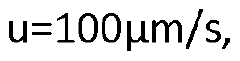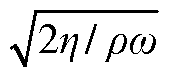Using HPLC Analytics to Maximize Separation Purity
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC Technology Evolution and Objectives
High-Performance Liquid Chromatography (HPLC) has evolved significantly since its inception in the late 1960s, transforming from rudimentary separation techniques to sophisticated analytical platforms. The journey began with the development of packed columns and basic UV detection systems, which laid the foundation for modern chromatographic analysis. By the 1980s, advancements in stationary phase chemistry and pump technology enabled higher pressures and improved separation capabilities, marking the transition to what we now recognize as modern HPLC.
The 1990s witnessed the integration of computerized systems and automation, significantly enhancing reproducibility and throughput. This period also saw the emergence of specialized HPLC variants such as reversed-phase, normal-phase, and ion-exchange chromatography, each addressing specific separation challenges. The early 2000s brought Ultra-High Performance Liquid Chromatography (UHPLC), capable of operating at pressures exceeding 15,000 psi, dramatically improving resolution and reducing analysis time.
Recent technological innovations have focused on miniaturization, with nano-HPLC and micro-HPLC systems offering enhanced sensitivity for trace analysis while consuming minimal sample volumes and solvents. Concurrently, detector technology has progressed from simple UV-Vis systems to sophisticated mass spectrometry interfaces, enabling not just separation but also structural identification of complex analytes.
The primary objective in HPLC development has consistently been maximizing separation purity—the ability to achieve complete resolution between closely related compounds. This goal remains paramount as industries from pharmaceuticals to environmental monitoring require increasingly precise analytical capabilities. Current research aims to enhance separation efficiency through novel stationary phases, including monolithic columns, superficially porous particles, and functionalized materials that offer unique selectivity profiles.
Another critical objective is improving detection sensitivity and specificity, particularly for complex biological samples where target analytes may be present in minute quantities among numerous interferents. This has driven the development of multi-dimensional HPLC techniques that combine orthogonal separation mechanisms to resolve previously inseparable compounds.
Sustainability represents an emerging focus in HPLC evolution, with green chemistry principles driving research toward reduced solvent consumption, environmentally friendly mobile phases, and energy-efficient instrumentation. These efforts align with broader industry trends toward sustainable laboratory practices while maintaining or enhancing analytical performance.
The future trajectory of HPLC technology points toward intelligent systems incorporating machine learning algorithms for method development and optimization, predictive maintenance, and automated troubleshooting. These advancements aim to democratize high-purity separations, making sophisticated analytical capabilities accessible to researchers across diverse fields and expertise levels.
The 1990s witnessed the integration of computerized systems and automation, significantly enhancing reproducibility and throughput. This period also saw the emergence of specialized HPLC variants such as reversed-phase, normal-phase, and ion-exchange chromatography, each addressing specific separation challenges. The early 2000s brought Ultra-High Performance Liquid Chromatography (UHPLC), capable of operating at pressures exceeding 15,000 psi, dramatically improving resolution and reducing analysis time.
Recent technological innovations have focused on miniaturization, with nano-HPLC and micro-HPLC systems offering enhanced sensitivity for trace analysis while consuming minimal sample volumes and solvents. Concurrently, detector technology has progressed from simple UV-Vis systems to sophisticated mass spectrometry interfaces, enabling not just separation but also structural identification of complex analytes.
The primary objective in HPLC development has consistently been maximizing separation purity—the ability to achieve complete resolution between closely related compounds. This goal remains paramount as industries from pharmaceuticals to environmental monitoring require increasingly precise analytical capabilities. Current research aims to enhance separation efficiency through novel stationary phases, including monolithic columns, superficially porous particles, and functionalized materials that offer unique selectivity profiles.
Another critical objective is improving detection sensitivity and specificity, particularly for complex biological samples where target analytes may be present in minute quantities among numerous interferents. This has driven the development of multi-dimensional HPLC techniques that combine orthogonal separation mechanisms to resolve previously inseparable compounds.
Sustainability represents an emerging focus in HPLC evolution, with green chemistry principles driving research toward reduced solvent consumption, environmentally friendly mobile phases, and energy-efficient instrumentation. These efforts align with broader industry trends toward sustainable laboratory practices while maintaining or enhancing analytical performance.
The future trajectory of HPLC technology points toward intelligent systems incorporating machine learning algorithms for method development and optimization, predictive maintenance, and automated troubleshooting. These advancements aim to democratize high-purity separations, making sophisticated analytical capabilities accessible to researchers across diverse fields and expertise levels.
Market Analysis for High-Purity Separation Demands
The global market for high-purity separation technologies has experienced significant growth over the past decade, driven primarily by increasing demands in pharmaceutical, biotechnology, and chemical industries. The HPLC (High-Performance Liquid Chromatography) analytics segment specifically has shown a compound annual growth rate of 6.8% between 2018 and 2023, with projections indicating continued expansion through 2030.
Pharmaceutical applications represent the largest market share for high-purity separation technologies, accounting for approximately 45% of the total market value. This dominance stems from stringent regulatory requirements for drug purity and the growing complexity of pharmaceutical compounds requiring advanced separation techniques. The biologics sector, in particular, has emerged as a key growth driver due to the inherent challenges in purifying large biomolecules.
Biotechnology research institutions constitute the second-largest market segment, with academic and industrial research facilities increasingly adopting sophisticated HPLC systems for both routine and specialized analyses. The food and beverage industry has also shown accelerated adoption rates, particularly for detecting contaminants and ensuring product quality at parts-per-billion levels.
Geographically, North America leads the market with approximately 38% share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region demonstrates the highest growth rate, particularly in China and India, where expanding pharmaceutical manufacturing capabilities and increasing R&D investments are creating substantial demand for high-purity separation technologies.
Customer requirements have evolved significantly, with end-users increasingly prioritizing higher resolution, greater sensitivity, and improved reproducibility in separation processes. There is growing demand for HPLC systems capable of ultra-high performance (UHPLC) that can achieve superior separation efficiency while reducing analysis time and solvent consumption.
The consumables segment, including columns, filters, and solvents, represents a substantial recurring revenue stream, estimated at 60% of the total HPLC market value. Column technology innovations, particularly sub-2-micron particle columns and monolithic columns, have seen increased adoption rates due to their enhanced separation capabilities.
Market trends indicate growing interest in integrated analytical platforms that combine HPLC with mass spectrometry or other detection methods, providing comprehensive analytical solutions. Additionally, there is increasing demand for automated systems with reduced operator intervention requirements, particularly in high-throughput environments such as pharmaceutical quality control laboratories.
Pharmaceutical applications represent the largest market share for high-purity separation technologies, accounting for approximately 45% of the total market value. This dominance stems from stringent regulatory requirements for drug purity and the growing complexity of pharmaceutical compounds requiring advanced separation techniques. The biologics sector, in particular, has emerged as a key growth driver due to the inherent challenges in purifying large biomolecules.
Biotechnology research institutions constitute the second-largest market segment, with academic and industrial research facilities increasingly adopting sophisticated HPLC systems for both routine and specialized analyses. The food and beverage industry has also shown accelerated adoption rates, particularly for detecting contaminants and ensuring product quality at parts-per-billion levels.
Geographically, North America leads the market with approximately 38% share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region demonstrates the highest growth rate, particularly in China and India, where expanding pharmaceutical manufacturing capabilities and increasing R&D investments are creating substantial demand for high-purity separation technologies.
Customer requirements have evolved significantly, with end-users increasingly prioritizing higher resolution, greater sensitivity, and improved reproducibility in separation processes. There is growing demand for HPLC systems capable of ultra-high performance (UHPLC) that can achieve superior separation efficiency while reducing analysis time and solvent consumption.
The consumables segment, including columns, filters, and solvents, represents a substantial recurring revenue stream, estimated at 60% of the total HPLC market value. Column technology innovations, particularly sub-2-micron particle columns and monolithic columns, have seen increased adoption rates due to their enhanced separation capabilities.
Market trends indicate growing interest in integrated analytical platforms that combine HPLC with mass spectrometry or other detection methods, providing comprehensive analytical solutions. Additionally, there is increasing demand for automated systems with reduced operator intervention requirements, particularly in high-throughput environments such as pharmaceutical quality control laboratories.
Current HPLC Limitations and Technical Barriers
Despite significant advancements in HPLC technology, several critical limitations and technical barriers continue to challenge the achievement of maximum separation purity. Column efficiency remains a fundamental constraint, with theoretical plate counts typically limited to 15,000-25,000 plates per column in conventional systems. This limitation directly impacts resolution capabilities, particularly when analyzing complex samples with structurally similar compounds or isomers that exhibit nearly identical physicochemical properties.
Peak capacity presents another significant challenge, especially in one-dimensional separations. Even optimized systems struggle to resolve more than 200-300 compounds in a single run, making comprehensive analysis of biological samples containing thousands of components virtually impossible without multi-dimensional approaches. This limitation becomes particularly evident in proteomics, metabolomics, and natural product analysis.
Sample matrix effects continue to compromise separation purity, with co-eluting matrix components causing ion suppression or enhancement in LC-MS applications. These effects can significantly distort quantitative measurements and lead to false positive or negative results, particularly in complex biological or environmental samples where target analytes exist at trace levels.
Pressure limitations in conventional HPLC systems (typically 400-600 bar) restrict flow rates and column particle size options, forcing compromises between analysis speed and separation efficiency. While UHPLC systems operating at pressures up to 1500 bar have addressed this partially, they introduce additional challenges including system durability issues, frictional heating effects, and increased backpressure that can affect chromatographic performance.
Detector sensitivity and selectivity constraints further impact separation purity assessment. UV-Vis detectors, while robust, lack specificity for compounds with similar chromophores. Mass spectrometric detection offers improved selectivity but suffers from matrix effects and ionization suppression. Additionally, the dynamic range limitations of most detectors (typically 103-106) make it difficult to accurately quantify both major and minor components in a single analysis.
Carryover effects between injections represent another persistent challenge, particularly with hydrophobic compounds that adsorb to system components. Even trace carryover can significantly impact purity determinations in subsequent analyses, especially in quality control applications where detection of low-level impurities is critical.
Finally, method transferability issues between different HPLC systems, columns, and laboratories introduce variability that compromises reproducible purity assessments. Subtle differences in instrument design, column manufacturing, and operating conditions can significantly alter separation selectivity and resolution, making standardization of purity determinations challenging across different analytical platforms.
Peak capacity presents another significant challenge, especially in one-dimensional separations. Even optimized systems struggle to resolve more than 200-300 compounds in a single run, making comprehensive analysis of biological samples containing thousands of components virtually impossible without multi-dimensional approaches. This limitation becomes particularly evident in proteomics, metabolomics, and natural product analysis.
Sample matrix effects continue to compromise separation purity, with co-eluting matrix components causing ion suppression or enhancement in LC-MS applications. These effects can significantly distort quantitative measurements and lead to false positive or negative results, particularly in complex biological or environmental samples where target analytes exist at trace levels.
Pressure limitations in conventional HPLC systems (typically 400-600 bar) restrict flow rates and column particle size options, forcing compromises between analysis speed and separation efficiency. While UHPLC systems operating at pressures up to 1500 bar have addressed this partially, they introduce additional challenges including system durability issues, frictional heating effects, and increased backpressure that can affect chromatographic performance.
Detector sensitivity and selectivity constraints further impact separation purity assessment. UV-Vis detectors, while robust, lack specificity for compounds with similar chromophores. Mass spectrometric detection offers improved selectivity but suffers from matrix effects and ionization suppression. Additionally, the dynamic range limitations of most detectors (typically 103-106) make it difficult to accurately quantify both major and minor components in a single analysis.
Carryover effects between injections represent another persistent challenge, particularly with hydrophobic compounds that adsorb to system components. Even trace carryover can significantly impact purity determinations in subsequent analyses, especially in quality control applications where detection of low-level impurities is critical.
Finally, method transferability issues between different HPLC systems, columns, and laboratories introduce variability that compromises reproducible purity assessments. Subtle differences in instrument design, column manufacturing, and operating conditions can significantly alter separation selectivity and resolution, making standardization of purity determinations challenging across different analytical platforms.
State-of-the-Art HPLC Separation Methodologies
01 HPLC method development for improved separation
Development of specialized HPLC methods to enhance separation efficiency and resolution for complex mixtures. These methods involve optimizing mobile phase composition, gradient elution parameters, and column selection to achieve better peak separation. Advanced techniques include adjusting pH, temperature, and flow rate to maximize resolution between closely eluting compounds, resulting in more accurate purity determinations.- HPLC method development for purity analysis: High-performance liquid chromatography (HPLC) methods can be developed specifically for purity analysis of various compounds. These methods involve optimizing parameters such as mobile phase composition, column selection, and detection techniques to achieve effective separation of target analytes from impurities. The development process typically includes validation steps to ensure accuracy, precision, and reproducibility of the analytical results for determining product purity.
- Column technology for improved separation: Advanced column technologies play a crucial role in enhancing HPLC separation efficiency for purity analysis. These include specialized stationary phases, particle sizes, and column dimensions designed to improve resolution between closely related compounds. Innovations in column technology such as core-shell particles, monolithic columns, and functionalized silica materials enable better peak separation, reduced analysis time, and improved detection of trace impurities in complex samples.
- Detection systems for purity determination: Various detection systems can be coupled with HPLC for accurate purity determination. These include UV-Vis detectors, diode array detectors (DAD), mass spectrometry (MS), evaporative light scattering detectors (ELSD), and fluorescence detectors. The choice of detector depends on the chemical properties of the analytes and impurities. Advanced detection systems allow for improved sensitivity, specificity, and the ability to identify and quantify unknown impurities, enhancing the overall reliability of purity assessments.
- Sample preparation techniques for purity analysis: Effective sample preparation is essential for accurate HPLC purity analysis. Techniques include filtration, solid-phase extraction, liquid-liquid extraction, and derivatization to remove interfering substances and concentrate analytes of interest. Proper sample preparation enhances chromatographic performance by reducing matrix effects, preventing column contamination, and improving detection limits. These techniques are critical for ensuring reliable and reproducible purity determinations across different sample types.
- Validation and standardization of HPLC purity methods: Validation and standardization protocols ensure the reliability of HPLC methods for purity analysis. These include evaluating parameters such as linearity, accuracy, precision, specificity, detection limit, quantitation limit, and robustness. Standardized approaches to method validation help establish consistency across different laboratories and ensure compliance with regulatory requirements. Reference standards and system suitability tests are employed to verify the performance of the analytical system before and during analysis.
02 Column technology for enhanced purity analysis
Innovative column technologies designed specifically for high-resolution purity analysis. These columns feature specialized stationary phases with improved selectivity for different compound classes. Advancements include monolithic columns, core-shell particles, and functionalized silica that provide superior separation performance, reduced analysis time, and enhanced detection of impurities at trace levels.Expand Specific Solutions03 Detection systems for purity determination
Advanced detection systems integrated with HPLC for comprehensive purity assessment. These include diode array detectors (DAD), mass spectrometry (MS), evaporative light scattering detectors (ELSD), and fluorescence detectors that enable multi-dimensional analysis of sample purity. The combination of multiple detection methods allows for more accurate identification and quantification of impurities, particularly for compounds with similar chromatographic properties.Expand Specific Solutions04 Sample preparation techniques for purity analysis
Specialized sample preparation methods to enhance HPLC purity analysis. These techniques include solid-phase extraction, liquid-liquid extraction, and filtration protocols designed to remove matrix interferences and concentrate analytes of interest. Advanced sample clean-up procedures improve chromatographic performance by reducing column fouling and baseline noise, leading to more accurate purity determinations and longer column lifetimes.Expand Specific Solutions05 Validation and standardization of HPLC purity methods
Comprehensive validation protocols and standardization approaches for HPLC purity testing. These include system suitability tests, method validation parameters (accuracy, precision, linearity, range, specificity), and quality control procedures to ensure reliable and reproducible purity results. Standardized methods incorporate robustness testing and forced degradation studies to verify that the analytical procedure can accurately detect and quantify impurities under various conditions.Expand Specific Solutions
Leading HPLC Equipment and Solution Providers
The HPLC analytics market for separation purity is in a mature growth phase with an estimated global market size of $4-5 billion, expanding at 5-7% annually. The competitive landscape is dominated by established players like Waters Technology and Agilent Technologies, who lead with comprehensive HPLC solutions and significant R&D investments. The technology has reached high maturity with recent innovations focusing on ultra-high performance systems, automation, and integration with mass spectrometry. Mid-tier competitors include Hitachi High-Tech and BASF, while academic institutions like Vrije Universiteit Brussel and University of Washington contribute to technological advancement through research partnerships. The market is seeing increased specialization in pharmaceutical applications, with companies like AbbVie and Galapagos NV driving demand for higher purity separation techniques.
Waters Technology Corp.
Technical Solution: Waters Technology has developed the ACQUITY UPLC and Arc HPLC systems specifically designed to maximize separation purity. Their technology incorporates sub-2-micron particle columns that significantly enhance resolution and sensitivity compared to traditional HPLC systems[1]. The ACQUITY Premier System features MaxPeak High-Performance Surface (HPS) technology that reduces unwanted interactions between sample analytes and metal surfaces in the flow path, resulting in improved peak shapes and recovery for challenging compounds[2]. Waters' Empower Chromatography Data Software provides advanced method development tools with automated method optimization capabilities that can systematically evaluate multiple chromatographic parameters (mobile phase composition, pH, temperature, gradient profiles) to identify optimal separation conditions[3]. Their columns incorporate hybrid particle technology that combines the mechanical strength of silica with the chemical stability of polymers, allowing operation across a wider pH range (2-12) for improved selectivity options.
Strengths: Industry-leading resolution capabilities with their UPLC technology; comprehensive software integration for method development; extensive column chemistry options for diverse applications. Weaknesses: Higher initial investment costs compared to basic HPLC systems; proprietary consumables can increase operational expenses; complex systems may require specialized training for optimal utilization.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has pioneered the InfinityLab LC series that incorporates multiple technological innovations to maximize separation purity. Their systems feature Intelligent System Emulation Technology (ISET) that allows method transfer between different instruments while maintaining chromatographic results, ensuring consistent separation quality across platforms[1]. Agilent's Dual C18 Selectivity technology provides orthogonal separation mechanisms on a single column, enabling improved resolution for complex samples without changing column hardware[2]. Their InfinityLab Poroshell 120 columns utilize superficially porous particles with a solid core and porous outer layer, delivering the efficiency of sub-2-micron particles with lower backpressure, allowing for faster separations without compromising resolution[3]. Agilent has also developed Advanced Solvent Modulation technology that enables precise gradient formation and mobile phase blending, minimizing baseline disturbances during gradient elution and improving quantitative accuracy for trace analysis. Their OpenLab CDS software incorporates automated peak integration algorithms and custom calculation capabilities for enhanced data analysis and reporting of separation results.
Strengths: Exceptional instrument-to-instrument reproducibility through ISET technology; wide range of column chemistries optimized for specific applications; comprehensive method development tools with built-in intelligence. Weaknesses: Premium pricing structure may be prohibitive for smaller laboratories; proprietary software ecosystems can create integration challenges with third-party systems; some advanced features require significant user expertise to fully utilize.
Critical Patents and Innovations in Column Technology
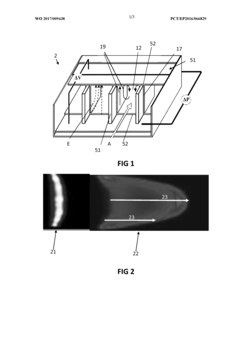
High-performance liquid chromatography with a controllable transverse flow inducer
PatentWO2017009438A1
Innovation
- The use of a controllable transverse flow inducer, such as an array of electrodes generating an alternating current electrokinetic field, to create micro-scale vortices that reduce dispersion and enhance mass transfer between support structures in the chromatography column, allowing for efficient separation without permanent surface charges and minimizing direct contact with electrodes.
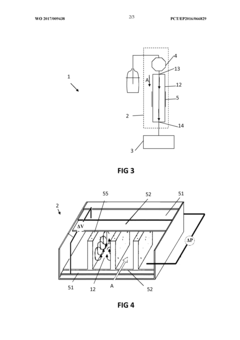
High-performance liquid chromatography with a controllable transverse flow inducer
PatentActiveEP3322978A1
Innovation
- The use of a controllable transverse flow inducer, which generates micro-scale vortices through alternating current electrokinetics, allowing for orthogonal flow induction independent of axial velocity, reducing dispersion by combining pressure and electro-osmotic flow, and enabling retention modulation without permanent surface charges.
Quality Control Standards and Validation Protocols
Quality control standards and validation protocols are essential components in HPLC analytics for maximizing separation purity. The implementation of robust quality control measures ensures consistent and reliable analytical results across different batches, operators, and time periods. These standards must align with international regulatory frameworks such as ICH (International Council for Harmonisation) guidelines, particularly ICH Q2(R1) for analytical method validation, and comply with pharmacopeia requirements including USP, EP, and JP standards.
Method validation represents the cornerstone of quality assurance in HPLC separation processes. A comprehensive validation protocol typically encompasses several critical parameters: specificity, linearity, range, accuracy, precision, detection limit, quantitation limit, and robustness. For separation purity applications, specificity validation becomes particularly crucial as it demonstrates the method's ability to unambiguously assess the analyte in the presence of expected impurities, degradation products, and matrix components.
System suitability tests (SSTs) serve as ongoing quality control measures that verify the chromatographic system's performance before and during analysis. Key SST parameters include resolution between critical pairs, tailing factor, theoretical plate count, and retention time reproducibility. These parameters must be established with appropriate acceptance criteria based on the separation's critical quality attributes and the intended application of the analytical method.
Calibration procedures represent another vital aspect of quality control in HPLC analytics. Regular calibration using certified reference standards ensures measurement accuracy and traceability to recognized standards. For complex separations, the use of multiple calibration points across the expected concentration range helps establish reliable quantitative relationships and identify potential non-linear responses that could affect separation purity assessment.
Documentation and change control protocols are equally important for maintaining analytical method integrity. All validation activities, calibration records, maintenance logs, and method modifications must be thoroughly documented following GMP (Good Manufacturing Practice) principles. Any changes to established methods require appropriate revalidation to demonstrate that separation purity is not compromised by the modifications.
Continuous monitoring through statistical process control techniques enables early detection of analytical drift or system deterioration that could impact separation purity. Implementation of control charts for critical method parameters, combined with regular proficiency testing and inter-laboratory comparisons, provides objective evidence of ongoing method performance and laboratory competence in achieving maximum separation purity.
Method validation represents the cornerstone of quality assurance in HPLC separation processes. A comprehensive validation protocol typically encompasses several critical parameters: specificity, linearity, range, accuracy, precision, detection limit, quantitation limit, and robustness. For separation purity applications, specificity validation becomes particularly crucial as it demonstrates the method's ability to unambiguously assess the analyte in the presence of expected impurities, degradation products, and matrix components.
System suitability tests (SSTs) serve as ongoing quality control measures that verify the chromatographic system's performance before and during analysis. Key SST parameters include resolution between critical pairs, tailing factor, theoretical plate count, and retention time reproducibility. These parameters must be established with appropriate acceptance criteria based on the separation's critical quality attributes and the intended application of the analytical method.
Calibration procedures represent another vital aspect of quality control in HPLC analytics. Regular calibration using certified reference standards ensures measurement accuracy and traceability to recognized standards. For complex separations, the use of multiple calibration points across the expected concentration range helps establish reliable quantitative relationships and identify potential non-linear responses that could affect separation purity assessment.
Documentation and change control protocols are equally important for maintaining analytical method integrity. All validation activities, calibration records, maintenance logs, and method modifications must be thoroughly documented following GMP (Good Manufacturing Practice) principles. Any changes to established methods require appropriate revalidation to demonstrate that separation purity is not compromised by the modifications.
Continuous monitoring through statistical process control techniques enables early detection of analytical drift or system deterioration that could impact separation purity. Implementation of control charts for critical method parameters, combined with regular proficiency testing and inter-laboratory comparisons, provides objective evidence of ongoing method performance and laboratory competence in achieving maximum separation purity.
Green Chemistry Applications in Modern HPLC
Green chemistry principles have increasingly become integral to modern High-Performance Liquid Chromatography (HPLC) methodologies, particularly in maximizing separation purity while minimizing environmental impact. The integration of green chemistry with HPLC represents a significant paradigm shift in analytical chemistry, moving from traditional solvent-intensive approaches toward more sustainable practices.
The primary focus of green HPLC involves reducing or eliminating hazardous solvents through several innovative approaches. Solvent reduction strategies include the implementation of micro and nano-HPLC systems that dramatically decrease mobile phase consumption while maintaining separation efficiency. These miniaturized systems can achieve comparable analytical results with solvent volumes reduced by factors of 10-100 compared to conventional systems.
Water-based mobile phases have emerged as environmentally friendly alternatives to traditional organic solvents. Recent advancements in stationary phase technology have enabled effective separations using predominantly aqueous mobile phases, particularly through the development of hydrophilic interaction liquid chromatography (HILIC) and temperature-responsive stationary phases that change selectivity with minimal solvent modification.
Supercritical fluid chromatography (SFC) represents another significant green chemistry application, utilizing supercritical CO2 as the primary mobile phase component. This approach substantially reduces organic solvent consumption while offering unique selectivity advantages for certain compound classes. Modern SFC instrumentation has overcome historical limitations, making this technique increasingly viable for routine analytical applications.
Recyclable solvent systems have been developed to minimize waste generation in preparative HPLC applications. These closed-loop systems capture, purify, and reuse mobile phases, reducing both environmental impact and operational costs in large-scale separations. Implementation of such systems has demonstrated solvent consumption reductions of up to 80% in industrial applications.
Biodegradable stationary phases represent an emerging frontier in green HPLC. Research into cellulose-based, chitosan-derived, and other biopolymer stationary phases has yielded promising results for specific separation challenges while offering improved end-of-life disposal options compared to traditional silica-based materials.
The pharmaceutical industry has been particularly proactive in adopting green HPLC methodologies, driven by both environmental considerations and regulatory pressures. Several major pharmaceutical companies have published case studies demonstrating successful method development using green chemistry principles without compromising analytical performance or validation requirements.
The primary focus of green HPLC involves reducing or eliminating hazardous solvents through several innovative approaches. Solvent reduction strategies include the implementation of micro and nano-HPLC systems that dramatically decrease mobile phase consumption while maintaining separation efficiency. These miniaturized systems can achieve comparable analytical results with solvent volumes reduced by factors of 10-100 compared to conventional systems.
Water-based mobile phases have emerged as environmentally friendly alternatives to traditional organic solvents. Recent advancements in stationary phase technology have enabled effective separations using predominantly aqueous mobile phases, particularly through the development of hydrophilic interaction liquid chromatography (HILIC) and temperature-responsive stationary phases that change selectivity with minimal solvent modification.
Supercritical fluid chromatography (SFC) represents another significant green chemistry application, utilizing supercritical CO2 as the primary mobile phase component. This approach substantially reduces organic solvent consumption while offering unique selectivity advantages for certain compound classes. Modern SFC instrumentation has overcome historical limitations, making this technique increasingly viable for routine analytical applications.
Recyclable solvent systems have been developed to minimize waste generation in preparative HPLC applications. These closed-loop systems capture, purify, and reuse mobile phases, reducing both environmental impact and operational costs in large-scale separations. Implementation of such systems has demonstrated solvent consumption reductions of up to 80% in industrial applications.
Biodegradable stationary phases represent an emerging frontier in green HPLC. Research into cellulose-based, chitosan-derived, and other biopolymer stationary phases has yielded promising results for specific separation challenges while offering improved end-of-life disposal options compared to traditional silica-based materials.
The pharmaceutical industry has been particularly proactive in adopting green HPLC methodologies, driven by both environmental considerations and regulatory pressures. Several major pharmaceutical companies have published case studies demonstrating successful method development using green chemistry principles without compromising analytical performance or validation requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!