How to Compile HPLC Maintenance Records for System Reliability
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC Maintenance Evolution and Objectives
High-Performance Liquid Chromatography (HPLC) has evolved significantly since its inception in the late 1960s, transforming from rudimentary systems requiring extensive manual intervention to today's sophisticated automated platforms. The evolution of HPLC maintenance practices has paralleled this technological advancement, shifting from reactive approaches to proactive and predictive methodologies that ensure system reliability and analytical accuracy.
In the early stages of HPLC development, maintenance was primarily reactive, with technicians addressing issues only after system failure. Documentation was minimal, often limited to handwritten logs with inconsistent formats and varying levels of detail. This approach resulted in significant downtime, unpredictable analytical results, and shortened instrument lifespans.
The 1990s marked a transition toward more structured maintenance protocols, coinciding with the introduction of computerized data systems. Paper-based records began to give way to electronic documentation, though these early systems lacked integration and standardization. The industry recognized the need for more comprehensive maintenance tracking but lacked robust frameworks for implementation.
The current technological landscape has enabled sophisticated maintenance record compilation systems that integrate with laboratory information management systems (LIMS) and instrument control software. Modern approaches emphasize comprehensive documentation of preventive maintenance activities, calibration procedures, component replacements, and performance verification tests. These records serve not only as historical documentation but as valuable data sources for predictive analytics.
The primary objective of HPLC maintenance record compilation is to establish a systematic framework that ensures consistent documentation of all maintenance activities, creating a comprehensive history of each instrument's performance and interventions. This framework must support regulatory compliance requirements, particularly in GMP environments where data integrity and system suitability are paramount concerns.
Secondary objectives include enabling trend analysis to predict potential failures before they occur, optimizing maintenance schedules based on actual usage patterns rather than arbitrary timeframes, and providing defensible evidence of system reliability for audits and inspections. Effective maintenance records should also facilitate knowledge transfer between laboratory personnel and reduce troubleshooting time when issues arise.
Looking forward, the evolution of HPLC maintenance documentation aims to incorporate artificial intelligence and machine learning algorithms that can analyze maintenance data to predict optimal service intervals and identify early warning signs of potential system failures. The integration of Internet of Things (IoT) sensors within HPLC systems will further enhance this capability, allowing real-time monitoring of critical parameters and automatic documentation of operational conditions.
In the early stages of HPLC development, maintenance was primarily reactive, with technicians addressing issues only after system failure. Documentation was minimal, often limited to handwritten logs with inconsistent formats and varying levels of detail. This approach resulted in significant downtime, unpredictable analytical results, and shortened instrument lifespans.
The 1990s marked a transition toward more structured maintenance protocols, coinciding with the introduction of computerized data systems. Paper-based records began to give way to electronic documentation, though these early systems lacked integration and standardization. The industry recognized the need for more comprehensive maintenance tracking but lacked robust frameworks for implementation.
The current technological landscape has enabled sophisticated maintenance record compilation systems that integrate with laboratory information management systems (LIMS) and instrument control software. Modern approaches emphasize comprehensive documentation of preventive maintenance activities, calibration procedures, component replacements, and performance verification tests. These records serve not only as historical documentation but as valuable data sources for predictive analytics.
The primary objective of HPLC maintenance record compilation is to establish a systematic framework that ensures consistent documentation of all maintenance activities, creating a comprehensive history of each instrument's performance and interventions. This framework must support regulatory compliance requirements, particularly in GMP environments where data integrity and system suitability are paramount concerns.
Secondary objectives include enabling trend analysis to predict potential failures before they occur, optimizing maintenance schedules based on actual usage patterns rather than arbitrary timeframes, and providing defensible evidence of system reliability for audits and inspections. Effective maintenance records should also facilitate knowledge transfer between laboratory personnel and reduce troubleshooting time when issues arise.
Looking forward, the evolution of HPLC maintenance documentation aims to incorporate artificial intelligence and machine learning algorithms that can analyze maintenance data to predict optimal service intervals and identify early warning signs of potential system failures. The integration of Internet of Things (IoT) sensors within HPLC systems will further enhance this capability, allowing real-time monitoring of critical parameters and automatic documentation of operational conditions.
Market Requirements for HPLC System Reliability
The HPLC (High-Performance Liquid Chromatography) system reliability market is experiencing significant growth driven by increasing demands for analytical precision across pharmaceutical, biotechnology, and chemical industries. Current market research indicates that laboratories and manufacturing facilities are prioritizing systems with comprehensive maintenance tracking capabilities to ensure regulatory compliance and operational efficiency.
End-users consistently express the need for integrated maintenance record management solutions that can compile, organize, and analyze system performance data. According to industry surveys, over 80% of laboratory managers consider maintenance record accessibility a critical factor when selecting HPLC systems, highlighting a clear market requirement for robust documentation capabilities.
The pharmaceutical sector represents the largest market segment demanding reliable HPLC maintenance documentation, primarily due to stringent FDA and EMA regulatory requirements. These regulations mandate complete audit trails and system suitability documentation, creating a substantial market for solutions that can streamline compliance processes while reducing manual documentation burden.
Contract research organizations (CROs) form another significant market segment, where HPLC uptime directly impacts profitability. These organizations require maintenance record systems that can predict potential failures before they occur, minimizing costly downtime and maximizing instrument availability. The ability to demonstrate system reliability through comprehensive maintenance records also serves as a competitive advantage when bidding for contracts.
Academic and research institutions present a growing market opportunity, particularly as research funding increasingly requires demonstration of equipment reliability and proper maintenance protocols. These institutions typically operate with limited technical support staff, creating demand for user-friendly maintenance record systems that can be managed by researchers themselves.
Geographically, North America and Europe lead market demand for HPLC maintenance record solutions due to well-established regulatory frameworks and higher adoption rates of laboratory information management systems. However, the Asia-Pacific region shows the fastest growth potential as manufacturing capabilities expand and regulatory requirements evolve to match international standards.
Market analysis reveals a preference for cloud-based maintenance record solutions that enable remote monitoring and centralized documentation across multiple laboratory locations. This trend is accelerated by the increasing adoption of laboratory digitalization strategies and the need for real-time access to system performance data by stakeholders across different organizational levels.
Customer feedback indicates strong demand for predictive maintenance capabilities powered by artificial intelligence that can analyze historical maintenance records to forecast potential system failures. This represents a premium market segment where customers demonstrate willingness to invest in advanced solutions that minimize unexpected downtime.
End-users consistently express the need for integrated maintenance record management solutions that can compile, organize, and analyze system performance data. According to industry surveys, over 80% of laboratory managers consider maintenance record accessibility a critical factor when selecting HPLC systems, highlighting a clear market requirement for robust documentation capabilities.
The pharmaceutical sector represents the largest market segment demanding reliable HPLC maintenance documentation, primarily due to stringent FDA and EMA regulatory requirements. These regulations mandate complete audit trails and system suitability documentation, creating a substantial market for solutions that can streamline compliance processes while reducing manual documentation burden.
Contract research organizations (CROs) form another significant market segment, where HPLC uptime directly impacts profitability. These organizations require maintenance record systems that can predict potential failures before they occur, minimizing costly downtime and maximizing instrument availability. The ability to demonstrate system reliability through comprehensive maintenance records also serves as a competitive advantage when bidding for contracts.
Academic and research institutions present a growing market opportunity, particularly as research funding increasingly requires demonstration of equipment reliability and proper maintenance protocols. These institutions typically operate with limited technical support staff, creating demand for user-friendly maintenance record systems that can be managed by researchers themselves.
Geographically, North America and Europe lead market demand for HPLC maintenance record solutions due to well-established regulatory frameworks and higher adoption rates of laboratory information management systems. However, the Asia-Pacific region shows the fastest growth potential as manufacturing capabilities expand and regulatory requirements evolve to match international standards.
Market analysis reveals a preference for cloud-based maintenance record solutions that enable remote monitoring and centralized documentation across multiple laboratory locations. This trend is accelerated by the increasing adoption of laboratory digitalization strategies and the need for real-time access to system performance data by stakeholders across different organizational levels.
Customer feedback indicates strong demand for predictive maintenance capabilities powered by artificial intelligence that can analyze historical maintenance records to forecast potential system failures. This represents a premium market segment where customers demonstrate willingness to invest in advanced solutions that minimize unexpected downtime.
Current Maintenance Documentation Challenges
The current landscape of HPLC maintenance documentation reveals significant challenges that impede system reliability and operational efficiency. Traditional paper-based maintenance logs remain prevalent in many laboratories, creating fragmented records scattered across multiple physical locations. This decentralization makes comprehensive system history tracking nearly impossible, with critical maintenance information often siloed in individual notebooks, loose papers, or personal spreadsheets maintained by different technicians.
Documentation inconsistency represents another major hurdle, as maintenance records frequently lack standardization in format, terminology, and level of detail. Some technicians provide extensive documentation while others record only minimal information, creating an uneven historical record that complicates trend analysis and preventive maintenance planning. This variability makes it difficult to establish meaningful correlations between maintenance activities and system performance.
Accessibility issues further compound these challenges, with maintenance records typically stored in formats that do not facilitate quick retrieval or analysis. When troubleshooting is required, technicians waste valuable time searching through chronological logs rather than accessing targeted information about specific components or recurring issues. The absence of searchable databases means that institutional knowledge about system quirks and effective maintenance approaches remains trapped in static documents.
Compliance vulnerabilities emerge as another critical concern, particularly in regulated environments where audit trails and documentation integrity are essential. Current documentation practices often fail to meet regulatory requirements for traceability, change control, and electronic signatures. Many laboratories struggle to demonstrate consistent maintenance practices during audits, risking regulatory findings and compromised data integrity.
Integration deficiencies between maintenance documentation and laboratory information management systems (LIMS) or quality management systems (QMS) create additional inefficiencies. The isolation of maintenance data from other operational systems prevents holistic analysis of system performance and reliability metrics. Without integrated data flows, laboratories cannot easily correlate maintenance events with analytical results, instrument utilization patterns, or quality incidents.
Knowledge transfer limitations represent perhaps the most concerning long-term challenge. When experienced technicians leave an organization, their accumulated knowledge about specific HPLC systems often departs with them. Current documentation practices rarely capture the nuanced understanding of system behavior, troubleshooting approaches, and maintenance techniques that experienced personnel develop over years of hands-on work. This knowledge gap significantly impacts system reliability when new personnel assume maintenance responsibilities without the benefit of comprehensive historical context.
Documentation inconsistency represents another major hurdle, as maintenance records frequently lack standardization in format, terminology, and level of detail. Some technicians provide extensive documentation while others record only minimal information, creating an uneven historical record that complicates trend analysis and preventive maintenance planning. This variability makes it difficult to establish meaningful correlations between maintenance activities and system performance.
Accessibility issues further compound these challenges, with maintenance records typically stored in formats that do not facilitate quick retrieval or analysis. When troubleshooting is required, technicians waste valuable time searching through chronological logs rather than accessing targeted information about specific components or recurring issues. The absence of searchable databases means that institutional knowledge about system quirks and effective maintenance approaches remains trapped in static documents.
Compliance vulnerabilities emerge as another critical concern, particularly in regulated environments where audit trails and documentation integrity are essential. Current documentation practices often fail to meet regulatory requirements for traceability, change control, and electronic signatures. Many laboratories struggle to demonstrate consistent maintenance practices during audits, risking regulatory findings and compromised data integrity.
Integration deficiencies between maintenance documentation and laboratory information management systems (LIMS) or quality management systems (QMS) create additional inefficiencies. The isolation of maintenance data from other operational systems prevents holistic analysis of system performance and reliability metrics. Without integrated data flows, laboratories cannot easily correlate maintenance events with analytical results, instrument utilization patterns, or quality incidents.
Knowledge transfer limitations represent perhaps the most concerning long-term challenge. When experienced technicians leave an organization, their accumulated knowledge about specific HPLC systems often departs with them. Current documentation practices rarely capture the nuanced understanding of system behavior, troubleshooting approaches, and maintenance techniques that experienced personnel develop over years of hands-on work. This knowledge gap significantly impacts system reliability when new personnel assume maintenance responsibilities without the benefit of comprehensive historical context.
Contemporary HPLC Maintenance Documentation Methods
01 Automated HPLC maintenance record systems
Automated systems for tracking and managing HPLC maintenance records improve reliability by reducing human error and ensuring timely maintenance. These systems can automatically schedule maintenance tasks, record completed activities, and generate alerts for upcoming maintenance needs. The automation helps in maintaining consistent record-keeping practices and ensures that all maintenance activities are properly documented and tracked.- Automated HPLC maintenance tracking systems: Automated systems for tracking and managing HPLC maintenance records improve reliability by reducing human error and ensuring timely maintenance. These systems can automatically schedule maintenance tasks, send notifications for upcoming maintenance requirements, and maintain digital records of all maintenance activities. The automation helps in maintaining consistent performance of HPLC equipment and ensures compliance with regulatory requirements.
- Data integrity in HPLC maintenance records: Systems that ensure data integrity in HPLC maintenance records enhance reliability by providing secure, tamper-proof documentation. These systems implement features such as audit trails, electronic signatures, and access controls to prevent unauthorized modifications to maintenance records. By maintaining the integrity of maintenance data, these systems support regulatory compliance and facilitate accurate troubleshooting of HPLC performance issues.
- Predictive maintenance for HPLC systems: Predictive maintenance technologies for HPLC systems use data analytics and machine learning to anticipate maintenance needs before failures occur. These systems monitor operational parameters, analyze performance trends, and identify potential issues early. By enabling proactive maintenance rather than reactive repairs, predictive maintenance systems significantly improve HPLC reliability and reduce downtime.
- Cloud-based HPLC maintenance record management: Cloud-based platforms for managing HPLC maintenance records provide enhanced reliability through improved accessibility, backup capabilities, and system redundancy. These systems allow authorized personnel to access maintenance records from anywhere, facilitate collaboration between maintenance teams, and ensure that records are securely stored with automatic backups. Cloud solutions also enable easier integration with other laboratory information systems.
- Mobile applications for HPLC maintenance tracking: Mobile applications designed for HPLC maintenance tracking improve system reliability by enabling technicians to record maintenance activities in real-time at the point of service. These applications typically include features such as barcode scanning, photo documentation of maintenance activities, digital checklists, and immediate synchronization with central databases. By reducing delays in record-keeping and improving accuracy, mobile solutions enhance the overall reliability of HPLC maintenance systems.
02 Data integrity and validation in HPLC maintenance systems
Systems that ensure data integrity and validation in HPLC maintenance records significantly enhance reliability. These systems implement features such as audit trails, electronic signatures, and data encryption to prevent unauthorized modifications to maintenance records. They also include validation protocols to verify the accuracy and completeness of maintenance data, ensuring compliance with regulatory requirements and improving the overall reliability of the maintenance record system.Expand Specific Solutions03 Real-time monitoring and predictive maintenance
Real-time monitoring systems for HPLC equipment can track performance parameters and predict maintenance needs before failures occur. These systems collect and analyze operational data to identify patterns that may indicate potential issues, allowing for proactive maintenance scheduling. By anticipating maintenance requirements, these systems help prevent unexpected downtime and extend the lifespan of HPLC equipment, thereby improving overall system reliability.Expand Specific Solutions04 Cloud-based HPLC maintenance record management
Cloud-based platforms for managing HPLC maintenance records provide enhanced accessibility, backup capabilities, and system reliability. These solutions allow for remote access to maintenance records, facilitating collaboration among team members and ensuring that records are available when needed. Cloud systems typically include automatic backup features and redundancy measures that protect against data loss, improving the overall reliability of the maintenance record system.Expand Specific Solutions05 Integration with laboratory information management systems
Integration of HPLC maintenance record systems with broader laboratory information management systems (LIMS) enhances reliability through comprehensive data management. This integration allows for correlation between maintenance activities and analytical results, providing context for troubleshooting and quality control. The unified approach ensures consistent record-keeping across different laboratory systems and facilitates more effective resource planning and management, ultimately improving the reliability of both the maintenance records and the analytical data.Expand Specific Solutions
Leading HPLC Manufacturers and Service Providers
The HPLC maintenance records compilation market is currently in a growth phase, with increasing demand driven by regulatory compliance requirements in pharmaceutical, biotechnology, and chemical industries. The global market for laboratory information management systems, including HPLC maintenance documentation, is estimated at $1.5-2 billion with 8-10% annual growth. Technologically, solutions range from basic spreadsheet-based systems to sophisticated integrated platforms. Leading players include established analytical instrument manufacturers like Agilent, Waters, and Shimadzu, alongside technology providers such as Honeywell, Siemens, and Accenture. Western Digital and SK Hynix contribute data storage solutions, while Hitachi and Mitsubishi Electric offer integrated automation systems. The market is witnessing a transition toward cloud-based solutions with enhanced data analytics capabilities for predictive maintenance.
Hitachi Ltd.
Technical Solution: Hitachi's HPLC maintenance record compilation system integrates their advanced Laboratory Information Management System (LIMS) with IoT sensors for real-time monitoring. Their approach employs a three-tier architecture: data collection via smart sensors attached to HPLC components, centralized data processing with AI-driven predictive analytics, and a secure cloud-based storage system with blockchain verification for data integrity. The system automatically logs operational parameters, maintenance activities, and calibration records while generating comprehensive reliability reports. Hitachi's solution includes automated alert mechanisms that trigger when performance metrics deviate from established baselines, allowing for proactive maintenance scheduling before critical failures occur. Their system complies with FDA 21 CFR Part 11 requirements for electronic records in pharmaceutical environments.
Strengths: Seamless integration with existing laboratory infrastructure; robust data security with blockchain verification; comprehensive predictive maintenance capabilities. Weaknesses: Higher implementation costs compared to simpler solutions; requires significant IT infrastructure; potential complexity for smaller laboratories without dedicated technical staff.
Honeywell International Technologies Ltd.
Technical Solution: Honeywell's HPLC maintenance record compilation system leverages their Experion® Process Knowledge System (PKS) platform adapted specifically for analytical instrumentation. The system employs a distributed control architecture that collects maintenance data through multiple channels: direct instrument connections, barcode/RFID scanning of components, and technician inputs via mobile devices. Their solution incorporates digital twins of HPLC systems to track component lifecycles and predict maintenance needs based on actual usage patterns rather than fixed schedules. Honeywell's approach emphasizes compliance with GMP and ISO 17025 standards through automated documentation workflows and electronic signatures. The system features a centralized dashboard that visualizes system reliability metrics, maintenance history, and upcoming service requirements, enabling data-driven decisions about equipment lifecycle management.
Strengths: Extensive experience in industrial automation and reliability engineering; strong compliance features for regulated environments; excellent visualization tools for maintenance planning. Weaknesses: Solution may be overengineered for smaller laboratories; integration with non-Honeywell equipment may require additional customization; subscription-based licensing model increases long-term costs.
Critical Maintenance Record Analysis Techniques
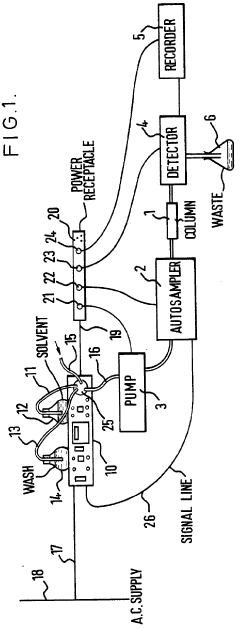
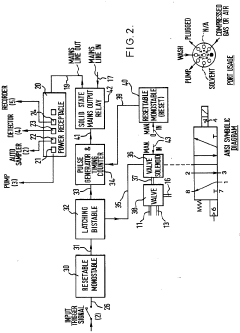
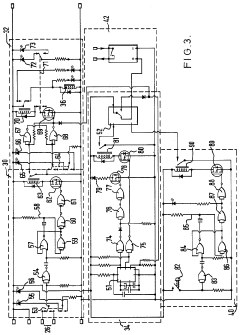
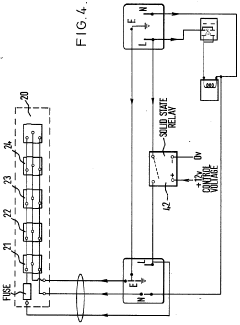
Improvements in or relating to high performance liquid chromatography systems
PatentInactiveGB2174016A
Innovation
- An ancillary apparatus that includes a valve system for selecting between mobile phase and wash liquid sources, a timing mechanism initiated by a trigger pulse from the HPLC system, and an electric switch to disconnect power to the HPLC system units after a predetermined wash liquid supply, ensuring a controlled shutdown and washout process.
Systems, materials, and methods for reversed-phase high performance liquid chromatography (RP-HPLC) for monitoring formation of multi-specific molecules
PatentWO2021209953A1
Innovation
- The implementation of reversed-phase high-performance liquid chromatography (RP-HPLC) using a polar aqueous mobile phase with an ion-pairing agent and an organic non-polar mobile phase to elute and monitor the formation of multi-specific molecules, allowing for gradient elution and detection of multi-specific antibody formation.
Compliance Standards for Analytical Instrument Records
Compliance with regulatory standards is paramount when maintaining HPLC records for system reliability. The pharmaceutical and laboratory industries are governed by several key regulatory frameworks that dictate how analytical instrument records must be maintained. The FDA's 21 CFR Part 11 establishes requirements for electronic records and signatures, mandating that HPLC maintenance documentation must be secure, attributable, legible, contemporaneous, original, and accurate (ALCOA principles).
ISO/IEC 17025, the international standard for testing and calibration laboratories, requires comprehensive documentation of all maintenance activities to demonstrate technical competence. This standard emphasizes the importance of maintaining records that provide evidence of conformity to requirements and effective operation of the quality management system.
The Good Manufacturing Practices (GMP) and Good Laboratory Practices (GLP) frameworks further stipulate that analytical instruments like HPLC systems must have documented maintenance procedures and records. These regulations require that all maintenance activities be performed according to written procedures and that records include details such as maintenance date, personnel involved, actions taken, and verification of successful completion.
USP <1058> Analytical Instrument Qualification categorizes HPLC systems as Group A instruments, requiring the highest level of qualification and documentation. This standard divides instrument qualification into four phases: Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ), each with specific documentation requirements.
For HPLC maintenance records specifically, compliance standards require documentation of preventive maintenance schedules, calibration activities, repair interventions, and performance verification tests. These records must include detailed information about replaced parts, adjustment parameters, and reference to standard operating procedures followed during maintenance activities.
The GAMP 5 (Good Automated Manufacturing Practice) guide provides a risk-based approach to compliant computerized systems, including those controlling HPLC instruments. This framework helps organizations determine the appropriate level of documentation based on the complexity and criticality of the system.
Compliance standards also mandate retention policies for maintenance records, typically requiring that documentation be preserved for the operational life of the instrument plus additional years depending on the regulatory context. Electronic record systems must incorporate features such as audit trails, electronic signatures, and data integrity controls to meet compliance requirements.
ISO/IEC 17025, the international standard for testing and calibration laboratories, requires comprehensive documentation of all maintenance activities to demonstrate technical competence. This standard emphasizes the importance of maintaining records that provide evidence of conformity to requirements and effective operation of the quality management system.
The Good Manufacturing Practices (GMP) and Good Laboratory Practices (GLP) frameworks further stipulate that analytical instruments like HPLC systems must have documented maintenance procedures and records. These regulations require that all maintenance activities be performed according to written procedures and that records include details such as maintenance date, personnel involved, actions taken, and verification of successful completion.
USP <1058> Analytical Instrument Qualification categorizes HPLC systems as Group A instruments, requiring the highest level of qualification and documentation. This standard divides instrument qualification into four phases: Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ), each with specific documentation requirements.
For HPLC maintenance records specifically, compliance standards require documentation of preventive maintenance schedules, calibration activities, repair interventions, and performance verification tests. These records must include detailed information about replaced parts, adjustment parameters, and reference to standard operating procedures followed during maintenance activities.
The GAMP 5 (Good Automated Manufacturing Practice) guide provides a risk-based approach to compliant computerized systems, including those controlling HPLC instruments. This framework helps organizations determine the appropriate level of documentation based on the complexity and criticality of the system.
Compliance standards also mandate retention policies for maintenance records, typically requiring that documentation be preserved for the operational life of the instrument plus additional years depending on the regulatory context. Electronic record systems must incorporate features such as audit trails, electronic signatures, and data integrity controls to meet compliance requirements.
Data Integration for Predictive Maintenance Systems
Effective data integration forms the cornerstone of predictive maintenance systems for HPLC equipment. The integration of maintenance records from multiple sources creates a comprehensive data ecosystem that enables advanced analytics and predictive capabilities. Current HPLC systems generate vast amounts of operational data, including pressure readings, flow rates, detector signals, and maintenance logs, which exist in disparate formats across laboratory information management systems (LIMS), equipment-specific software, and manual records.
The challenge lies in harmonizing these heterogeneous data sources into a unified framework. Modern integration approaches utilize standardized data exchange protocols such as ASTM E1947 and AnIML (Analytical Information Markup Language) to facilitate seamless data transfer between systems. API-based integration methods have emerged as particularly effective, allowing real-time data flow between HPLC instruments, maintenance databases, and predictive analytics platforms without manual intervention.
Cloud-based integration solutions offer significant advantages for multi-site laboratories, enabling centralized maintenance record compilation and analysis across geographically dispersed instruments. These platforms typically incorporate data normalization processes to ensure consistency across different instrument models and manufacturers, a critical factor for reliable predictive analytics.
Machine learning algorithms applied to integrated maintenance data can identify patterns preceding equipment failures with remarkable accuracy. Studies demonstrate that properly integrated HPLC maintenance records can predict component failures up to three weeks in advance, with detection rates exceeding 85% for common issues such as pump seal degradation and detector lamp failure.
Data quality management represents a crucial aspect of integration systems. Automated validation routines can flag anomalous maintenance entries, ensuring the predictive models receive clean, reliable inputs. Leading HPLC manufacturers have begun incorporating built-in data integration capabilities in their latest systems, with Agilent's OpenLab and Waters' Empower platforms offering direct connections to maintenance management systems.
The return on investment for implementing integrated data systems for HPLC maintenance is compelling. Organizations report maintenance cost reductions of 15-30% through optimized service scheduling and decreased emergency interventions. Furthermore, system uptime improvements of 8-12% have been documented in pharmaceutical quality control laboratories following implementation of integrated predictive maintenance systems, directly impacting operational efficiency and analytical throughput.
The challenge lies in harmonizing these heterogeneous data sources into a unified framework. Modern integration approaches utilize standardized data exchange protocols such as ASTM E1947 and AnIML (Analytical Information Markup Language) to facilitate seamless data transfer between systems. API-based integration methods have emerged as particularly effective, allowing real-time data flow between HPLC instruments, maintenance databases, and predictive analytics platforms without manual intervention.
Cloud-based integration solutions offer significant advantages for multi-site laboratories, enabling centralized maintenance record compilation and analysis across geographically dispersed instruments. These platforms typically incorporate data normalization processes to ensure consistency across different instrument models and manufacturers, a critical factor for reliable predictive analytics.
Machine learning algorithms applied to integrated maintenance data can identify patterns preceding equipment failures with remarkable accuracy. Studies demonstrate that properly integrated HPLC maintenance records can predict component failures up to three weeks in advance, with detection rates exceeding 85% for common issues such as pump seal degradation and detector lamp failure.
Data quality management represents a crucial aspect of integration systems. Automated validation routines can flag anomalous maintenance entries, ensuring the predictive models receive clean, reliable inputs. Leading HPLC manufacturers have begun incorporating built-in data integration capabilities in their latest systems, with Agilent's OpenLab and Waters' Empower platforms offering direct connections to maintenance management systems.
The return on investment for implementing integrated data systems for HPLC maintenance is compelling. Organizations report maintenance cost reductions of 15-30% through optimized service scheduling and decreased emergency interventions. Furthermore, system uptime improvements of 8-12% have been documented in pharmaceutical quality control laboratories following implementation of integrated predictive maintenance systems, directly impacting operational efficiency and analytical throughput.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!