacific (24%, with the fastest growth rate), and rest of the world (9%). Notably, emerging economies are showing accelerated adoption rates as their pharmaceutical and biotechnology sectors expand and regulatory frameworks mature.
Industry analysts project that technologies enabling superior flow stability assessment and control will command premium pricing, with customers demonstrating willingness to invest 15-20% more for systems offering documented improvements in flow precision and stability monitoring capabilities.
Current Challenges in HPLC Flow Rate Consistency
Despite significant advancements in HPLC technology, maintaining consistent flow rate stability remains a persistent challenge in analytical laboratories. Modern HPLC systems face several technical obstacles that impact flow precision and ultimately affect chromatographic reproducibility. These challenges stem from both mechanical limitations and external factors that collectively compromise the reliability of analytical results.
Pump design limitations represent a fundamental challenge, with reciprocating pistons creating inherent pulsations that disrupt smooth flow. Even in high-end systems equipped with pulse dampeners, residual fluctuations can persist, particularly at low flow rates where minor variations have proportionally greater impact. These pulsations become especially problematic when working with highly sensitive detectors or when analyzing trace components.
Backpressure fluctuations constitute another significant obstacle to flow stability. As columns age or become partially blocked with particulates, pressure variations develop across the system. These variations can trigger compensatory responses from the pump, leading to inconsistent flow delivery. Similarly, gradient formation introduces additional complexity, as mixing different solvents with varying viscosities can create pressure differentials that the system must continuously adjust for.
Temperature effects further complicate flow stability maintenance. Ambient temperature fluctuations affect solvent viscosity and pump performance, while thermal expansion of components can alter internal volumes and flow paths. These temperature-related variations are particularly challenging in laboratories without precise environmental controls or during extended analytical runs where conditions may change throughout the day.
Mobile phase degassing issues represent another technical hurdle. Dissolved gases can form bubbles under pressure changes, creating flow irregularities and detector noise. While modern systems incorporate degassing units, their efficiency varies based on solvent composition and operational conditions. Incomplete degassing remains a common source of flow instability, especially when working with volatile organic solvents.
System compliance and elasticity effects also contribute to flow inconsistencies. Pressure fluctuations cause minute expansions in system components like tubing, fittings, and seals. These elastic deformations absorb and release energy, creating secondary flow variations that are difficult to predict or control. The cumulative effect becomes more pronounced in systems with higher operating pressures or those utilizing components near their pressure limits.
Electronic control limitations further exacerbate flow stability challenges. While modern HPLC systems employ sophisticated feedback mechanisms, sensor resolution, processing delays, and control algorithm limitations can still result in suboptimal flow corrections. These electronic constraints become particularly evident during rapid gradient changes or when precise micro-flow control is required for specialized applications.
Pump design limitations represent a fundamental challenge, with reciprocating pistons creating inherent pulsations that disrupt smooth flow. Even in high-end systems equipped with pulse dampeners, residual fluctuations can persist, particularly at low flow rates where minor variations have proportionally greater impact. These pulsations become especially problematic when working with highly sensitive detectors or when analyzing trace components.
Backpressure fluctuations constitute another significant obstacle to flow stability. As columns age or become partially blocked with particulates, pressure variations develop across the system. These variations can trigger compensatory responses from the pump, leading to inconsistent flow delivery. Similarly, gradient formation introduces additional complexity, as mixing different solvents with varying viscosities can create pressure differentials that the system must continuously adjust for.
Temperature effects further complicate flow stability maintenance. Ambient temperature fluctuations affect solvent viscosity and pump performance, while thermal expansion of components can alter internal volumes and flow paths. These temperature-related variations are particularly challenging in laboratories without precise environmental controls or during extended analytical runs where conditions may change throughout the day.
Mobile phase degassing issues represent another technical hurdle. Dissolved gases can form bubbles under pressure changes, creating flow irregularities and detector noise. While modern systems incorporate degassing units, their efficiency varies based on solvent composition and operational conditions. Incomplete degassing remains a common source of flow instability, especially when working with volatile organic solvents.
System compliance and elasticity effects also contribute to flow inconsistencies. Pressure fluctuations cause minute expansions in system components like tubing, fittings, and seals. These elastic deformations absorb and release energy, creating secondary flow variations that are difficult to predict or control. The cumulative effect becomes more pronounced in systems with higher operating pressures or those utilizing components near their pressure limits.
Electronic control limitations further exacerbate flow stability challenges. While modern HPLC systems employ sophisticated feedback mechanisms, sensor resolution, processing delays, and control algorithm limitations can still result in suboptimal flow corrections. These electronic constraints become particularly evident during rapid gradient changes or when precise micro-flow control is required for specialized applications.
Modern Flow Stability Assessment Methodologies
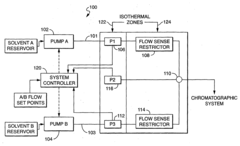
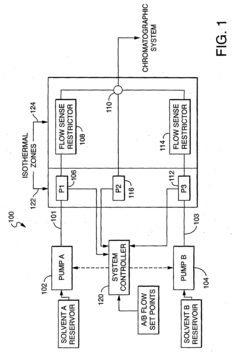
01 Flow control mechanisms for HPLC stability
Various flow control mechanisms are employed to maintain stable flow rates in HPLC systems. These include precision pumps, flow regulators, and feedback control systems that continuously monitor and adjust flow parameters. Advanced flow control technologies help minimize pulsation, ensure consistent mobile phase delivery, and maintain pressure stability throughout the chromatographic process, resulting in improved separation efficiency and reproducibility of analytical results.- Flow control mechanisms for HPLC stability: Various flow control mechanisms are employed to maintain stable flow rates in HPLC systems. These include precision pumps, flow regulators, and feedback control systems that continuously monitor and adjust flow parameters. Advanced flow control technologies help minimize pulsation and ensure consistent chromatographic performance by maintaining pressure stability throughout the analysis, which is critical for reproducible retention times and peak shapes.
- Temperature control systems for flow stability: Temperature fluctuations can significantly impact HPLC flow stability by affecting mobile phase viscosity and column backpressure. Specialized temperature control systems maintain consistent thermal conditions for both the mobile phase and column environment. These systems include column ovens, mobile phase pre-heaters, and thermal insulation technologies that minimize the effects of ambient temperature variations, resulting in more stable flow rates and improved chromatographic reproducibility.
- Mobile phase degassing techniques: Dissolved gases in the mobile phase can cause flow instability through bubble formation and cavitation in the pump heads. Various degassing techniques are implemented to remove these gases, including vacuum degassing, helium sparging, and ultrasonic degassing systems. Effective degassing prevents flow interruptions, ensures consistent delivery of mobile phase, and maintains stable baseline signals, all of which contribute to improved chromatographic performance and system reliability.
- Advanced pump designs for flow precision: Innovative pump designs specifically address flow stability challenges in HPLC systems. These include dual-piston reciprocating pumps with precise cam mechanisms, quaternary gradient systems with electronic flow control, and microfluidic pump technologies. Advanced pump designs incorporate features such as active damping, electronic compressibility compensation, and real-time flow correction algorithms to deliver pulse-free flow even under changing backpressure conditions.
- Flow monitoring and feedback systems: Real-time flow monitoring and feedback systems continuously measure actual flow rates and compare them to setpoints. These systems employ various sensors including optical flow meters, pressure transducers, and thermal mass flow sensors to detect deviations. When variations are detected, automated correction mechanisms adjust pump parameters to maintain stable flow. Some advanced systems incorporate machine learning algorithms to predict and preemptively correct flow instabilities before they affect chromatographic separation.
02 Temperature control systems for flow stability
Temperature fluctuations can significantly impact HPLC flow stability by affecting mobile phase viscosity and column backpressure. Specialized temperature control systems maintain consistent thermal conditions throughout the chromatographic system, including column compartments, mobile phase reservoirs, and flow paths. These systems employ precise heating/cooling elements, thermal insulation, and temperature sensors to ensure stable flow characteristics and reproducible retention times during analysis.Expand Specific Solutions03 Mobile phase composition and degassing techniques
The composition and preparation of mobile phases significantly affect HPLC flow stability. Proper degassing techniques remove dissolved gases that can form bubbles and disrupt flow consistency. Methods include helium sparging, vacuum degassing, and online degassing modules. Additionally, careful selection of solvent mixtures with compatible physicochemical properties and appropriate buffer systems helps maintain homogeneous flow characteristics and prevents precipitation or phase separation that could obstruct flow paths.Expand Specific Solutions04 Pressure regulation and pulsation dampening
Effective pressure regulation and pulsation dampening are crucial for maintaining HPLC flow stability. Systems incorporate pressure sensors, dampeners, and regulators to minimize flow fluctuations caused by pump mechanics. Advanced dampening technologies absorb pressure waves and smooth flow delivery, while pressure monitoring systems provide real-time feedback for flow adjustments. These components work together to ensure consistent mobile phase delivery and stable baseline performance during chromatographic separations.Expand Specific Solutions05 System integration and automation for flow stability
Integrated HPLC systems with automated flow control features enhance overall flow stability. These systems incorporate intelligent software algorithms that continuously monitor multiple parameters including pressure, temperature, and flow rate, making real-time adjustments to maintain optimal conditions. Automated calibration routines, leak detection systems, and predictive maintenance features help prevent flow disruptions. The integration of multiple stability-enhancing components into cohesive systems results in more reliable chromatographic performance and reproducible analytical results.Expand Specific Solutions
Leading Manufacturers in HPLC Instrumentation
The HPLC flow stability assessment market is currently in a mature growth phase, characterized by established technologies and ongoing innovations. The global market size for HPLC systems is estimated at $4-5 billion, with flow stability components representing a significant segment due to their critical role in analytical precision. Leading players include Agilent Technologies, Waters Technology, and Shimadzu Corp., who dominate with comprehensive solutions integrating advanced flow control technologies. Mid-tier competitors like Dionex Softron (Thermo Fisher) and Flux Instruments offer specialized solutions with unique microflow capabilities. The technology landscape shows high maturity with innovations focusing on miniaturization, precision enhancement, and integration with digital systems, as evidenced by recent developments from Agilent and Waters featuring sub-microliter flow accuracy and real-time monitoring capabilities.
Agilent Technologies, Inc.
Technical Solution: Agilent's approach to HPLC flow stability centers on their Intelligent System Emulation Technology (ISET), which digitally models mechanical flow behavior to compensate for physical limitations. Their 1290 Infinity II LC system incorporates advanced hydraulic design with Jet Weaver II mixers and active damping technology that minimizes pressure ripples. The system utilizes microprocessor-controlled variable-speed pistons with electronic compressibility compensation that continuously adjusts for solvent compressibility changes. Agilent's proprietary algorithms predict and correct flow deviations before they occur, maintaining flow precision to within ±0.07% RSD. Their systems also feature real-time flow sensors that provide continuous feedback to the pump control mechanisms, enabling immediate correction of any deviations from the set parameters.
Strengths: Industry-leading flow precision (±0.07% RSD), predictive correction algorithms, and comprehensive system diagnostics that identify potential issues before they affect chromatography. Weaknesses: Higher initial investment cost compared to basic systems, and proprietary technology creates potential vendor lock-in for consumables and service.
Waters Technology Corp.
Technical Solution: Waters addresses HPLC flow stability through their patented FlexFlow technology in their ACQUITY UPLC systems. This technology utilizes quaternary solvent management with independent variable-stroke, serial-connected flow paths that minimize pressure fluctuations. Their systems incorporate advanced electronic pressure compensation (EPC) that dynamically adjusts to changing backpressure conditions. Waters' approach includes precision-engineered ceramic pump heads with near-zero compliance characteristics and temperature-controlled flow paths that minimize thermal expansion effects on flow rates. Their systems feature real-time flow monitoring with closed-loop feedback control that can detect and correct deviations as small as 0.1 μL/min. Additionally, Waters implements predictive maintenance algorithms that monitor pump seal wear patterns to prevent flow instability before it occurs.
Strengths: Superior performance at ultra-high pressures (up to 15,000 psi), exceptional flow precision for nano/micro flow applications, and advanced diagnostics that predict maintenance needs. Weaknesses: Complex system architecture requires specialized training, and premium pricing positions these systems at the higher end of the market.
Critical Patents in Flow Rate Control Systems
Closed Loop Flow Control Of A HPLC Constant Flow Pump To Enable Low-Flow Operation
PatentInactiveUS20100116745A1
Innovation
- Modifying commercially available analytical-scale HPLC pumps with hardware and firmware changes, such as increased micro-stepping resolution in stepper motor drive mechanisms, and using delta-P type flow meters with a fluidic cross pressure transducer to enable precise flow control at nano-scale rates without complex calibration routines.
Liquid chromatograph and analyzing system
PatentWO2002101381A1
Innovation
- A liquid chromatograph configuration with a branched eluent flow path and eluent storage means allows for sequential selection and delivery of eluents with different compositions, using a high-pressure liquid-feeding system to maintain a constant low flow rate, and includes a valve system for easy replenishment and selection of eluents, ensuring stable and accurate gradient elution.
Validation Standards and Compliance Requirements
Validation of HPLC flow stability requires adherence to stringent regulatory frameworks established by international organizations. The United States Pharmacopeia (USP) and European Pharmacopoeia (EP) provide comprehensive guidelines for HPLC system validation, specifically addressing flow rate accuracy and precision requirements. According to USP <621>, flow rate precision must demonstrate a relative standard deviation (RSD) of less than 2.0% across multiple measurements, while EP 2.2.46 mandates flow accuracy within ±1.0% of the set value for analytical applications.
The International Conference on Harmonization (ICH) guidelines, particularly ICH Q2(R1), outline validation parameters relevant to flow stability assessment, including specificity, linearity, accuracy, precision, detection limit, quantitation limit, range, and robustness. For HPLC flow validation specifically, precision and robustness are critical parameters that must be thoroughly documented to demonstrate system suitability.
FDA 21 CFR Part 11 compliance requirements apply to electronic records generated during flow stability testing, necessitating secure data handling, audit trails, and electronic signatures. Similarly, EU GMP Annex 11 provides parallel guidance for European markets, emphasizing data integrity throughout the validation process.
ISO 17025 accreditation standards for testing laboratories establish additional requirements for method validation and equipment qualification that directly impact flow stability assessment protocols. These standards mandate regular calibration, performance verification, and documentation of measurement uncertainty for all critical parameters, including flow rate.
The ASTM E2500 standard on specification, design, and verification of pharmaceutical manufacturing systems provides a risk-based approach to validation that can be applied to HPLC flow stability assessment. This approach emphasizes critical quality attributes and process parameters that must be controlled to ensure consistent product quality.
Implementation of these standards requires a structured validation master plan (VMP) that includes installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) protocols specifically addressing flow stability. The OQ protocol must include tests for flow accuracy, precision, and linearity across the operational range, while PQ should demonstrate consistent performance under actual operating conditions.
Compliance with these validation standards necessitates comprehensive documentation, including standard operating procedures (SOPs) for flow stability testing, calibration records, training documentation, and change control procedures. Regular system suitability testing (SST) must be performed to verify ongoing compliance with established acceptance criteria for flow stability parameters.
The International Conference on Harmonization (ICH) guidelines, particularly ICH Q2(R1), outline validation parameters relevant to flow stability assessment, including specificity, linearity, accuracy, precision, detection limit, quantitation limit, range, and robustness. For HPLC flow validation specifically, precision and robustness are critical parameters that must be thoroughly documented to demonstrate system suitability.
FDA 21 CFR Part 11 compliance requirements apply to electronic records generated during flow stability testing, necessitating secure data handling, audit trails, and electronic signatures. Similarly, EU GMP Annex 11 provides parallel guidance for European markets, emphasizing data integrity throughout the validation process.
ISO 17025 accreditation standards for testing laboratories establish additional requirements for method validation and equipment qualification that directly impact flow stability assessment protocols. These standards mandate regular calibration, performance verification, and documentation of measurement uncertainty for all critical parameters, including flow rate.
The ASTM E2500 standard on specification, design, and verification of pharmaceutical manufacturing systems provides a risk-based approach to validation that can be applied to HPLC flow stability assessment. This approach emphasizes critical quality attributes and process parameters that must be controlled to ensure consistent product quality.
Implementation of these standards requires a structured validation master plan (VMP) that includes installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) protocols specifically addressing flow stability. The OQ protocol must include tests for flow accuracy, precision, and linearity across the operational range, while PQ should demonstrate consistent performance under actual operating conditions.
Compliance with these validation standards necessitates comprehensive documentation, including standard operating procedures (SOPs) for flow stability testing, calibration records, training documentation, and change control procedures. Regular system suitability testing (SST) must be performed to verify ongoing compliance with established acceptance criteria for flow stability parameters.
Environmental Factors Affecting Flow Stability
Environmental conditions play a crucial role in determining the stability and consistency of HPLC flow delivery systems. Temperature fluctuations represent one of the most significant environmental factors affecting flow stability. When ambient temperature changes occur in the laboratory environment, they can directly impact the viscosity of mobile phases, causing variations in flow resistance and ultimately leading to inconsistent flow rates. Research indicates that even minor temperature variations of 2-3°C can result in measurable flow rate deviations, particularly in high-precision applications.
Barometric pressure changes, though often overlooked, can significantly influence HPLC system performance. These variations affect the behavior of pumps, particularly in systems utilizing vacuum degassers or operating near their pressure limits. Studies have demonstrated that atmospheric pressure fluctuations during weather pattern changes can introduce up to 1-2% variability in flow rates for certain pump designs, necessitating compensation mechanisms in critical applications.
Electrical supply stability represents another critical environmental factor. Voltage fluctuations, power surges, and electromagnetic interference can disrupt the precise electronic control systems governing modern HPLC pumps. Unstable power supplies may cause erratic pump motor behavior, leading to pulsations and irregular flow patterns that compromise analytical precision. Implementation of uninterruptible power supplies (UPS) with power conditioning capabilities has been shown to improve flow stability by 15-20% in environments with problematic electrical infrastructure.
Vibration and mechanical disturbances transmitted through laboratory benches or from nearby equipment can directly impact pump performance. High-frequency vibrations may interfere with pressure sensors and feedback mechanisms, while low-frequency disturbances can create resonance effects within the hydraulic system. Specialized anti-vibration platforms have demonstrated effectiveness in isolating HPLC systems from environmental vibrations, with studies showing up to 30% improvement in flow precision for sensitive applications.
Humidity levels affect both electronic components and mobile phase properties. Excessive humidity can lead to condensation on electronic circuits, potentially causing short circuits or corrosion over time. Additionally, hygroscopic mobile phase components may absorb atmospheric moisture, altering their chemical properties and flow characteristics. Controlled laboratory environments maintaining 40-60% relative humidity represent the optimal range for HPLC system operation according to multiple manufacturer recommendations and research studies.
Air quality factors, including particulate matter and chemical contaminants, can impact system performance through mechanical interference with moving parts or chemical interaction with mobile phases. HEPA-filtered environments have demonstrated superior long-term stability for HPLC systems, particularly in industrial settings where airborne contaminants are prevalent.
Barometric pressure changes, though often overlooked, can significantly influence HPLC system performance. These variations affect the behavior of pumps, particularly in systems utilizing vacuum degassers or operating near their pressure limits. Studies have demonstrated that atmospheric pressure fluctuations during weather pattern changes can introduce up to 1-2% variability in flow rates for certain pump designs, necessitating compensation mechanisms in critical applications.
Electrical supply stability represents another critical environmental factor. Voltage fluctuations, power surges, and electromagnetic interference can disrupt the precise electronic control systems governing modern HPLC pumps. Unstable power supplies may cause erratic pump motor behavior, leading to pulsations and irregular flow patterns that compromise analytical precision. Implementation of uninterruptible power supplies (UPS) with power conditioning capabilities has been shown to improve flow stability by 15-20% in environments with problematic electrical infrastructure.
Vibration and mechanical disturbances transmitted through laboratory benches or from nearby equipment can directly impact pump performance. High-frequency vibrations may interfere with pressure sensors and feedback mechanisms, while low-frequency disturbances can create resonance effects within the hydraulic system. Specialized anti-vibration platforms have demonstrated effectiveness in isolating HPLC systems from environmental vibrations, with studies showing up to 30% improvement in flow precision for sensitive applications.
Humidity levels affect both electronic components and mobile phase properties. Excessive humidity can lead to condensation on electronic circuits, potentially causing short circuits or corrosion over time. Additionally, hygroscopic mobile phase components may absorb atmospheric moisture, altering their chemical properties and flow characteristics. Controlled laboratory environments maintaining 40-60% relative humidity represent the optimal range for HPLC system operation according to multiple manufacturer recommendations and research studies.
Air quality factors, including particulate matter and chemical contaminants, can impact system performance through mechanical interference with moving parts or chemical interaction with mobile phases. HEPA-filtered environments have demonstrated superior long-term stability for HPLC systems, particularly in industrial settings where airborne contaminants are prevalent.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!