HPLC vs CE Efficiency: Assessing Sample Purity Reach
SEP 19, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC and CE Technology Evolution and Objectives
High-Performance Liquid Chromatography (HPLC) and Capillary Electrophoresis (CE) have evolved significantly since their inception, representing two cornerstone analytical techniques in modern laboratory science. HPLC emerged in the late 1960s as an advancement of traditional column chromatography, with the first commercial HPLC system introduced by Waters Corporation in 1969. This innovation marked a paradigm shift in analytical chemistry by enabling faster, more precise separations under high pressure.
CE technology developed later, gaining prominence in the 1980s as researchers sought alternatives to gel electrophoresis for biomolecule analysis. The technique's evolution was accelerated by advancements in capillary manufacturing and detection systems, allowing for increasingly sensitive analyses with minimal sample volumes—a critical advantage over traditional methods.
The technological trajectory of both methods has been characterized by continuous improvements in sensitivity, resolution, and automation. HPLC systems have progressed from conventional designs to ultra-high-performance liquid chromatography (UHPLC), featuring sub-2μm particle columns that dramatically enhance separation efficiency and reduce analysis time. Similarly, CE has evolved to incorporate various modes including capillary zone electrophoresis (CZE), micellar electrokinetic chromatography (MEKC), and capillary gel electrophoresis (CGE).
Recent technological advancements have focused on miniaturization, with nano-HPLC and microchip CE systems representing the cutting edge of separation science. These innovations address the growing demand for analyses of increasingly complex samples with limited availability, particularly in proteomics and metabolomics research.
The primary objective in comparing HPLC and CE efficiency for sample purity assessment is to establish a comprehensive framework for selecting the optimal analytical technique based on specific sample characteristics and purity requirements. This includes evaluating separation mechanisms, detection limits, sample throughput, and the ability to resolve closely related compounds or impurities.
Additional objectives include quantifying the relative advantages of each technique across different application domains, from pharmaceutical quality control to environmental monitoring and clinical diagnostics. Understanding how each technology performs under varying conditions enables more informed decision-making in analytical method development.
The technological evolution continues with integration of advanced detection systems, including mass spectrometry, which has dramatically expanded the capabilities of both HPLC and CE. These hyphenated techniques offer unprecedented insights into sample composition and purity, though they present unique challenges in method development and data interpretation.
Looking forward, the field is moving toward greater integration of artificial intelligence and machine learning algorithms to optimize separation parameters and interpret complex analytical data, potentially revolutionizing how scientists approach sample purity assessment across diverse industries.
CE technology developed later, gaining prominence in the 1980s as researchers sought alternatives to gel electrophoresis for biomolecule analysis. The technique's evolution was accelerated by advancements in capillary manufacturing and detection systems, allowing for increasingly sensitive analyses with minimal sample volumes—a critical advantage over traditional methods.
The technological trajectory of both methods has been characterized by continuous improvements in sensitivity, resolution, and automation. HPLC systems have progressed from conventional designs to ultra-high-performance liquid chromatography (UHPLC), featuring sub-2μm particle columns that dramatically enhance separation efficiency and reduce analysis time. Similarly, CE has evolved to incorporate various modes including capillary zone electrophoresis (CZE), micellar electrokinetic chromatography (MEKC), and capillary gel electrophoresis (CGE).
Recent technological advancements have focused on miniaturization, with nano-HPLC and microchip CE systems representing the cutting edge of separation science. These innovations address the growing demand for analyses of increasingly complex samples with limited availability, particularly in proteomics and metabolomics research.
The primary objective in comparing HPLC and CE efficiency for sample purity assessment is to establish a comprehensive framework for selecting the optimal analytical technique based on specific sample characteristics and purity requirements. This includes evaluating separation mechanisms, detection limits, sample throughput, and the ability to resolve closely related compounds or impurities.
Additional objectives include quantifying the relative advantages of each technique across different application domains, from pharmaceutical quality control to environmental monitoring and clinical diagnostics. Understanding how each technology performs under varying conditions enables more informed decision-making in analytical method development.
The technological evolution continues with integration of advanced detection systems, including mass spectrometry, which has dramatically expanded the capabilities of both HPLC and CE. These hyphenated techniques offer unprecedented insights into sample composition and purity, though they present unique challenges in method development and data interpretation.
Looking forward, the field is moving toward greater integration of artificial intelligence and machine learning algorithms to optimize separation parameters and interpret complex analytical data, potentially revolutionizing how scientists approach sample purity assessment across diverse industries.
Market Demand for Advanced Analytical Separation Methods
The analytical separation methods market is experiencing robust growth driven by increasing demands across pharmaceutical, biotechnology, and clinical diagnostic sectors. Current market valuations place the global analytical instrumentation market at approximately 85 billion USD, with separation technologies representing a significant segment growing at a compound annual rate of 6-7%. Within this landscape, both High-Performance Liquid Chromatography (HPLC) and Capillary Electrophoresis (CE) have established themselves as critical technologies for sample purity assessment.
Pharmaceutical companies constitute the largest market segment, where stringent regulatory requirements necessitate advanced analytical methods for drug development and quality control. The FDA and EMA continue to raise standards for analytical testing, creating sustained demand for higher resolution separation technologies. This regulatory pressure has translated into market expansion, with pharmaceutical analytical testing services growing at nearly 8% annually.
Biotechnology applications represent the fastest-growing segment, particularly in protein and nucleic acid analysis, where sample purity determination directly impacts research outcomes and product quality. The rise of biologics and biosimilars has intensified the need for sophisticated separation methods capable of detecting minute impurities in complex biological matrices.
Academic and research institutions form another substantial market segment, collectively investing billions in analytical equipment annually. Their requirements focus on versatility and research-grade performance rather than throughput, creating distinct market demands compared to industrial applications.
Geographically, North America leads the market with approximately 40% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region demonstrates the highest growth rate, driven by expanding pharmaceutical manufacturing, contract research organizations, and increasing R&D investments in China, India, and South Korea.
Customer preferences are evolving toward integrated analytical systems that combine separation technologies with advanced detection methods. There is growing demand for systems offering higher sensitivity, improved resolution, faster analysis times, and reduced sample volume requirements. Additionally, automation capabilities and data integration with laboratory information management systems have become key purchasing factors.
Cost considerations remain significant market drivers, with total cost of ownership increasingly influencing purchasing decisions. While HPLC systems generally command higher initial investments, CE systems often present advantages in operational costs and consumables, creating distinct market positioning for each technology based on customer priorities and application requirements.
Pharmaceutical companies constitute the largest market segment, where stringent regulatory requirements necessitate advanced analytical methods for drug development and quality control. The FDA and EMA continue to raise standards for analytical testing, creating sustained demand for higher resolution separation technologies. This regulatory pressure has translated into market expansion, with pharmaceutical analytical testing services growing at nearly 8% annually.
Biotechnology applications represent the fastest-growing segment, particularly in protein and nucleic acid analysis, where sample purity determination directly impacts research outcomes and product quality. The rise of biologics and biosimilars has intensified the need for sophisticated separation methods capable of detecting minute impurities in complex biological matrices.
Academic and research institutions form another substantial market segment, collectively investing billions in analytical equipment annually. Their requirements focus on versatility and research-grade performance rather than throughput, creating distinct market demands compared to industrial applications.
Geographically, North America leads the market with approximately 40% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region demonstrates the highest growth rate, driven by expanding pharmaceutical manufacturing, contract research organizations, and increasing R&D investments in China, India, and South Korea.
Customer preferences are evolving toward integrated analytical systems that combine separation technologies with advanced detection methods. There is growing demand for systems offering higher sensitivity, improved resolution, faster analysis times, and reduced sample volume requirements. Additionally, automation capabilities and data integration with laboratory information management systems have become key purchasing factors.
Cost considerations remain significant market drivers, with total cost of ownership increasingly influencing purchasing decisions. While HPLC systems generally command higher initial investments, CE systems often present advantages in operational costs and consumables, creating distinct market positioning for each technology based on customer priorities and application requirements.
Current Challenges in Chromatographic and Electrophoretic Techniques
Despite significant advancements in analytical chemistry, both High-Performance Liquid Chromatography (HPLC) and Capillary Electrophoresis (CE) face persistent challenges that limit their effectiveness in assessing sample purity. These challenges span technical, operational, and fundamental aspects of the methodologies, creating barriers to achieving optimal analytical outcomes.
Resolution limitations remain a critical issue for both techniques. While modern HPLC columns can achieve impressive theoretical plate counts, complex biological samples often contain closely related compounds with similar physicochemical properties that prove difficult to separate completely. CE systems, though theoretically capable of higher resolution, struggle with reproducibility issues that compromise this advantage in routine applications.
Sample matrix effects present significant obstacles, particularly when analyzing biological or environmental samples. In HPLC, matrix components can cause ion suppression, adsorption to stationary phases, or column fouling, leading to reduced sensitivity and shortened column lifespans. CE is even more susceptible to matrix effects due to its lower sample capacity, with proteins and other high-molecular-weight compounds potentially altering the electroosmotic flow and disrupting separations.
Detection sensitivity disparities between the techniques create application-specific challenges. HPLC generally offers superior sensitivity with conventional detectors, but CE's smaller sample volumes inherently limit detection capabilities, particularly when analyzing trace components in complex matrices. This sensitivity gap narrows with specialized detection methods but remains a fundamental constraint.
Method development complexity continues to challenge analysts. HPLC method optimization requires balancing multiple parameters including mobile phase composition, flow rate, temperature, and column chemistry. CE adds additional variables such as buffer composition, applied voltage, and capillary coating considerations, making systematic optimization time-consuming and often empirical rather than theoretical.
Throughput and automation limitations affect both techniques differently. HPLC typically requires longer analysis times due to pressure constraints and column equilibration requirements. While CE offers faster separations, it struggles with automation reliability, particularly in high-throughput environments where robust performance is essential.
Quantification accuracy presents ongoing challenges, especially at trace levels. Both techniques face issues with linear dynamic range limitations, though HPLC generally provides wider working ranges. CE suffers from injection volume variability that can compromise quantitative precision, requiring careful internal standardization protocols that add complexity to analytical workflows.
Instrument robustness differences impact routine laboratory operations. HPLC systems have matured to provide consistent performance across various applications, while CE instruments remain more sensitive to environmental conditions and operator expertise, limiting widespread adoption despite theoretical advantages in certain applications.
Resolution limitations remain a critical issue for both techniques. While modern HPLC columns can achieve impressive theoretical plate counts, complex biological samples often contain closely related compounds with similar physicochemical properties that prove difficult to separate completely. CE systems, though theoretically capable of higher resolution, struggle with reproducibility issues that compromise this advantage in routine applications.
Sample matrix effects present significant obstacles, particularly when analyzing biological or environmental samples. In HPLC, matrix components can cause ion suppression, adsorption to stationary phases, or column fouling, leading to reduced sensitivity and shortened column lifespans. CE is even more susceptible to matrix effects due to its lower sample capacity, with proteins and other high-molecular-weight compounds potentially altering the electroosmotic flow and disrupting separations.
Detection sensitivity disparities between the techniques create application-specific challenges. HPLC generally offers superior sensitivity with conventional detectors, but CE's smaller sample volumes inherently limit detection capabilities, particularly when analyzing trace components in complex matrices. This sensitivity gap narrows with specialized detection methods but remains a fundamental constraint.
Method development complexity continues to challenge analysts. HPLC method optimization requires balancing multiple parameters including mobile phase composition, flow rate, temperature, and column chemistry. CE adds additional variables such as buffer composition, applied voltage, and capillary coating considerations, making systematic optimization time-consuming and often empirical rather than theoretical.
Throughput and automation limitations affect both techniques differently. HPLC typically requires longer analysis times due to pressure constraints and column equilibration requirements. While CE offers faster separations, it struggles with automation reliability, particularly in high-throughput environments where robust performance is essential.
Quantification accuracy presents ongoing challenges, especially at trace levels. Both techniques face issues with linear dynamic range limitations, though HPLC generally provides wider working ranges. CE suffers from injection volume variability that can compromise quantitative precision, requiring careful internal standardization protocols that add complexity to analytical workflows.
Instrument robustness differences impact routine laboratory operations. HPLC systems have matured to provide consistent performance across various applications, while CE instruments remain more sensitive to environmental conditions and operator expertise, limiting widespread adoption despite theoretical advantages in certain applications.
Comparative Analysis of HPLC and CE Methodologies
01 Advanced separation techniques for improved sample purity
High-Performance Liquid Chromatography (HPLC) and Capillary Electrophoresis (CE) can be enhanced through advanced separation techniques to achieve higher sample purity. These techniques include optimized column selection, buffer composition adjustments, and gradient elution methods that effectively separate complex mixtures. By implementing these advanced separation approaches, researchers can significantly improve resolution and reduce interference from contaminants, leading to more accurate analytical results and higher sample purity.- Advanced column technologies for improved separation efficiency: Novel column designs and materials enhance separation efficiency in both HPLC and CE systems. These advancements include specialized stationary phases, monolithic columns, and modified capillaries that improve resolution, reduce analysis time, and increase sample purity. The technologies enable better separation of complex mixtures and detection of trace impurities, significantly improving the overall analytical performance.
- Sample preparation techniques for enhanced purity: Various sample preparation methods are developed to improve sample purity before HPLC and CE analysis. These techniques include filtration systems, solid-phase extraction, liquid-liquid extraction, and pre-concentration methods that remove interfering substances and concentrate analytes of interest. Proper sample preparation significantly reduces matrix effects and improves detection limits, resulting in more accurate and reliable analytical results.
- Integration of detection systems for improved sensitivity: Advanced detection systems integrated with HPLC and CE improve sensitivity and selectivity in analytical measurements. These include mass spectrometry coupling, diode array detection, fluorescence detection, and electrochemical detection systems that enhance the ability to identify and quantify trace components in complex samples. The integration allows for lower detection limits and better characterization of sample purity.
- Automated systems and microfluidic technologies: Automated sample handling systems and microfluidic platforms improve reproducibility and efficiency in HPLC and CE analyses. These technologies include robotic sample preparation, automated injection systems, and lab-on-a-chip devices that minimize human error and sample contamination. The miniaturization and automation lead to faster analyses, reduced solvent consumption, and improved sample throughput while maintaining high separation efficiency.
- Method optimization strategies for specific applications: Specialized method development approaches optimize HPLC and CE conditions for specific sample types and analytical goals. These strategies include gradient optimization, buffer selection, temperature control, and voltage programming that enhance separation efficiency for particular analytes. The optimization techniques are tailored to specific industries such as pharmaceuticals, environmental analysis, and food safety, ensuring maximum sample purity and analytical efficiency for the intended application.
02 Innovative detection systems for enhanced efficiency
Integration of innovative detection systems with HPLC and CE technologies enables more efficient sample analysis and purity determination. These systems include advanced UV-Vis detectors, mass spectrometry coupling, fluorescence detection, and electrochemical sensors that provide higher sensitivity and selectivity. The improved detection capabilities allow for identification of trace impurities and more accurate quantification of target compounds, thereby enhancing the overall efficiency of analytical processes and ensuring higher sample purity standards.Expand Specific Solutions03 Automated sample preparation and handling systems
Automated sample preparation and handling systems significantly improve the efficiency and reproducibility of HPLC and CE analyses. These systems incorporate robotic sample loading, precise injection mechanisms, and automated fraction collection that minimize human error and sample contamination. The automation of these critical steps ensures consistent sample treatment, reduces analysis time, and increases throughput while maintaining high purity standards across multiple samples.Expand Specific Solutions04 Novel stationary phases and buffer compositions
Development of novel stationary phases and optimized buffer compositions has revolutionized HPLC and CE separation capabilities. These innovations include specialized polymer-based matrices, functionalized silica materials, and custom-designed buffer systems that provide enhanced selectivity for specific compounds. The tailored interaction between samples and these advanced materials allows for better resolution of complex mixtures, improved peak shapes, and ultimately higher sample purity in analytical and preparative applications.Expand Specific Solutions05 Miniaturization and microfluidic technologies
Miniaturization and microfluidic technologies have transformed HPLC and CE systems, offering advantages in efficiency and sample purity. These compact systems require smaller sample volumes, reduce solvent consumption, and provide faster analysis times while maintaining or improving separation performance. The reduced internal volumes minimize sample dilution and dispersion, resulting in sharper peaks, better resolution, and ultimately higher purity of isolated compounds with improved detection limits.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Analytical Chemistry
The HPLC vs CE efficiency landscape is currently in a mature development phase, with the global analytical instrumentation market exceeding $5 billion annually. High-Performance Liquid Chromatography (HPLC) dominates with established technical maturity, evidenced by significant innovations from industry leaders like Bio-Rad Laboratories, Genentech, and Daiichi Sankyo. Capillary Electrophoresis (CE) represents a growing segment with approximately 15% market share, showing increasing adoption for specialized applications. Academic institutions including The Regents of the University of California and Zhejiang University are advancing CE technology, while pharmaceutical companies such as Takeda, Vertex, and Novozymes are implementing both technologies in complementary roles for comprehensive sample purity assessment, reflecting the industry's trend toward integrated analytical approaches.
The Regents of the University of California
Technical Solution: The University of California system has made significant contributions to both HPLC and CE technologies through its research institutions. Their innovations in HPLC include the development of ultra-high efficiency monolithic columns that achieve theoretical plate counts exceeding 200,000 plates/meter while maintaining lower backpressure than comparable particulate columns. UC researchers pioneered temperature-responsive chromatography using specially modified stationary phases that change selectivity with small temperature variations, enabling fine-tuned separations without changing mobile phase composition. For capillary electrophoresis, UC laboratories developed advanced microchip CE platforms that integrate sample preparation, separation, and detection in devices smaller than a credit card, reducing sample volume requirements to picoliters and analysis times to seconds. Their innovations in CE detection include ultra-sensitive laser-induced fluorescence systems achieving detection limits in the attomole range, approximately 1000 times more sensitive than conventional UV detection in HPLC. UC researchers have also developed hybrid CE-MS interfaces that overcome traditional compatibility challenges, allowing direct coupling of high-efficiency CE separations with the identification power of mass spectrometry.
Strengths: UC technologies excel in pushing the theoretical limits of separation efficiency, particularly for CE where their systems achieve some of the highest plate counts reported in literature. Their microfluidic platforms offer exceptional performance for limited sample volumes and rapid analysis requirements. Weaknesses: Many of the most advanced technologies remain primarily research tools rather than robust commercial platforms. The specialized nature of some innovations requires significant expertise for implementation, limiting widespread adoption outside academic settings. Some approaches prioritize theoretical performance over practical considerations like throughput and ease of use.
Genentech, Inc.
Technical Solution: Genentech has developed sophisticated analytical platforms combining both HPLC and CE technologies specifically optimized for biotherapeutic characterization and purity assessment. Their HPLC approach utilizes ultra-high performance sub-2μm particle columns coupled with mass spectrometry, achieving peak capacities exceeding 500 for complex protein samples. Their proprietary multi-attribute monitoring methodology integrates quantitative and qualitative assessments of product variants and impurities in a single HPLC-MS workflow, reducing analysis time by approximately 60% compared to traditional multi-method approaches. For CE analysis, Genentech employs capillary isoelectric focusing (cIEF) and capillary zone electrophoresis (CZE) with advanced detection systems that can resolve charge variants differing by as little as 0.02 pH units. Their CE systems achieve theoretical plate counts exceeding 1 million plates/meter, substantially outperforming HPLC for certain applications. Genentech has pioneered orthogonal approaches that leverage both technologies' strengths, using HPLC for robust quantitation of size variants and hydrophobicity-based separations, while employing CE for high-resolution charge variant analysis and glycan characterization.
Strengths: Genentech's analytical platforms excel in characterizing complex biotherapeutics with exceptional resolution and sensitivity. Their integrated data analysis systems provide comprehensive product quality profiles from complementary separation techniques. Their methods are specifically optimized for regulatory submissions with validated procedures that meet ICH guidelines. Weaknesses: Their highly specialized approaches require significant expertise and sophisticated instrumentation. The methods are primarily optimized for protein therapeutics and may require substantial modification for other sample types. The high-end MS-coupled systems represent significant capital investments compared to standard purity assessment technologies.
Key Innovations in Sample Purity Detection Technologies
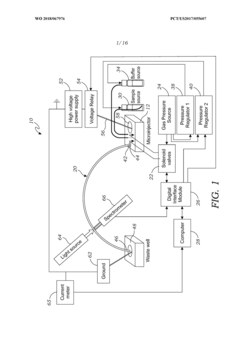
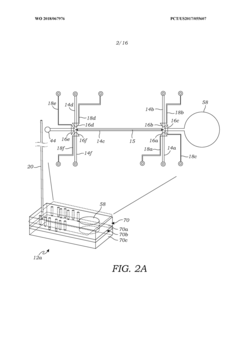
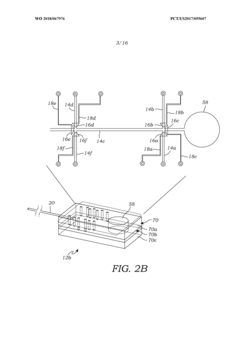
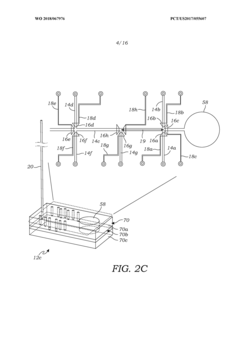
Volumetric micro-injector for capillary electrophoresis
PatentWO2018067976A1
Innovation
- A volumetric micro-injector for CE is developed, featuring a microfluidic injector chip with a defined volume channel and on-chip micro-valves, allowing for precise control of sample injection, eliminating biases and achieving high repeatability by isolating a plug of sample between valves and applying electrophoretic potential for separation.
Method Validation and Quality Assurance Protocols
Method validation and quality assurance protocols are essential components in analytical chemistry, particularly when comparing high-performance liquid chromatography (HPLC) and capillary electrophoresis (CE) for sample purity assessment. These protocols ensure that analytical methods consistently produce reliable and accurate results across different laboratories and over time.
For HPLC validation, the process typically begins with specificity testing to confirm the method's ability to unambiguously assess the analyte in the presence of expected impurities. This is followed by linearity assessment across a minimum of five concentration levels, covering 80-120% of the target concentration. Precision studies must address both repeatability (intra-day) and intermediate precision (inter-day) variations, with acceptance criteria generally set at RSD ≤2% for assay methods.
CE validation protocols share similar parameters but require additional considerations due to the technique's unique characteristics. Migration time reproducibility becomes critical, with acceptance criteria typically more stringent than retention time variations in HPLC. System suitability tests for CE must include buffer conductivity monitoring and capillary conditioning procedures to ensure consistent electroosmotic flow.
Accuracy verification for both techniques requires recovery studies using spiked samples at multiple concentration levels. However, matrix effects assessment becomes particularly important for CE due to its higher sensitivity to sample ionic strength variations. Robustness testing parameters differ significantly between the two techniques - HPLC focuses on mobile phase composition and column temperature, while CE emphasizes buffer pH, applied voltage, and capillary temperature.
Quality control procedures for routine analysis must include regular system suitability tests with appropriate reference standards. For HPLC, these typically involve column efficiency, tailing factor, and resolution parameters. CE quality control emphasizes migration time reproducibility and peak area precision. Both techniques require regular calibration with certified reference materials traceable to international standards.
Documentation requirements for both techniques must comply with regulatory guidelines such as ICH Q2(R1), USP <1225>, and FDA Guidance for Industry. However, CE methods often require more extensive documentation of method development due to the technique's higher sensitivity to experimental conditions. Ongoing method performance verification through control charting becomes essential for both techniques, with warning and action limits established based on validation data.
Transfer of validated methods between laboratories requires comprehensive protocols addressing instrument specifications, analyst training, and comparative testing. This aspect becomes particularly critical when comparing HPLC and CE efficiency, as inter-laboratory reproducibility can vary significantly between these techniques due to differences in instrumentation standardization and operator expertise.
For HPLC validation, the process typically begins with specificity testing to confirm the method's ability to unambiguously assess the analyte in the presence of expected impurities. This is followed by linearity assessment across a minimum of five concentration levels, covering 80-120% of the target concentration. Precision studies must address both repeatability (intra-day) and intermediate precision (inter-day) variations, with acceptance criteria generally set at RSD ≤2% for assay methods.
CE validation protocols share similar parameters but require additional considerations due to the technique's unique characteristics. Migration time reproducibility becomes critical, with acceptance criteria typically more stringent than retention time variations in HPLC. System suitability tests for CE must include buffer conductivity monitoring and capillary conditioning procedures to ensure consistent electroosmotic flow.
Accuracy verification for both techniques requires recovery studies using spiked samples at multiple concentration levels. However, matrix effects assessment becomes particularly important for CE due to its higher sensitivity to sample ionic strength variations. Robustness testing parameters differ significantly between the two techniques - HPLC focuses on mobile phase composition and column temperature, while CE emphasizes buffer pH, applied voltage, and capillary temperature.
Quality control procedures for routine analysis must include regular system suitability tests with appropriate reference standards. For HPLC, these typically involve column efficiency, tailing factor, and resolution parameters. CE quality control emphasizes migration time reproducibility and peak area precision. Both techniques require regular calibration with certified reference materials traceable to international standards.
Documentation requirements for both techniques must comply with regulatory guidelines such as ICH Q2(R1), USP <1225>, and FDA Guidance for Industry. However, CE methods often require more extensive documentation of method development due to the technique's higher sensitivity to experimental conditions. Ongoing method performance verification through control charting becomes essential for both techniques, with warning and action limits established based on validation data.
Transfer of validated methods between laboratories requires comprehensive protocols addressing instrument specifications, analyst training, and comparative testing. This aspect becomes particularly critical when comparing HPLC and CE efficiency, as inter-laboratory reproducibility can vary significantly between these techniques due to differences in instrumentation standardization and operator expertise.
Environmental Impact and Green Chemistry Considerations
The environmental impact of analytical techniques has become increasingly important in modern laboratory practices, with sustainability considerations now playing a crucial role in method selection. When comparing High-Performance Liquid Chromatography (HPLC) and Capillary Electrophoresis (CE) for sample purity assessment, significant differences emerge in their environmental footprints.
HPLC systems typically consume substantial volumes of organic solvents, with a standard analysis requiring 0.5-2 liters of mobile phase per day. These solvents—often acetonitrile, methanol, and tetrahydrofuran—present environmental concerns including toxicity, flammability, and disposal challenges. The environmental burden extends to the energy consumption of HPLC systems, which require high-pressure pumps operating continuously, resulting in electricity usage of approximately 1-2 kWh per day of operation.
In contrast, CE demonstrates considerably greener credentials. The technique utilizes minimal solvent volumes—typically less than 10 mL per day—and predominantly employs aqueous buffer systems rather than organic solvents. This represents a 50-100 fold reduction in solvent consumption compared to HPLC. Additionally, CE systems operate at lower pressures and require less energy, with typical consumption around 0.2-0.5 kWh per day.
Waste generation presents another significant environmental consideration. HPLC methods produce substantial volumes of hazardous waste requiring specialized disposal procedures, while CE generates minimal waste streams that are often less environmentally problematic. Recent life cycle assessment studies indicate that CE has approximately 30-40% lower environmental impact across multiple ecological indicators including carbon footprint and aquatic toxicity potential.
The principles of green chemistry further highlight CE's advantages. CE aligns with several key green chemistry principles, including prevention of waste, atom economy, and safer solvent selection. Recent innovations in both techniques have focused on environmental improvements, with "green HPLC" approaches incorporating solvent recycling systems and miniaturized formats. Similarly, CE has seen advancements in microchip formats that further reduce resource requirements.
Laboratory adoption of greener analytical techniques faces practical challenges including method validation requirements and regulatory considerations. However, the pharmaceutical industry has begun incorporating environmental impact assessments into analytical method selection, with several major companies publishing sustainability metrics for their analytical procedures. This trend suggests growing recognition of environmental considerations as a legitimate factor in choosing between HPLC and CE for purity assessment applications.
HPLC systems typically consume substantial volumes of organic solvents, with a standard analysis requiring 0.5-2 liters of mobile phase per day. These solvents—often acetonitrile, methanol, and tetrahydrofuran—present environmental concerns including toxicity, flammability, and disposal challenges. The environmental burden extends to the energy consumption of HPLC systems, which require high-pressure pumps operating continuously, resulting in electricity usage of approximately 1-2 kWh per day of operation.
In contrast, CE demonstrates considerably greener credentials. The technique utilizes minimal solvent volumes—typically less than 10 mL per day—and predominantly employs aqueous buffer systems rather than organic solvents. This represents a 50-100 fold reduction in solvent consumption compared to HPLC. Additionally, CE systems operate at lower pressures and require less energy, with typical consumption around 0.2-0.5 kWh per day.
Waste generation presents another significant environmental consideration. HPLC methods produce substantial volumes of hazardous waste requiring specialized disposal procedures, while CE generates minimal waste streams that are often less environmentally problematic. Recent life cycle assessment studies indicate that CE has approximately 30-40% lower environmental impact across multiple ecological indicators including carbon footprint and aquatic toxicity potential.
The principles of green chemistry further highlight CE's advantages. CE aligns with several key green chemistry principles, including prevention of waste, atom economy, and safer solvent selection. Recent innovations in both techniques have focused on environmental improvements, with "green HPLC" approaches incorporating solvent recycling systems and miniaturized formats. Similarly, CE has seen advancements in microchip formats that further reduce resource requirements.
Laboratory adoption of greener analytical techniques faces practical challenges including method validation requirements and regulatory considerations. However, the pharmaceutical industry has begun incorporating environmental impact assessments into analytical method selection, with several major companies publishing sustainability metrics for their analytical procedures. This trend suggests growing recognition of environmental considerations as a legitimate factor in choosing between HPLC and CE for purity assessment applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!