Comparing HPLC and GC: Retention Time and Effectiveness
SEP 19, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chromatography Evolution and Objectives
Chromatography has evolved significantly since its inception in the early 20th century, transforming from rudimentary separation techniques to sophisticated analytical methodologies essential in modern scientific research and industrial applications. The journey began with Mikhail Tsvet's pioneering work in 1903, where he separated plant pigments using calcium carbonate columns and petroleum ether, coining the term "chromatography" from the Greek words for "color writing." This fundamental discovery laid the groundwork for decades of innovation.
By the 1940s, Martin and Synge developed partition chromatography, earning them the Nobel Prize and establishing theoretical principles that would later enable the creation of High-Performance Liquid Chromatography (HPLC) and Gas Chromatography (GC). The 1950s witnessed the emergence of GC as a practical analytical tool, while HPLC gained prominence in the 1970s with the development of high-pressure pumps and more efficient column packings.
The technological evolution of both HPLC and GC has been characterized by continuous improvements in column technology, detection methods, and automation capabilities. Modern HPLC systems feature ultra-high-performance capabilities (UHPLC) with sub-2-micron particles, enabling faster analyses and superior resolution. Similarly, GC has advanced with capillary columns offering exceptional separation efficiency and specialized detectors providing remarkable sensitivity and selectivity.
Retention time, a critical parameter in both techniques, represents the time a compound takes to travel through the chromatographic system. In HPLC, retention is primarily influenced by the compound's interaction with the stationary phase and mobile phase composition, while in GC, it depends largely on boiling points and the compound's affinity for the stationary phase under temperature-controlled conditions.
The primary objective of comparing HPLC and GC retention times and effectiveness is to establish optimal analytical methodologies for specific applications. This comparison aims to determine which technique provides superior separation efficiency, sensitivity, selectivity, and reproducibility for particular compound classes. Additionally, it seeks to identify the practical limitations of each method regarding sample types, analysis time, and operational costs.
Future technological trends point toward miniaturization, increased automation, and integration with advanced data analysis tools. The development of hybrid techniques combining chromatography with mass spectrometry and other detection methods continues to expand analytical capabilities. Green chromatography approaches are also gaining momentum, focusing on reducing solvent consumption and environmental impact while maintaining analytical performance.
The ultimate goal of this technical research is to provide comprehensive guidance for selecting the most appropriate chromatographic technique based on specific analytical requirements, sample characteristics, and operational constraints, thereby optimizing analytical outcomes across various scientific and industrial applications.
By the 1940s, Martin and Synge developed partition chromatography, earning them the Nobel Prize and establishing theoretical principles that would later enable the creation of High-Performance Liquid Chromatography (HPLC) and Gas Chromatography (GC). The 1950s witnessed the emergence of GC as a practical analytical tool, while HPLC gained prominence in the 1970s with the development of high-pressure pumps and more efficient column packings.
The technological evolution of both HPLC and GC has been characterized by continuous improvements in column technology, detection methods, and automation capabilities. Modern HPLC systems feature ultra-high-performance capabilities (UHPLC) with sub-2-micron particles, enabling faster analyses and superior resolution. Similarly, GC has advanced with capillary columns offering exceptional separation efficiency and specialized detectors providing remarkable sensitivity and selectivity.
Retention time, a critical parameter in both techniques, represents the time a compound takes to travel through the chromatographic system. In HPLC, retention is primarily influenced by the compound's interaction with the stationary phase and mobile phase composition, while in GC, it depends largely on boiling points and the compound's affinity for the stationary phase under temperature-controlled conditions.
The primary objective of comparing HPLC and GC retention times and effectiveness is to establish optimal analytical methodologies for specific applications. This comparison aims to determine which technique provides superior separation efficiency, sensitivity, selectivity, and reproducibility for particular compound classes. Additionally, it seeks to identify the practical limitations of each method regarding sample types, analysis time, and operational costs.
Future technological trends point toward miniaturization, increased automation, and integration with advanced data analysis tools. The development of hybrid techniques combining chromatography with mass spectrometry and other detection methods continues to expand analytical capabilities. Green chromatography approaches are also gaining momentum, focusing on reducing solvent consumption and environmental impact while maintaining analytical performance.
The ultimate goal of this technical research is to provide comprehensive guidance for selecting the most appropriate chromatographic technique based on specific analytical requirements, sample characteristics, and operational constraints, thereby optimizing analytical outcomes across various scientific and industrial applications.
Market Applications and Analytical Demands
The analytical instrumentation market has witnessed substantial growth driven by increasing demands across pharmaceutical, environmental, food safety, and petrochemical sectors. High Performance Liquid Chromatography (HPLC) and Gas Chromatography (GC) represent cornerstone technologies within this landscape, each addressing specific analytical needs with distinct market applications.
The pharmaceutical industry constitutes the largest market segment for HPLC systems, valued at approximately $4.5 billion globally, with a compound annual growth rate of 6.8%. This dominance stems from HPLC's superior capability in analyzing non-volatile, thermally unstable compounds prevalent in drug development and quality control processes. Particularly, the rise in biopharmaceuticals has accelerated demand for specialized HPLC techniques like size-exclusion and affinity chromatography.
Conversely, GC systems find their strongest application in petrochemical and environmental testing markets, collectively representing a $2.7 billion segment. The petrochemical industry relies heavily on GC for hydrocarbon analysis, while environmental monitoring agencies utilize GC for detecting volatile organic compounds (VOCs) and persistent organic pollutants in various matrices.
The food and beverage industry presents a rapidly expanding market for both technologies, with HPLC preferred for nutritional component analysis and GC favored for flavor, fragrance, and contaminant detection. This sector has shown increased analytical demands following stricter regulatory frameworks implemented across major markets including the EU, US, and China.
Clinical diagnostics represents an emerging application area, particularly for HPLC, with growing utilization in therapeutic drug monitoring, vitamin analysis, and metabolic disorder screening. This segment is projected to grow at 8.2% annually through 2028, outpacing the overall market average.
Regional analysis reveals North America maintains the largest market share at 38%, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region demonstrates the highest growth trajectory, driven by expanding pharmaceutical manufacturing, environmental concerns, and food safety initiatives in China and India.
The analytical demands across these markets increasingly emphasize higher throughput, improved sensitivity, and enhanced resolution. Ultra-high performance liquid chromatography (UHPLC) has emerged as a response to these requirements, offering significantly reduced retention times while maintaining separation efficiency. Similarly, comprehensive two-dimensional gas chromatography (GC×GC) addresses complex sample analysis needs in petroleum, environmental, and food safety applications.
Miniaturization represents another significant trend, with portable and compact chromatography systems gaining traction for on-site analysis in environmental monitoring, food safety inspection, and forensic applications, addressing the growing demand for rapid, field-deployable analytical capabilities.
The pharmaceutical industry constitutes the largest market segment for HPLC systems, valued at approximately $4.5 billion globally, with a compound annual growth rate of 6.8%. This dominance stems from HPLC's superior capability in analyzing non-volatile, thermally unstable compounds prevalent in drug development and quality control processes. Particularly, the rise in biopharmaceuticals has accelerated demand for specialized HPLC techniques like size-exclusion and affinity chromatography.
Conversely, GC systems find their strongest application in petrochemical and environmental testing markets, collectively representing a $2.7 billion segment. The petrochemical industry relies heavily on GC for hydrocarbon analysis, while environmental monitoring agencies utilize GC for detecting volatile organic compounds (VOCs) and persistent organic pollutants in various matrices.
The food and beverage industry presents a rapidly expanding market for both technologies, with HPLC preferred for nutritional component analysis and GC favored for flavor, fragrance, and contaminant detection. This sector has shown increased analytical demands following stricter regulatory frameworks implemented across major markets including the EU, US, and China.
Clinical diagnostics represents an emerging application area, particularly for HPLC, with growing utilization in therapeutic drug monitoring, vitamin analysis, and metabolic disorder screening. This segment is projected to grow at 8.2% annually through 2028, outpacing the overall market average.
Regional analysis reveals North America maintains the largest market share at 38%, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region demonstrates the highest growth trajectory, driven by expanding pharmaceutical manufacturing, environmental concerns, and food safety initiatives in China and India.
The analytical demands across these markets increasingly emphasize higher throughput, improved sensitivity, and enhanced resolution. Ultra-high performance liquid chromatography (UHPLC) has emerged as a response to these requirements, offering significantly reduced retention times while maintaining separation efficiency. Similarly, comprehensive two-dimensional gas chromatography (GC×GC) addresses complex sample analysis needs in petroleum, environmental, and food safety applications.
Miniaturization represents another significant trend, with portable and compact chromatography systems gaining traction for on-site analysis in environmental monitoring, food safety inspection, and forensic applications, addressing the growing demand for rapid, field-deployable analytical capabilities.
HPLC vs GC: Technical Limitations and Challenges
High-performance liquid chromatography (HPLC) and gas chromatography (GC) represent two fundamental analytical techniques in modern chemistry, each with distinct technical limitations and challenges. Despite their widespread adoption, both methods face significant constraints that impact their effectiveness in various applications.
HPLC systems encounter challenges related to pressure limitations, with conventional systems typically operating below 6,000 psi. This constraint restricts column efficiency and resolution capabilities, particularly when analyzing complex mixtures. Ultra-high-performance liquid chromatography (UHPLC) has emerged to address this limitation, but introduces additional challenges including system compatibility issues and increased backpressure management requirements.
Mobile phase considerations present another significant challenge for HPLC. The selection of appropriate solvents must balance analyte solubility, detector compatibility, and column chemistry. Additionally, mobile phase degassing remains critical to prevent bubble formation that can disrupt detection systems and compromise quantitative analysis.
Column degradation in HPLC systems occurs through mechanisms including silica dissolution at high pH, stationary phase collapse under pressure, and contamination from sample matrices. This degradation manifests as peak broadening, retention time shifts, and decreased resolution, ultimately requiring costly column replacement.
For GC systems, temperature limitations represent a primary constraint. Most conventional columns cannot withstand temperatures exceeding 350-400°C, restricting analysis to compounds with sufficient volatility below these thresholds. High-boiling point compounds often require derivatization, adding complexity and potential for error to analytical workflows.
Carrier gas selection and management present ongoing challenges in GC. While hydrogen offers superior efficiency, safety concerns limit its adoption in many laboratories. Helium, though safer, faces global supply shortages and increasing costs, forcing laboratories to consider alternative gases that may compromise separation efficiency.
Sample introduction in GC presents technical hurdles, particularly for thermally labile compounds that may degrade during injection. Split/splitless injection techniques require careful optimization to prevent discrimination effects that can skew quantitative results, especially for wide-boiling-point range samples.
Detection sensitivity varies significantly between techniques. GC typically offers superior detection limits for volatile compounds when coupled with sensitive detectors like electron capture or mass spectrometry. However, HPLC generally provides better performance for polar, non-volatile, and thermally unstable compounds, though often with higher detection limits.
Both techniques face challenges in achieving consistent retention times. In HPLC, variations in mobile phase composition, temperature fluctuations, and column aging contribute to retention time drift. GC systems experience similar issues due to carrier gas flow inconsistencies, column degradation, and temperature program reproducibility limitations, all of which complicate qualitative identification and quantitative analysis.
HPLC systems encounter challenges related to pressure limitations, with conventional systems typically operating below 6,000 psi. This constraint restricts column efficiency and resolution capabilities, particularly when analyzing complex mixtures. Ultra-high-performance liquid chromatography (UHPLC) has emerged to address this limitation, but introduces additional challenges including system compatibility issues and increased backpressure management requirements.
Mobile phase considerations present another significant challenge for HPLC. The selection of appropriate solvents must balance analyte solubility, detector compatibility, and column chemistry. Additionally, mobile phase degassing remains critical to prevent bubble formation that can disrupt detection systems and compromise quantitative analysis.
Column degradation in HPLC systems occurs through mechanisms including silica dissolution at high pH, stationary phase collapse under pressure, and contamination from sample matrices. This degradation manifests as peak broadening, retention time shifts, and decreased resolution, ultimately requiring costly column replacement.
For GC systems, temperature limitations represent a primary constraint. Most conventional columns cannot withstand temperatures exceeding 350-400°C, restricting analysis to compounds with sufficient volatility below these thresholds. High-boiling point compounds often require derivatization, adding complexity and potential for error to analytical workflows.
Carrier gas selection and management present ongoing challenges in GC. While hydrogen offers superior efficiency, safety concerns limit its adoption in many laboratories. Helium, though safer, faces global supply shortages and increasing costs, forcing laboratories to consider alternative gases that may compromise separation efficiency.
Sample introduction in GC presents technical hurdles, particularly for thermally labile compounds that may degrade during injection. Split/splitless injection techniques require careful optimization to prevent discrimination effects that can skew quantitative results, especially for wide-boiling-point range samples.
Detection sensitivity varies significantly between techniques. GC typically offers superior detection limits for volatile compounds when coupled with sensitive detectors like electron capture or mass spectrometry. However, HPLC generally provides better performance for polar, non-volatile, and thermally unstable compounds, though often with higher detection limits.
Both techniques face challenges in achieving consistent retention times. In HPLC, variations in mobile phase composition, temperature fluctuations, and column aging contribute to retention time drift. GC systems experience similar issues due to carrier gas flow inconsistencies, column degradation, and temperature program reproducibility limitations, all of which complicate qualitative identification and quantitative analysis.
Current Retention Time Optimization Approaches
01 HPLC method optimization for improved retention time accuracy
High-Performance Liquid Chromatography (HPLC) methods can be optimized to improve retention time accuracy and effectiveness. This involves adjusting parameters such as mobile phase composition, flow rate, column temperature, and pH to enhance separation efficiency. Optimized HPLC methods provide better resolution, sensitivity, and reproducibility, which are crucial for accurate compound identification and quantification in complex samples.- HPLC method optimization for improved retention time accuracy: High-Performance Liquid Chromatography (HPLC) methods can be optimized to improve retention time accuracy and effectiveness. This involves adjusting parameters such as mobile phase composition, flow rate, column temperature, and pH to enhance separation efficiency. Optimized HPLC methods provide better resolution, shorter analysis times, and more reliable identification of compounds based on their retention characteristics.
- GC retention time correlation with compound properties: Gas Chromatography (GC) retention times correlate with specific compound properties such as boiling point, molecular weight, and polarity. This relationship allows for predictive analysis and compound identification. By understanding these correlations, researchers can develop more effective analytical methods and improve the accuracy of compound identification in complex mixtures.
- Combined HPLC-GC techniques for comprehensive analysis: Combining HPLC and GC techniques provides comprehensive analytical capabilities for complex samples. This approach leverages the strengths of both methods: HPLC's ability to analyze non-volatile compounds and GC's superior separation of volatile components. Integrated systems or sequential analysis protocols enable more complete characterization of samples, improved detection limits, and enhanced identification of compounds with varying physicochemical properties.
- Retention time standardization and calibration methods: Standardization and calibration methods are essential for ensuring reliable retention time data in both HPLC and GC analyses. These methods include the use of internal standards, retention indices, and reference compounds to normalize retention times across different instruments and conditions. Proper standardization improves the reproducibility of analyses, facilitates inter-laboratory comparisons, and enhances the effectiveness of compound identification.
- Advanced detection systems for enhancing chromatographic effectiveness: Advanced detection systems significantly enhance the effectiveness of both HPLC and GC analyses. These include mass spectrometry (MS), diode array detection (DAD), flame ionization detection (FID), and other specialized detectors. Integration of these detection technologies with chromatographic methods improves sensitivity, selectivity, and the ability to identify compounds based on both retention time and spectral characteristics, leading to more comprehensive analytical results.
02 GC retention time correlation with compound properties
Gas Chromatography (GC) retention times correlate with specific compound properties such as volatility, polarity, and molecular weight. This relationship can be utilized to predict compound behavior and improve analytical effectiveness. By understanding these correlations, analysts can develop more efficient separation methods, optimize temperature programs, and select appropriate stationary phases to enhance the resolution and identification of target compounds.Expand Specific Solutions03 Combined HPLC-GC techniques for comprehensive analysis
Combining HPLC and GC techniques provides comprehensive analytical capabilities for complex samples. This approach leverages the strengths of both methods: HPLC for non-volatile, polar compounds and GC for volatile, thermally stable compounds. Integrated systems or sequential analysis protocols enable more complete characterization of samples, improved compound identification, and enhanced quantification accuracy across a wider range of chemical properties.Expand Specific Solutions04 Retention time standardization and calibration methods
Standardization and calibration methods are essential for ensuring reliable retention time data in both HPLC and GC analyses. These methods include the use of internal standards, retention indices, and reference compounds to normalize retention times across different instruments and conditions. Proper calibration enhances the reproducibility of analyses, facilitates inter-laboratory comparisons, and improves the accuracy of compound identification in complex matrices.Expand Specific Solutions05 Advanced detection systems for enhanced chromatographic effectiveness
Advanced detection systems significantly enhance the effectiveness of both HPLC and GC analyses. These include mass spectrometry (MS), diode array detection (DAD), flame ionization detection (FID), and other specialized detectors that provide additional structural information beyond retention time. The integration of these detection technologies with chromatographic separation improves compound identification confidence, lowers detection limits, and enables more accurate quantification, particularly for complex or trace-level analyses.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The HPLC and GC analytical techniques market is in a mature growth phase, with an estimated global value exceeding $10 billion. Agilent Technologies and Thermo Fisher Scientific dominate this landscape, with significant contributions from F. Hoffmann-La Roche and Merck Patent GmbH. The technology has reached high maturity levels, evidenced by widespread adoption in pharmaceutical companies like Bristol Myers Squibb and Ironwood Pharmaceuticals for retention time analysis and compound separation. Academic institutions including the University of Zurich and University of Queensland contribute to ongoing methodological improvements. The competitive advantage increasingly shifts toward integrated software solutions and specialized applications, with companies like Hitachi and Samsung Electronics incorporating AI-driven analytics to enhance chromatography effectiveness.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has developed comprehensive HPLC and GC solutions with advanced retention time precision technologies. Their InfinityLab LC Series offers sub-second retention time reproducibility through precise solvent mixing and temperature control systems. Their Intelligent System Emulation Technology (ISET) allows method transfer between different instruments while maintaining consistent retention times. For GC analysis, Agilent's Intuvo 9000 GC system features direct heating technology that eliminates traditional column ovens, reducing thermal mass and enabling faster temperature ramping with more stable retention times. Their J&W GC columns provide industry-leading inertness and column-to-column reproducibility with retention time RSDs typically below 0.5%. Agilent's OpenLab CDS software incorporates retention time locking (RTL) algorithms that automatically adjust parameters to maintain consistent retention times despite column aging or maintenance procedures.
Strengths: Industry-leading retention time precision and reproducibility across both platforms; comprehensive software integration for method development and transfer; extensive column chemistry options for diverse applications. Weaknesses: Higher initial investment costs compared to some competitors; proprietary consumables can increase operational expenses; complex systems may require specialized training.
Bristol Myers Squibb Co.
Technical Solution: Bristol Myers Squibb has developed sophisticated analytical platforms comparing HPLC and GC technologies for pharmaceutical development and manufacturing quality control. Their approach includes automated method development systems that systematically evaluate retention behavior across both techniques to determine optimal separation strategies for complex drug substances and products. BMS has implemented Quality by Design (QbD) principles incorporating retention time robustness testing under varied conditions to establish method control strategies. Their analytical laboratories utilize dual-platform workflows with standardized sample preparation protocols that minimize variability between techniques. BMS has pioneered the application of ultra-high performance supercritical fluid chromatography (UHPSFC) as a complementary technique that bridges the capabilities of HPLC and GC, offering unique selectivity with retention characteristics influenced by both partition and adsorption mechanisms. Their analytical method lifecycle management system includes continuous monitoring of retention time trends to detect system performance changes before they impact data quality.
Strengths: Extensive experience with regulatory compliance for chromatographic methods; sophisticated method development capabilities; strong integration of analytical data with manufacturing processes. Weaknesses: Technologies primarily developed for internal use rather than commercial applications; solutions often customized for specific products rather than general-purpose applications; limited public disclosure of proprietary methodologies.
Key Separation Mechanism Innovations
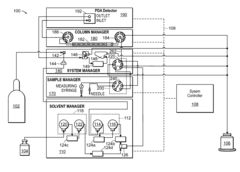
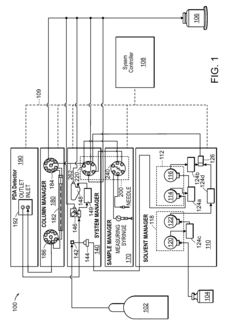
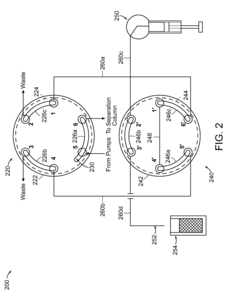
Gas liquid separator and associated methods
PatentActiveEP3532835A1
Innovation
- Incorporating sensors to monitor solvent and gas levels within the chamber, coupled with a pressure regulator or on/off valve to adjust pressure and flow, ensuring balanced separation by preventing gas from being discharged through the solvent outlet and solvent from being discharged through the gas outlet.
System and method for rapid analysis of polycyclic aromatic hydrocarbons
PatentActiveUS20150089997A1
Innovation
- A CO2-based chromatography system using a stationary phase with particle sizes of 0.5 to 3.5 μm and a mobile phase with a pre-column dwell volume of 75 μL to 500 μL, allowing for rapid and accurate detection of PAHs without derivatization and solvent exchange, using a mixture of liquid CO2 and modifiers to achieve high resolution chromatograms in less than 5 minutes.
Method Validation and Quality Control Standards
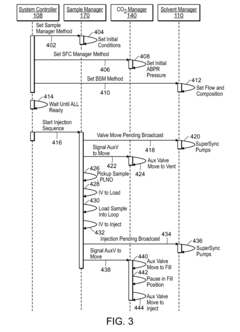
Method validation is a critical component in analytical chemistry, particularly when comparing techniques like HPLC (High-Performance Liquid Chromatography) and GC (Gas Chromatography). Both methodologies require rigorous validation protocols to ensure reliable retention time measurements and overall effectiveness in analytical applications.
The validation process for HPLC and GC methods follows internationally recognized guidelines established by regulatory bodies such as ICH (International Conference on Harmonization), FDA (Food and Drug Administration), and USP (United States Pharmacopeia). These standards typically require assessment of specificity, linearity, accuracy, precision, detection limit, quantitation limit, range, robustness, and system suitability.
For retention time validation, system suitability tests are particularly important. These tests verify that the chromatographic system is performing adequately before and during analysis. Key parameters include retention time reproducibility, resolution between critical pairs, tailing factor, and theoretical plate count. HPLC methods typically require RSD (Relative Standard Deviation) values below 1% for retention time reproducibility, while GC methods may allow slightly higher variability due to temperature programming effects.
Quality control standards differ somewhat between HPLC and GC applications. HPLC quality control often emphasizes mobile phase composition consistency, pump pressure stability, and detector response linearity. GC quality control focuses more on temperature program reproducibility, carrier gas flow stability, and injection technique consistency. Both techniques require regular calibration using certified reference materials traceable to international standards.
Internal quality control procedures for both techniques include the use of control charts to monitor system performance over time. These charts track critical parameters such as retention time drift, peak area response, and resolution between adjacent peaks. Statistical process control methodologies help identify trends before they become problematic, allowing preventive maintenance rather than reactive troubleshooting.
Method transfer protocols represent another important aspect of validation, particularly when comparing analytical techniques across different laboratories. These protocols ensure that methods developed on one instrument can be successfully implemented on another with equivalent results. For HPLC and GC comparisons, this often involves collaborative studies where identical samples are analyzed using both techniques to establish correlation factors and identify potential systematic biases.
Robustness testing evaluates how small, deliberate variations in method parameters affect retention time and overall effectiveness. For HPLC, this might include changes in mobile phase composition, pH, column temperature, and flow rate. For GC, robustness testing typically examines variations in temperature program, carrier gas flow, and injection parameters. Methods that demonstrate high robustness are generally preferred for routine quality control applications.
The validation process for HPLC and GC methods follows internationally recognized guidelines established by regulatory bodies such as ICH (International Conference on Harmonization), FDA (Food and Drug Administration), and USP (United States Pharmacopeia). These standards typically require assessment of specificity, linearity, accuracy, precision, detection limit, quantitation limit, range, robustness, and system suitability.
For retention time validation, system suitability tests are particularly important. These tests verify that the chromatographic system is performing adequately before and during analysis. Key parameters include retention time reproducibility, resolution between critical pairs, tailing factor, and theoretical plate count. HPLC methods typically require RSD (Relative Standard Deviation) values below 1% for retention time reproducibility, while GC methods may allow slightly higher variability due to temperature programming effects.
Quality control standards differ somewhat between HPLC and GC applications. HPLC quality control often emphasizes mobile phase composition consistency, pump pressure stability, and detector response linearity. GC quality control focuses more on temperature program reproducibility, carrier gas flow stability, and injection technique consistency. Both techniques require regular calibration using certified reference materials traceable to international standards.
Internal quality control procedures for both techniques include the use of control charts to monitor system performance over time. These charts track critical parameters such as retention time drift, peak area response, and resolution between adjacent peaks. Statistical process control methodologies help identify trends before they become problematic, allowing preventive maintenance rather than reactive troubleshooting.
Method transfer protocols represent another important aspect of validation, particularly when comparing analytical techniques across different laboratories. These protocols ensure that methods developed on one instrument can be successfully implemented on another with equivalent results. For HPLC and GC comparisons, this often involves collaborative studies where identical samples are analyzed using both techniques to establish correlation factors and identify potential systematic biases.
Robustness testing evaluates how small, deliberate variations in method parameters affect retention time and overall effectiveness. For HPLC, this might include changes in mobile phase composition, pH, column temperature, and flow rate. For GC, robustness testing typically examines variations in temperature program, carrier gas flow, and injection parameters. Methods that demonstrate high robustness are generally preferred for routine quality control applications.
Environmental Impact and Green Chromatography
Chromatographic techniques, while essential for analytical chemistry, have significant environmental implications that warrant careful consideration. Both High-Performance Liquid Chromatography (HPLC) and Gas Chromatography (GC) consume substantial resources and generate waste that impacts our ecosystem. HPLC typically requires large volumes of organic solvents, with a single analysis potentially consuming 0.5-2 liters of solvents like acetonitrile, methanol, or tetrahydrofuran—chemicals that pose environmental hazards when improperly disposed.
GC systems, while using fewer solvents, rely on carrier gases and often require sample derivatization using toxic reagents. Additionally, both techniques consume significant electrical energy during operation, with HPLC systems requiring 1-3 kWh per day and GC systems consuming 1.5-4 kWh daily, contributing to their carbon footprint.
The emergence of green chromatography represents a paradigm shift toward environmentally responsible analytical practices. This approach encompasses several strategies to reduce environmental impact while maintaining analytical effectiveness. Miniaturization technologies like micro-HPLC and capillary GC have reduced solvent and carrier gas consumption by up to 90% compared to conventional systems, while maintaining comparable retention time precision and separation effectiveness.
Solvent selection innovations have introduced greener alternatives to traditional toxic organic solvents. Water-based mobile phases, supercritical CO2, and bio-derived solvents like ethyl lactate have demonstrated promising separation capabilities in HPLC applications, reducing hazardous waste generation while achieving retention time stability within acceptable parameters for many applications.
Energy efficiency improvements have also been implemented in modern chromatographic systems. Low-thermal-mass GC ovens reduce power consumption by 40-60%, while ambient temperature HPLC techniques eliminate the need for column heating in certain applications. These advancements maintain separation effectiveness while significantly reducing the carbon footprint of analytical procedures.
Waste management protocols have evolved to include solvent recycling systems that can recover up to 80% of HPLC mobile phases for reuse. Additionally, advanced waste treatment technologies neutralize toxic components before disposal, minimizing environmental contamination. These practices address the end-of-life concerns for chromatographic waste without compromising analytical quality.
The comparative environmental impact assessment between HPLC and GC reveals that the choice between these techniques should consider not only retention time and separation effectiveness but also ecological footprint. While GC generally consumes less solvent, its energy requirements and specialized gas needs present different environmental challenges than HPLC's solvent-intensive processes. The selection should be application-specific, with green chromatography principles applied to whichever technique is chosen.
GC systems, while using fewer solvents, rely on carrier gases and often require sample derivatization using toxic reagents. Additionally, both techniques consume significant electrical energy during operation, with HPLC systems requiring 1-3 kWh per day and GC systems consuming 1.5-4 kWh daily, contributing to their carbon footprint.
The emergence of green chromatography represents a paradigm shift toward environmentally responsible analytical practices. This approach encompasses several strategies to reduce environmental impact while maintaining analytical effectiveness. Miniaturization technologies like micro-HPLC and capillary GC have reduced solvent and carrier gas consumption by up to 90% compared to conventional systems, while maintaining comparable retention time precision and separation effectiveness.
Solvent selection innovations have introduced greener alternatives to traditional toxic organic solvents. Water-based mobile phases, supercritical CO2, and bio-derived solvents like ethyl lactate have demonstrated promising separation capabilities in HPLC applications, reducing hazardous waste generation while achieving retention time stability within acceptable parameters for many applications.
Energy efficiency improvements have also been implemented in modern chromatographic systems. Low-thermal-mass GC ovens reduce power consumption by 40-60%, while ambient temperature HPLC techniques eliminate the need for column heating in certain applications. These advancements maintain separation effectiveness while significantly reducing the carbon footprint of analytical procedures.
Waste management protocols have evolved to include solvent recycling systems that can recover up to 80% of HPLC mobile phases for reuse. Additionally, advanced waste treatment technologies neutralize toxic components before disposal, minimizing environmental contamination. These practices address the end-of-life concerns for chromatographic waste without compromising analytical quality.
The comparative environmental impact assessment between HPLC and GC reveals that the choice between these techniques should consider not only retention time and separation effectiveness but also ecological footprint. While GC generally consumes less solvent, its energy requirements and specialized gas needs present different environmental challenges than HPLC's solvent-intensive processes. The selection should be application-specific, with green chromatography principles applied to whichever technique is chosen.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!