Emerging Uses for Carbon Tetrachloride in Industrial Applications
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Industrial Applications: Background and Objectives
Carbon tetrachloride (CCl4) has a long and complex history in industrial applications, dating back to its discovery in the mid-19th century. Initially used as a solvent and cleaning agent, its versatility led to widespread adoption across various industries. However, concerns about its environmental impact and health risks led to significant restrictions on its use in the late 20th century.
Despite these limitations, recent years have seen a resurgence of interest in CCl4 for specialized industrial applications. This renewed focus is driven by advancements in containment technologies and a better understanding of its unique properties. The current technological landscape aims to harness these properties while mitigating associated risks.
The evolution of CCl4 applications has been marked by several key milestones. Its early use as a fire extinguishing agent and refrigerant gave way to more specialized roles in chemical synthesis and as a reagent in various industrial processes. The Montreal Protocol of 1987 significantly curtailed its use due to its ozone-depleting properties, leading to a sharp decline in production and application.
Today, the technological goals for CCl4 are multifaceted. Researchers and industry professionals are exploring ways to utilize its exceptional solvent properties and chemical reactivity in controlled, environmentally responsible ways. This includes developing closed-loop systems that prevent environmental release, as well as investigating its potential in emerging fields such as nanotechnology and advanced materials processing.
One of the primary objectives in current CCl4 research is to find alternatives for its traditional uses that maintain its effectiveness while reducing environmental impact. This involves exploring modified versions of the compound or entirely new substances that can replicate its desirable properties without the associated risks.
Another key goal is to improve detection and remediation technologies for CCl4 in the environment. As legacy contamination remains a concern, developing more efficient methods for identifying and cleaning up CCl4 pollution is crucial. This aligns with broader environmental protection efforts and supports the responsible use of the compound in modern industrial applications.
The technological trajectory for CCl4 also includes investigating its potential role in carbon capture and utilization technologies. Its unique chemical properties make it a candidate for processes that could help mitigate greenhouse gas emissions, presenting an intriguing possibility for turning a once-problematic substance into a tool for environmental benefit.
Despite these limitations, recent years have seen a resurgence of interest in CCl4 for specialized industrial applications. This renewed focus is driven by advancements in containment technologies and a better understanding of its unique properties. The current technological landscape aims to harness these properties while mitigating associated risks.
The evolution of CCl4 applications has been marked by several key milestones. Its early use as a fire extinguishing agent and refrigerant gave way to more specialized roles in chemical synthesis and as a reagent in various industrial processes. The Montreal Protocol of 1987 significantly curtailed its use due to its ozone-depleting properties, leading to a sharp decline in production and application.
Today, the technological goals for CCl4 are multifaceted. Researchers and industry professionals are exploring ways to utilize its exceptional solvent properties and chemical reactivity in controlled, environmentally responsible ways. This includes developing closed-loop systems that prevent environmental release, as well as investigating its potential in emerging fields such as nanotechnology and advanced materials processing.
One of the primary objectives in current CCl4 research is to find alternatives for its traditional uses that maintain its effectiveness while reducing environmental impact. This involves exploring modified versions of the compound or entirely new substances that can replicate its desirable properties without the associated risks.
Another key goal is to improve detection and remediation technologies for CCl4 in the environment. As legacy contamination remains a concern, developing more efficient methods for identifying and cleaning up CCl4 pollution is crucial. This aligns with broader environmental protection efforts and supports the responsible use of the compound in modern industrial applications.
The technological trajectory for CCl4 also includes investigating its potential role in carbon capture and utilization technologies. Its unique chemical properties make it a candidate for processes that could help mitigate greenhouse gas emissions, presenting an intriguing possibility for turning a once-problematic substance into a tool for environmental benefit.
Market Analysis for CCl4 in Industry
The market for carbon tetrachloride (CCl4) in industrial applications is undergoing significant changes due to environmental regulations and emerging technological advancements. Historically, CCl4 was widely used as a solvent, cleaning agent, and refrigerant. However, its ozone-depleting properties led to strict regulations under the Montreal Protocol, resulting in a sharp decline in its production and use.
Despite these restrictions, there is a growing interest in new industrial applications for CCl4, particularly in niche markets where its unique properties can be leveraged without significant environmental impact. The global market for CCl4 is currently driven by its use as a feedstock for the production of other chemicals, especially hydrofluorocarbons (HFCs) and their alternatives.
The pharmaceutical industry represents a promising growth sector for CCl4 usage. It is employed in the synthesis of various active pharmaceutical ingredients (APIs) and as a solvent in drug manufacturing processes. The increasing demand for complex pharmaceutical compounds is expected to drive the need for CCl4 in controlled laboratory environments.
In the electronics industry, CCl4 finds application in the production of semiconductors and optical fibers. Its high purity and specific chemical properties make it valuable in certain manufacturing processes where alternatives are less effective. As the demand for advanced electronic components continues to grow, this sector may contribute to a stable market for CCl4.
The agrochemical industry is another area where CCl4 maintains a presence, primarily as an intermediate in the production of pesticides and herbicides. While efforts are being made to develop more environmentally friendly alternatives, CCl4 remains important in certain synthesis routes.
Market analysis indicates that Asia-Pacific is the largest consumer of CCl4, followed by North America and Europe. The concentration of chemical manufacturing facilities in countries like China and India contributes significantly to this regional dominance. However, stringent regulations in developed countries are reshaping the global market dynamics, with a shift towards more controlled and specialized applications.
The future market for CCl4 is likely to be characterized by a balance between regulatory pressures and technological innovations. As industries seek to reduce their environmental footprint, there is ongoing research into developing closed-loop systems and recycling technologies that could allow for the continued use of CCl4 in critical applications while minimizing its release into the environment.
Despite these restrictions, there is a growing interest in new industrial applications for CCl4, particularly in niche markets where its unique properties can be leveraged without significant environmental impact. The global market for CCl4 is currently driven by its use as a feedstock for the production of other chemicals, especially hydrofluorocarbons (HFCs) and their alternatives.
The pharmaceutical industry represents a promising growth sector for CCl4 usage. It is employed in the synthesis of various active pharmaceutical ingredients (APIs) and as a solvent in drug manufacturing processes. The increasing demand for complex pharmaceutical compounds is expected to drive the need for CCl4 in controlled laboratory environments.
In the electronics industry, CCl4 finds application in the production of semiconductors and optical fibers. Its high purity and specific chemical properties make it valuable in certain manufacturing processes where alternatives are less effective. As the demand for advanced electronic components continues to grow, this sector may contribute to a stable market for CCl4.
The agrochemical industry is another area where CCl4 maintains a presence, primarily as an intermediate in the production of pesticides and herbicides. While efforts are being made to develop more environmentally friendly alternatives, CCl4 remains important in certain synthesis routes.
Market analysis indicates that Asia-Pacific is the largest consumer of CCl4, followed by North America and Europe. The concentration of chemical manufacturing facilities in countries like China and India contributes significantly to this regional dominance. However, stringent regulations in developed countries are reshaping the global market dynamics, with a shift towards more controlled and specialized applications.
The future market for CCl4 is likely to be characterized by a balance between regulatory pressures and technological innovations. As industries seek to reduce their environmental footprint, there is ongoing research into developing closed-loop systems and recycling technologies that could allow for the continued use of CCl4 in critical applications while minimizing its release into the environment.
Current Status and Challenges of CCl4 Usage
Carbon tetrachloride (CCl4) usage in industrial applications has undergone significant changes in recent years due to environmental and health concerns. Currently, the production and consumption of CCl4 are strictly regulated in many countries, with its use being phased out in numerous applications. However, some emerging industrial uses are being explored under controlled conditions.
The primary challenge facing CCl4 usage is its classification as an ozone-depleting substance under the Montreal Protocol. This international treaty has led to a dramatic reduction in CCl4 production and consumption since the 1990s. Despite these restrictions, CCl4 is still produced as a byproduct in the manufacture of other chlorinated compounds, creating a need for responsible management and potential repurposing.
In the chemical industry, CCl4 continues to be used as a feedstock for the production of certain chemicals, particularly hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFOs). These compounds are used as refrigerants and foam-blowing agents with lower ozone depletion potential. However, the challenge lies in minimizing emissions and ensuring closed-loop processes to prevent environmental release.
Another emerging application is in the semiconductor industry, where CCl4 is being investigated for its potential in plasma etching processes. The high selectivity and anisotropic etching capabilities of CCl4 make it attractive for certain niche applications in microelectronics manufacturing. However, stringent containment measures and emission controls are necessary to mitigate environmental risks.
In analytical chemistry, CCl4 still finds limited use as a solvent for infrared spectroscopy and other analytical techniques. The challenge here is to develop safer alternatives that can match the unique properties of CCl4 in these specialized applications.
The recycling and destruction of CCl4 present significant technical challenges. While incineration is an effective destruction method, it requires high temperatures and specialized facilities. Research is ongoing to develop more efficient and cost-effective destruction technologies, such as plasma arc decomposition and catalytic conversion processes.
Globally, the distribution of CCl4 usage varies significantly. Developed countries have largely phased out its use in most applications, while some developing nations still struggle with illegal production and use. This geographical disparity poses challenges for global enforcement and highlights the need for technology transfer and capacity building in less developed regions.
Looking forward, the key challenge for CCl4 usage lies in balancing its potential benefits in specific industrial applications with the imperative of environmental protection. This requires ongoing research into safer alternatives, development of more efficient containment and destruction technologies, and stricter global regulations to prevent unauthorized use and emissions.
The primary challenge facing CCl4 usage is its classification as an ozone-depleting substance under the Montreal Protocol. This international treaty has led to a dramatic reduction in CCl4 production and consumption since the 1990s. Despite these restrictions, CCl4 is still produced as a byproduct in the manufacture of other chlorinated compounds, creating a need for responsible management and potential repurposing.
In the chemical industry, CCl4 continues to be used as a feedstock for the production of certain chemicals, particularly hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFOs). These compounds are used as refrigerants and foam-blowing agents with lower ozone depletion potential. However, the challenge lies in minimizing emissions and ensuring closed-loop processes to prevent environmental release.
Another emerging application is in the semiconductor industry, where CCl4 is being investigated for its potential in plasma etching processes. The high selectivity and anisotropic etching capabilities of CCl4 make it attractive for certain niche applications in microelectronics manufacturing. However, stringent containment measures and emission controls are necessary to mitigate environmental risks.
In analytical chemistry, CCl4 still finds limited use as a solvent for infrared spectroscopy and other analytical techniques. The challenge here is to develop safer alternatives that can match the unique properties of CCl4 in these specialized applications.
The recycling and destruction of CCl4 present significant technical challenges. While incineration is an effective destruction method, it requires high temperatures and specialized facilities. Research is ongoing to develop more efficient and cost-effective destruction technologies, such as plasma arc decomposition and catalytic conversion processes.
Globally, the distribution of CCl4 usage varies significantly. Developed countries have largely phased out its use in most applications, while some developing nations still struggle with illegal production and use. This geographical disparity poses challenges for global enforcement and highlights the need for technology transfer and capacity building in less developed regions.
Looking forward, the key challenge for CCl4 usage lies in balancing its potential benefits in specific industrial applications with the imperative of environmental protection. This requires ongoing research into safer alternatives, development of more efficient containment and destruction technologies, and stricter global regulations to prevent unauthorized use and emissions.
Existing Industrial Solutions Utilizing CCl4
01 Production and purification of carbon tetrachloride
Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.- Production and purification of carbon tetrachloride: Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.
- Applications of carbon tetrachloride in chemical processes: Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in the production of other chemicals. Its unique properties make it valuable in specific industrial applications and chemical reactions.
- Environmental and safety considerations: Due to its environmental impact and health hazards, research focuses on alternatives to carbon tetrachloride and methods for its safe handling, storage, and disposal. This includes developing eco-friendly substitutes and improving safety protocols in industries where its use is still necessary.
- Detection and analysis methods: Various techniques and apparatus have been developed for detecting and analyzing carbon tetrachloride in different environments. These include spectroscopic methods, chromatography, and specialized sensors for monitoring its presence in air, water, or other media.
- Historical uses and patents: Early patents and historical documents reveal the diverse applications of carbon tetrachloride in the past, including its use in fire extinguishers, dry cleaning, and as a refrigerant. Many of these applications have since been phased out due to safety and environmental concerns.
02 Applications of carbon tetrachloride in chemical processes
Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in the production of other chemicals. Its unique properties make it valuable in specific industrial applications and chemical reactions.Expand Specific Solutions03 Environmental and safety considerations
Due to its environmental impact and potential health hazards, there are concerns and regulations surrounding the use of carbon tetrachloride. Alternative substances and methods are being developed to replace its use in various applications, and proper handling and disposal procedures are emphasized.Expand Specific Solutions04 Detection and analysis methods for carbon tetrachloride
Various analytical techniques and methods have been developed for detecting and quantifying carbon tetrachloride in different matrices, including environmental samples, industrial products, and biological specimens. These methods are crucial for monitoring and controlling its presence.Expand Specific Solutions05 Historical uses and developments
Carbon tetrachloride has a long history of industrial and commercial use. Early patents describe its applications in fire extinguishers, dry cleaning, and as a refrigerant. Over time, its use has evolved due to increased understanding of its properties and environmental impact.Expand Specific Solutions
Key Industry Players in CCl4 Production and Use
The emerging uses for carbon tetrachloride in industrial applications are in a nascent stage of development, with the market still relatively small but showing potential for growth. The technology's maturity is evolving, with several key players actively involved in research and development. Companies like DuPont de Nemours, Occidental Chemical Corp., and Wacker Chemie AG are at the forefront, leveraging their expertise in chemical manufacturing to explore new applications. Smaller firms such as Blue Cube IP LLC and Seerstone LLC are also contributing to innovation in this space. The competitive landscape is characterized by a mix of established chemical giants and specialized companies, indicating a growing interest in carbon tetrachloride's industrial potential despite environmental concerns.
Occidental Chemical Corp.
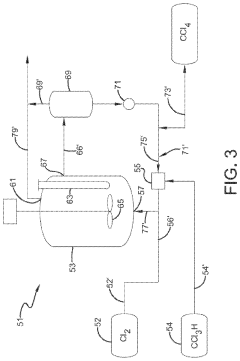
Technical Solution: Occidental Chemical Corp. has developed a closed-loop system for the use of carbon tetrachloride in the production of perchloroethylene and other chlorinated solvents. Their process involves the careful recycling and reuse of carbon tetrachloride within a controlled environment, minimizing emissions and maximizing efficiency[3]. The company has also explored the use of carbon tetrachloride as a feedstock for the production of high-purity silicon tetrachloride, which is crucial in the semiconductor and fiber optics industries[4]. This approach allows for the valorization of carbon tetrachloride while addressing environmental concerns.
Strengths: Efficient closed-loop processes, diversification of carbon tetrachloride applications, expertise in chlorinated compounds. Weaknesses: Dependence on strict environmental controls, potential market limitations due to regulatory restrictions.
Blue Cube IP LLC
Technical Solution: Blue Cube IP LLC has developed advanced purification techniques for carbon tetrachloride, enabling its use in high-precision applications such as analytical chemistry and pharmaceutical manufacturing. Their process involves multi-stage distillation and adsorption steps to remove impurities, resulting in ultra-high purity carbon tetrachloride suitable for critical industrial applications[9]. The company has also explored the use of carbon tetrachloride as a selective extraction solvent in the purification of complex organic compounds, leveraging its unique solvation properties[10].
Strengths: Expertise in purification technologies, high-value niche applications, ability to meet stringent quality requirements. Weaknesses: Limited market size for ultra-high purity products, potential regulatory restrictions on carbon tetrachloride use.
Innovative CCl4 Applications: Patents and Literature
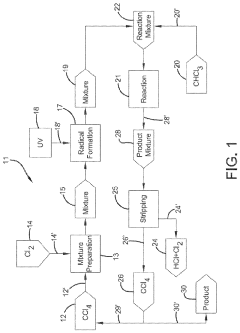
Photochlorination of partially-chlorinated chloromethanes to carbon tetrachloride
PatentActiveUS20240025823A1
Innovation
- A method involving the photochlorination of a chloromethanes stream containing chloroform, methyl chloride, and methylene chloride, combined with chlorine and additional carbon tetrachloride, and subjected to electromagnetic radiation to form carbon tetrachloride, achieving high conversion rates with reduced levels of unwanted chlorinated hydrocarbons.
Chlorinolysis process for producing carbon tetrachloride
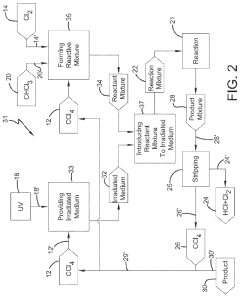
PatentActiveUS20210130266A1
Innovation
- A process involving a chlorination zone with chlorine, a C1 chlorinated compound, and a carbon/second chlorine source to produce a reaction mixture that favors the formation of carbon tetrachloride over perchloroethylene, using waste products as the carbon/second chlorine source to enhance efficiency and reduce impurity formation.
Environmental Impact and Regulations
Carbon tetrachloride, once widely used in various industrial applications, has been subject to stringent regulations due to its significant environmental impact. The Montreal Protocol, an international treaty designed to protect the ozone layer, has phased out the production and consumption of carbon tetrachloride in most countries since 1996. This regulatory action was taken in response to the compound's ozone-depleting properties and its contribution to global warming.
Despite these restrictions, carbon tetrachloride continues to be detected in the atmosphere at levels higher than expected, suggesting ongoing emissions from industrial sources. Recent studies have identified potential sources, including inadvertent production during chloromethane manufacturing and emissions from chlor-alkali plants. These findings highlight the need for improved monitoring and control measures in industrial processes.
The environmental persistence of carbon tetrachloride is a significant concern. With an atmospheric lifetime of approximately 26 years, it can accumulate in the environment and contribute to long-term ecological damage. Its ability to deplete stratospheric ozone has been well-documented, with a ozone depletion potential (ODP) of 0.72 relative to CFC-11. This impact on the ozone layer can lead to increased UV radiation reaching the Earth's surface, potentially harming ecosystems and human health.
Water contamination is another critical environmental issue associated with carbon tetrachloride. Its high solubility and mobility in groundwater systems make it a persistent pollutant in aquatic environments. Remediation of contaminated sites is challenging and often requires extensive and costly treatment processes.
Regulatory frameworks have evolved to address these environmental concerns. In addition to the Montreal Protocol, many countries have implemented national regulations to control the use and disposal of carbon tetrachloride. The United States Environmental Protection Agency (EPA) has classified it as a hazardous air pollutant and a priority pollutant under the Clean Water Act. The European Union has also imposed strict controls on its use and emission through the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation.
As industrial applications for carbon tetrachloride continue to emerge, regulatory bodies are faced with the challenge of balancing potential benefits against environmental risks. New uses must be carefully evaluated for their potential environmental impact, and stringent controls must be put in place to prevent releases into the environment. This may include the development of closed-loop systems, improved containment measures, and enhanced monitoring technologies.
The future of carbon tetrachloride in industrial applications will likely be shaped by ongoing scientific research, technological advancements in emission control, and evolving regulatory frameworks. As our understanding of its environmental impact grows, so too must our efforts to mitigate its negative effects while exploring safer alternatives for essential industrial processes.
Despite these restrictions, carbon tetrachloride continues to be detected in the atmosphere at levels higher than expected, suggesting ongoing emissions from industrial sources. Recent studies have identified potential sources, including inadvertent production during chloromethane manufacturing and emissions from chlor-alkali plants. These findings highlight the need for improved monitoring and control measures in industrial processes.
The environmental persistence of carbon tetrachloride is a significant concern. With an atmospheric lifetime of approximately 26 years, it can accumulate in the environment and contribute to long-term ecological damage. Its ability to deplete stratospheric ozone has been well-documented, with a ozone depletion potential (ODP) of 0.72 relative to CFC-11. This impact on the ozone layer can lead to increased UV radiation reaching the Earth's surface, potentially harming ecosystems and human health.
Water contamination is another critical environmental issue associated with carbon tetrachloride. Its high solubility and mobility in groundwater systems make it a persistent pollutant in aquatic environments. Remediation of contaminated sites is challenging and often requires extensive and costly treatment processes.
Regulatory frameworks have evolved to address these environmental concerns. In addition to the Montreal Protocol, many countries have implemented national regulations to control the use and disposal of carbon tetrachloride. The United States Environmental Protection Agency (EPA) has classified it as a hazardous air pollutant and a priority pollutant under the Clean Water Act. The European Union has also imposed strict controls on its use and emission through the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation.
As industrial applications for carbon tetrachloride continue to emerge, regulatory bodies are faced with the challenge of balancing potential benefits against environmental risks. New uses must be carefully evaluated for their potential environmental impact, and stringent controls must be put in place to prevent releases into the environment. This may include the development of closed-loop systems, improved containment measures, and enhanced monitoring technologies.
The future of carbon tetrachloride in industrial applications will likely be shaped by ongoing scientific research, technological advancements in emission control, and evolving regulatory frameworks. As our understanding of its environmental impact grows, so too must our efforts to mitigate its negative effects while exploring safer alternatives for essential industrial processes.
Safety Considerations for CCl4 Handling
Carbon tetrachloride (CCl4) is a highly toxic and potentially dangerous chemical compound that requires careful handling and stringent safety measures in industrial applications. The primary safety considerations for CCl4 handling revolve around protecting workers, preventing environmental contamination, and ensuring proper storage and disposal.
Personal protective equipment (PPE) is crucial when working with CCl4. Workers must wear impervious gloves, protective clothing, and eye protection to prevent skin and eye contact. Respiratory protection, such as a self-contained breathing apparatus (SCBA) or supplied-air respirator, is essential in areas where CCl4 vapors may be present, as inhalation can lead to severe health effects.
Proper ventilation is critical in areas where CCl4 is used or stored. Local exhaust ventilation systems should be installed to capture and remove CCl4 vapors at the source. Regular air monitoring should be conducted to ensure that exposure levels remain below established occupational exposure limits.
Spill prevention and containment measures are vital to minimize environmental contamination. CCl4 should be stored in tightly sealed containers in cool, well-ventilated areas away from incompatible materials. Secondary containment systems, such as spill trays or berms, should be used to prevent accidental releases from spreading.
Emergency response procedures must be established and regularly practiced. This includes having readily available spill clean-up materials, eye wash stations, and safety showers. Workers should be trained in proper spill response techniques and the use of emergency equipment.
Proper disposal of CCl4 and CCl4-contaminated materials is essential to prevent environmental pollution. CCl4 is considered a hazardous waste and must be disposed of in accordance with local, state, and federal regulations. Incineration at approved facilities is often the preferred method of disposal.
Regular safety training and education programs should be implemented to ensure that all workers are aware of the hazards associated with CCl4 and the proper safety procedures. This includes training on the proper use of PPE, handling techniques, emergency procedures, and the importance of following safety protocols.
Strict inventory control and management practices should be implemented to track CCl4 usage and storage. This helps prevent unauthorized access, reduces the risk of accidental releases, and ensures compliance with regulatory requirements.
Given the significant safety concerns associated with CCl4, industries should continually evaluate alternatives and explore safer substitutes whenever possible. This aligns with the broader trend towards green chemistry and sustainable industrial practices.
Personal protective equipment (PPE) is crucial when working with CCl4. Workers must wear impervious gloves, protective clothing, and eye protection to prevent skin and eye contact. Respiratory protection, such as a self-contained breathing apparatus (SCBA) or supplied-air respirator, is essential in areas where CCl4 vapors may be present, as inhalation can lead to severe health effects.
Proper ventilation is critical in areas where CCl4 is used or stored. Local exhaust ventilation systems should be installed to capture and remove CCl4 vapors at the source. Regular air monitoring should be conducted to ensure that exposure levels remain below established occupational exposure limits.
Spill prevention and containment measures are vital to minimize environmental contamination. CCl4 should be stored in tightly sealed containers in cool, well-ventilated areas away from incompatible materials. Secondary containment systems, such as spill trays or berms, should be used to prevent accidental releases from spreading.
Emergency response procedures must be established and regularly practiced. This includes having readily available spill clean-up materials, eye wash stations, and safety showers. Workers should be trained in proper spill response techniques and the use of emergency equipment.
Proper disposal of CCl4 and CCl4-contaminated materials is essential to prevent environmental pollution. CCl4 is considered a hazardous waste and must be disposed of in accordance with local, state, and federal regulations. Incineration at approved facilities is often the preferred method of disposal.
Regular safety training and education programs should be implemented to ensure that all workers are aware of the hazards associated with CCl4 and the proper safety procedures. This includes training on the proper use of PPE, handling techniques, emergency procedures, and the importance of following safety protocols.
Strict inventory control and management practices should be implemented to track CCl4 usage and storage. This helps prevent unauthorized access, reduces the risk of accidental releases, and ensures compliance with regulatory requirements.
Given the significant safety concerns associated with CCl4, industries should continually evaluate alternatives and explore safer substitutes whenever possible. This aligns with the broader trend towards green chemistry and sustainable industrial practices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!