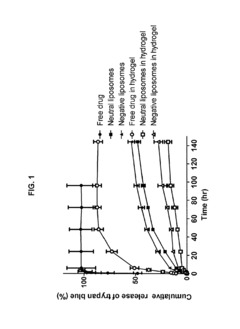
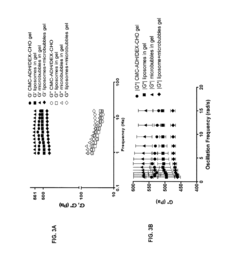
Engineered Living Materials For On-Demand Drug Delivery.
SEP 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ELM Drug Delivery Background and Objectives
Engineered Living Materials (ELMs) represent a revolutionary frontier in biotechnology, combining principles of synthetic biology with material science to create responsive, adaptive systems capable of sensing environmental changes and responding accordingly. The evolution of ELMs has progressed significantly over the past decade, transitioning from basic proof-of-concept demonstrations to increasingly sophisticated functional systems with potential real-world applications.
The field of drug delivery has historically faced significant challenges, including poor pharmacokinetics, off-target effects, and inability to respond dynamically to physiological changes. Traditional drug delivery systems often rely on passive diffusion or pre-programmed release mechanisms that cannot adapt to the body's changing needs. This limitation has driven researchers to explore more responsive and intelligent delivery platforms.
ELMs offer a promising solution by integrating living cells—typically bacteria, yeast, or mammalian cells—with structural materials to create hybrid systems capable of sensing physiological signals and producing therapeutic compounds on demand. The technological trajectory has evolved from simple encapsulation of microorganisms in hydrogels to more complex architectures with spatially organized cellular components and programmable genetic circuits.
The primary objective of ELM-based drug delivery systems is to achieve precise spatial and temporal control over therapeutic release, responding to specific biological triggers such as inflammation markers, metabolites, or pathogen signatures. This approach aims to minimize side effects while maximizing therapeutic efficacy through localized, context-dependent drug production.
Recent advances in genetic engineering tools, particularly CRISPR-Cas systems, have accelerated progress by enabling more sophisticated genetic programming of cellular components. Concurrently, developments in biomaterial science have yielded matrices with improved biocompatibility, mechanical properties, and functionalization capabilities, creating more hospitable environments for engineered cells.
The convergence of these technological advances has set the stage for ELMs that can potentially revolutionize treatment paradigms for chronic conditions requiring long-term medication, such as diabetes, autoimmune disorders, and certain cancers. The ultimate goal is to develop implantable or ingestible ELM systems that function as "living pharmacies," continuously monitoring the body's state and producing therapeutics only when and where needed.
Despite promising progress, significant challenges remain in ensuring long-term cell viability, preventing immune rejection, maintaining genetic stability, and addressing regulatory and safety concerns. The field is now at a critical juncture where fundamental research must transition toward translational applications with clear clinical relevance and practical implementation strategies.
The field of drug delivery has historically faced significant challenges, including poor pharmacokinetics, off-target effects, and inability to respond dynamically to physiological changes. Traditional drug delivery systems often rely on passive diffusion or pre-programmed release mechanisms that cannot adapt to the body's changing needs. This limitation has driven researchers to explore more responsive and intelligent delivery platforms.
ELMs offer a promising solution by integrating living cells—typically bacteria, yeast, or mammalian cells—with structural materials to create hybrid systems capable of sensing physiological signals and producing therapeutic compounds on demand. The technological trajectory has evolved from simple encapsulation of microorganisms in hydrogels to more complex architectures with spatially organized cellular components and programmable genetic circuits.
The primary objective of ELM-based drug delivery systems is to achieve precise spatial and temporal control over therapeutic release, responding to specific biological triggers such as inflammation markers, metabolites, or pathogen signatures. This approach aims to minimize side effects while maximizing therapeutic efficacy through localized, context-dependent drug production.
Recent advances in genetic engineering tools, particularly CRISPR-Cas systems, have accelerated progress by enabling more sophisticated genetic programming of cellular components. Concurrently, developments in biomaterial science have yielded matrices with improved biocompatibility, mechanical properties, and functionalization capabilities, creating more hospitable environments for engineered cells.
The convergence of these technological advances has set the stage for ELMs that can potentially revolutionize treatment paradigms for chronic conditions requiring long-term medication, such as diabetes, autoimmune disorders, and certain cancers. The ultimate goal is to develop implantable or ingestible ELM systems that function as "living pharmacies," continuously monitoring the body's state and producing therapeutics only when and where needed.
Despite promising progress, significant challenges remain in ensuring long-term cell viability, preventing immune rejection, maintaining genetic stability, and addressing regulatory and safety concerns. The field is now at a critical juncture where fundamental research must transition toward translational applications with clear clinical relevance and practical implementation strategies.
Market Analysis for On-Demand Drug Delivery Systems
The global market for on-demand drug delivery systems is experiencing robust growth, driven by increasing prevalence of chronic diseases, rising patient preference for minimally invasive therapies, and technological advancements in drug delivery mechanisms. The market was valued at approximately $28.7 billion in 2022 and is projected to reach $49.3 billion by 2030, representing a compound annual growth rate (CAGR) of 7.2% during the forecast period.
Engineered living materials (ELMs) for drug delivery represent a revolutionary segment within this market, combining synthetic biology with material science to create responsive therapeutic systems. This segment is currently in its nascent stage but demonstrates significant growth potential, with early-stage investments reaching $1.2 billion in 2022, a 45% increase from the previous year.
North America dominates the on-demand drug delivery market with approximately 42% market share, followed by Europe (28%) and Asia-Pacific (21%). The Asia-Pacific region is expected to witness the fastest growth due to improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about advanced drug delivery technologies.
By application segment, cancer therapy represents the largest market share (34%), followed by diabetes management (22%), pain management (18%), and infectious diseases (15%). The remaining 11% encompasses various therapeutic areas including autoimmune disorders and neurological conditions.
Consumer demand is increasingly shifting toward personalized medicine approaches, with 76% of healthcare providers reporting patient requests for customized treatment options. This trend particularly benefits ELM-based drug delivery systems, which can be engineered to respond to individual patient biomarkers and deliver therapeutic agents with unprecedented precision.
Key market drivers include the growing aging population, increasing incidence of chronic diseases, and rising demand for targeted drug delivery systems that minimize side effects. Additionally, favorable regulatory pathways for innovative drug delivery technologies in major markets are accelerating commercialization timelines.
Market challenges include high development costs, complex regulatory approval processes, and technical challenges in scaling production of living engineered materials. The average development timeline for bringing an ELM-based drug delivery system to market currently stands at 7-9 years, with development costs ranging from $80-120 million.
Despite these challenges, venture capital funding in this sector has shown remarkable growth, with $3.4 billion invested in startups focused on advanced drug delivery technologies in 2022, representing a 28% year-over-year increase.
Engineered living materials (ELMs) for drug delivery represent a revolutionary segment within this market, combining synthetic biology with material science to create responsive therapeutic systems. This segment is currently in its nascent stage but demonstrates significant growth potential, with early-stage investments reaching $1.2 billion in 2022, a 45% increase from the previous year.
North America dominates the on-demand drug delivery market with approximately 42% market share, followed by Europe (28%) and Asia-Pacific (21%). The Asia-Pacific region is expected to witness the fastest growth due to improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about advanced drug delivery technologies.
By application segment, cancer therapy represents the largest market share (34%), followed by diabetes management (22%), pain management (18%), and infectious diseases (15%). The remaining 11% encompasses various therapeutic areas including autoimmune disorders and neurological conditions.
Consumer demand is increasingly shifting toward personalized medicine approaches, with 76% of healthcare providers reporting patient requests for customized treatment options. This trend particularly benefits ELM-based drug delivery systems, which can be engineered to respond to individual patient biomarkers and deliver therapeutic agents with unprecedented precision.
Key market drivers include the growing aging population, increasing incidence of chronic diseases, and rising demand for targeted drug delivery systems that minimize side effects. Additionally, favorable regulatory pathways for innovative drug delivery technologies in major markets are accelerating commercialization timelines.
Market challenges include high development costs, complex regulatory approval processes, and technical challenges in scaling production of living engineered materials. The average development timeline for bringing an ELM-based drug delivery system to market currently stands at 7-9 years, with development costs ranging from $80-120 million.
Despite these challenges, venture capital funding in this sector has shown remarkable growth, with $3.4 billion invested in startups focused on advanced drug delivery technologies in 2022, representing a 28% year-over-year increase.
Current ELM Technology Landscape and Barriers
The field of Engineered Living Materials (ELMs) for drug delivery has witnessed significant advancements in recent years, yet faces substantial technical and regulatory challenges. Current ELM technologies primarily utilize genetically engineered microorganisms embedded within responsive matrices to create materials capable of sensing environmental cues and releasing therapeutic compounds accordingly. Leading approaches include bacterial cellulose-based systems, fungal mycelium composites, and mammalian cell-integrated hydrogels that respond to specific biochemical triggers.
Despite promising developments, several critical barriers impede widespread implementation. Foremost among these is the challenge of maintaining cell viability and functionality over extended periods. Most current systems demonstrate therapeutic efficacy for only days to weeks, falling short of the months or years required for chronic disease management. Environmental sensitivity further complicates deployment, as temperature fluctuations, nutrient availability, and competing microorganisms can compromise system performance in real-world settings.
Regulatory frameworks present another significant obstacle. Current regulations for living therapeutics were not designed with ELMs in mind, creating uncertainty regarding approval pathways. Concerns about horizontal gene transfer and environmental release of engineered organisms have prompted stringent containment requirements that limit practical applications. The FDA and EMA have yet to establish clear guidelines specifically addressing ELM-based drug delivery systems.
Technical limitations in genetic circuit design also constrain progress. While simple on-off drug release mechanisms have been demonstrated, sophisticated dose-responsive systems with feedback control remain elusive. Current genetic circuits often suffer from leaky expression, limited dynamic range, and poor tunability, resulting in suboptimal pharmacokinetic profiles compared to conventional drug delivery platforms.
Manufacturing scalability represents another substantial barrier. Laboratory-scale production methods typically involve complex bioreactor systems and aseptic processing that prove challenging to scale. Batch-to-batch variability in living components introduces quality control complications not encountered with traditional biomaterials. The absence of standardized characterization methods further hinders reproducible manufacturing.
Immunological considerations pose additional challenges, as host immune responses to both the engineered organisms and their matrices can trigger inflammation, reduce therapeutic efficacy, or cause adverse reactions. Current approaches to immunomodulation within ELMs remain rudimentary compared to established drug delivery technologies.
The integration of sensing and responsive elements within a single ELM system represents perhaps the most ambitious technical frontier. While proof-of-concept systems have demonstrated capabilities for detecting specific biomarkers and responding with drug release, creating closed-loop systems that can maintain therapeutic homeostasis remains beyond current technological capabilities.
Despite promising developments, several critical barriers impede widespread implementation. Foremost among these is the challenge of maintaining cell viability and functionality over extended periods. Most current systems demonstrate therapeutic efficacy for only days to weeks, falling short of the months or years required for chronic disease management. Environmental sensitivity further complicates deployment, as temperature fluctuations, nutrient availability, and competing microorganisms can compromise system performance in real-world settings.
Regulatory frameworks present another significant obstacle. Current regulations for living therapeutics were not designed with ELMs in mind, creating uncertainty regarding approval pathways. Concerns about horizontal gene transfer and environmental release of engineered organisms have prompted stringent containment requirements that limit practical applications. The FDA and EMA have yet to establish clear guidelines specifically addressing ELM-based drug delivery systems.
Technical limitations in genetic circuit design also constrain progress. While simple on-off drug release mechanisms have been demonstrated, sophisticated dose-responsive systems with feedback control remain elusive. Current genetic circuits often suffer from leaky expression, limited dynamic range, and poor tunability, resulting in suboptimal pharmacokinetic profiles compared to conventional drug delivery platforms.
Manufacturing scalability represents another substantial barrier. Laboratory-scale production methods typically involve complex bioreactor systems and aseptic processing that prove challenging to scale. Batch-to-batch variability in living components introduces quality control complications not encountered with traditional biomaterials. The absence of standardized characterization methods further hinders reproducible manufacturing.
Immunological considerations pose additional challenges, as host immune responses to both the engineered organisms and their matrices can trigger inflammation, reduce therapeutic efficacy, or cause adverse reactions. Current approaches to immunomodulation within ELMs remain rudimentary compared to established drug delivery technologies.
The integration of sensing and responsive elements within a single ELM system represents perhaps the most ambitious technical frontier. While proof-of-concept systems have demonstrated capabilities for detecting specific biomarkers and responding with drug release, creating closed-loop systems that can maintain therapeutic homeostasis remains beyond current technological capabilities.
Current ELM-Based Drug Release Mechanisms
01 Engineered living materials for controlled drug release
Engineered living materials (ELMs) can be designed to release therapeutic agents in a controlled manner. These materials integrate living cells with non-living components to create responsive systems that can deliver drugs based on specific environmental cues or programmed release profiles. The living components can be engineered to produce and release therapeutic molecules on demand, providing precise temporal control over drug delivery.- Engineered living materials for controlled drug release: Engineered living materials (ELMs) can be designed to release therapeutic agents in a controlled manner. These materials incorporate living cells that can produce and release drugs in response to specific stimuli. The living components can be engineered to sense environmental cues and respond by releasing therapeutic compounds at precise rates, allowing for sustained and targeted drug delivery. This approach offers advantages over traditional drug delivery systems by providing dynamic control over drug release profiles.
- Responsive biomaterials for on-demand drug delivery: Responsive biomaterials can be formulated to trigger drug release in response to specific physiological conditions or external stimuli. These smart materials can detect changes in pH, temperature, enzyme activity, or other biological signals to initiate drug release precisely when needed. By incorporating responsive elements into the material structure, these systems can provide on-demand drug delivery that adapts to the patient's physiological state, improving therapeutic efficacy while minimizing side effects.
- Cell-based delivery systems for therapeutic applications: Cell-based delivery systems utilize living cells as vehicles for drug production and delivery. These systems can be engineered to express therapeutic proteins or produce small molecule drugs directly at the target site. The cells can be encapsulated within protective matrices that allow nutrient exchange while preventing immune rejection. This approach enables localized, sustained drug delivery and can be particularly valuable for treating chronic conditions that require continuous therapy.
- Biofilm-based drug delivery platforms: Biofilm-based platforms leverage the natural properties of microbial communities to create drug delivery systems. These engineered biofilms can be designed to produce and release therapeutic compounds in a controlled manner. The extracellular matrix produced by the biofilm provides structural support and can modulate drug release kinetics. By engineering the composition and properties of the biofilm, these systems can deliver drugs with specific temporal and spatial patterns, offering new approaches for treating localized infections or other conditions.
- Integration of synthetic biology with material science for advanced drug delivery: The integration of synthetic biology with material science enables the development of sophisticated drug delivery systems. By combining engineered biological components with advanced materials, these hybrid systems can achieve functionalities not possible with either approach alone. Synthetic gene circuits can be designed to regulate drug production and release in response to specific signals, while material components provide structural support and additional control over drug diffusion. This interdisciplinary approach opens new possibilities for personalized medicine and targeted therapies.
02 Responsive biomaterials for on-demand drug delivery
Advanced biomaterials can be engineered to respond to specific stimuli such as pH changes, temperature fluctuations, or biochemical signals to trigger drug release. These smart materials incorporate responsive elements that undergo conformational or chemical changes when exposed to particular conditions, enabling precise control over when and where drugs are released. This approach allows for targeted therapy that minimizes side effects while maximizing therapeutic efficacy.Expand Specific Solutions03 Cell-based delivery systems for therapeutic applications
Living cells can be engineered to function as drug delivery vehicles, producing and secreting therapeutic molecules directly at target sites. These cell-based systems can be designed to respond to specific signals or operate continuously, providing sustained release of therapeutic agents. By incorporating these engineered cells into biocompatible matrices, long-term therapeutic effects can be achieved with minimal intervention, making them particularly valuable for chronic conditions requiring ongoing treatment.Expand Specific Solutions04 Biofilm-based drug delivery platforms
Bacterial biofilms can be engineered as living materials for controlled drug delivery applications. These structured communities of microorganisms produce extracellular matrices that can be modified to incorporate therapeutic agents. The biofilm architecture provides protection for the encapsulated drugs while allowing for controlled diffusion and release. By engineering the bacterial components, these systems can be programmed to respond to specific triggers and release drugs accordingly.Expand Specific Solutions05 Integration of synthetic biology with material science for programmable drug delivery
The convergence of synthetic biology and materials science enables the development of programmable drug delivery systems with unprecedented control. Genetically engineered cells can be incorporated into biomaterials to create hybrid systems that respond to specific signals and produce therapeutic molecules on demand. These integrated systems combine the adaptability of living organisms with the structural properties of engineered materials, offering new possibilities for personalized medicine and targeted therapies.Expand Specific Solutions
Key Industry Players in ELM Drug Delivery
The engineered living materials (ELM) for on-demand drug delivery field is currently in an early growth phase, characterized by significant academic research transitioning toward commercial applications. The market is projected to expand substantially as these technologies address critical challenges in targeted therapeutics and personalized medicine. While still emerging, the technology shows promising maturity levels with key players driving innovation across different segments. Academic institutions (Columbia University, University of North Carolina, Boston University) are establishing foundational research, while pharmaceutical companies (Sanofi, Pfizer) are investing in clinical applications. Specialized firms like Aquestive Therapeutics, MDimune, and Arsenal Medical are developing proprietary delivery platforms, with research organizations (INSERM, CNRS) providing collaborative support. This diverse ecosystem indicates a technology approaching inflection point between research prominence and commercial viability.
Sanofi-Aventis Deutschland GmbH
Technical Solution: Sanofi-Aventis Deutschland has developed a sophisticated engineered living materials platform called "BioResponsive Matrix Technology" (BRM-Tech) for targeted drug delivery applications. Their approach integrates genetically engineered probiotic bacteria with advanced biomaterials to create responsive drug delivery systems. The core technology employs Lactobacillus and Bifidobacterium strains that have been engineered to sense specific disease biomarkers and respond by producing therapeutic proteins or small molecules. These engineered probiotics are encapsulated within a proprietary multi-layered hydrogel matrix that provides protection from harsh environmental conditions while allowing selective permeability for nutrients and signaling molecules. The BRM-Tech system incorporates oxygen-scavenging components to maintain anaerobic conditions necessary for probiotic viability, extending the functional lifetime of the living material. Sanofi's platform includes programmable release mechanisms triggered by specific disease-associated enzymes that degrade portions of the matrix, enabling site-specific drug release. The company has demonstrated successful application of this technology for inflammatory bowel disease treatment, where the engineered living materials respond to inflammation markers by producing anti-inflammatory compounds directly at disease sites[4][6].
Strengths: Leverages established safety profile of probiotic bacteria; sophisticated multi-layered protection system enhances stability and shelf-life; targeted activation only at disease sites minimizes systemic side effects; potential for continuous, responsive drug production over extended periods. Weaknesses: Challenges in maintaining consistent bacterial viability during storage; complex manufacturing processes increase production costs; potential variability in therapeutic response between patients; regulatory pathway for living therapeutics remains challenging.
The University of North Carolina at Chapel Hill
Technical Solution: The University of North Carolina at Chapel Hill has developed an advanced engineered living materials platform called "CellMatrix" for controlled drug delivery applications. Their technology centers on mammalian cell-based systems embedded within specialized extracellular matrix (ECM) components that mimic native tissue environments. The CellMatrix platform utilizes genetically modified human cells (primarily mesenchymal stem cells and fibroblasts) engineered with synthetic gene circuits that respond to specific physiological signals by producing therapeutic proteins. These engineered cells are encapsulated within a composite hydrogel system containing both natural ECM proteins and synthetic polymers that provide structural support while facilitating nutrient diffusion and cell-cell communication. A key innovation in their approach is the incorporation of degradable microparticles loaded with growth factors and signaling molecules that are released over time to maintain cell viability and functionality. The university's research teams have demonstrated sustained therapeutic protein production for over six months in preclinical models, with the ability to modulate production rates using non-invasive stimuli such as light or ultrasound. Their system includes safety mechanisms such as inducible suicide genes that can be activated to eliminate the engineered cells if needed[7][9].
Strengths: Utilizes human cells with sophisticated protein production capabilities; excellent biocompatibility with host tissues; potential for long-term implantation and sustained drug delivery; ability to produce complex biologics including antibodies and growth factors. Weaknesses: Higher manufacturing complexity and cost compared to microbial systems; challenges in maintaining consistent cell phenotype over extended periods; potential immunogenicity concerns despite autologous approaches; more complex regulatory pathway compared to conventional drug delivery systems.
Critical Patents in Responsive Biomaterial Design
Composition for on-demand ultrasound-triggered drug delivery
PatentActiveUS10010709B2
Innovation
- An injectable or implantable drug delivery system utilizing a drug depot with encapsulating material and microbubbles that enhances ultrasound-triggered drug release, allowing for controlled and repeated drug delivery through modulation of ultrasound frequency, intensity, and duration, enabling precise dosage administration.
Drug delivery device, compositions and methods relating thereto
PatentWO2009023500A1
Innovation
- An implantable medical device with a flexible elongate element serving as a polymeric carrier for therapeutic agents, anchored to a vessel wall, which can be deployed using a shape memory material-based deployment device, allowing for controlled release of drugs over extended periods.
Regulatory Framework for Living Therapeutic Materials
The regulatory landscape for Engineered Living Materials (ELMs) used in drug delivery systems presents unique challenges that span multiple regulatory domains. Currently, these innovative therapeutic materials exist in a regulatory gray zone between traditional pharmaceuticals, medical devices, and biological products. The FDA has begun developing frameworks through its Center for Biologics Evaluation and Research (CBER) and Center for Drug Evaluation and Research (CDER), but comprehensive guidelines specific to living therapeutic materials remain incomplete.
Key regulatory considerations include genetic stability requirements, where manufacturers must demonstrate that engineered organisms maintain consistent therapeutic properties over time without unwanted mutations. Safety containment protocols represent another critical regulatory hurdle, requiring robust mechanisms to prevent uncontrolled proliferation of engineered cells within patients or environmental release.
The European Medicines Agency has adopted a case-by-case evaluation approach for these novel therapeutics, focusing on risk-benefit assessments that consider both immediate safety concerns and long-term ecological implications. Japan's PMDA has established an expedited pathway specifically for regenerative medicine products that could potentially accommodate certain ELM-based drug delivery systems.
Regulatory bodies worldwide are increasingly requiring comprehensive biocontainment strategies, including genetic kill switches and metabolic dependencies that prevent survival outside controlled environments. Documentation requirements for ELM-based therapies typically exceed those for conventional pharmaceuticals, with extensive characterization of genetic modifications, cellular behavior, and drug release kinetics.
International harmonization efforts are underway through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), which recently established a working group focused on cell-based advanced therapy medicinal products. This initiative aims to develop globally recognized standards for characterization, quality control, and safety assessment of living therapeutic materials.
Post-market surveillance requirements for ELM-based drug delivery systems are particularly stringent, often requiring long-term patient monitoring and environmental impact assessments. Regulatory pathways are evolving to accommodate these innovative therapies, with several countries implementing adaptive licensing approaches that allow for controlled market entry with ongoing evidence generation.
The regulatory framework continues to evolve as scientific understanding advances, with increasing emphasis on standardized characterization methods and validated biocontainment strategies. Successful navigation of this complex regulatory landscape requires early and frequent engagement with regulatory authorities through mechanisms like the FDA's INTERACT program and the EMA's Innovation Task Force.
Key regulatory considerations include genetic stability requirements, where manufacturers must demonstrate that engineered organisms maintain consistent therapeutic properties over time without unwanted mutations. Safety containment protocols represent another critical regulatory hurdle, requiring robust mechanisms to prevent uncontrolled proliferation of engineered cells within patients or environmental release.
The European Medicines Agency has adopted a case-by-case evaluation approach for these novel therapeutics, focusing on risk-benefit assessments that consider both immediate safety concerns and long-term ecological implications. Japan's PMDA has established an expedited pathway specifically for regenerative medicine products that could potentially accommodate certain ELM-based drug delivery systems.
Regulatory bodies worldwide are increasingly requiring comprehensive biocontainment strategies, including genetic kill switches and metabolic dependencies that prevent survival outside controlled environments. Documentation requirements for ELM-based therapies typically exceed those for conventional pharmaceuticals, with extensive characterization of genetic modifications, cellular behavior, and drug release kinetics.
International harmonization efforts are underway through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), which recently established a working group focused on cell-based advanced therapy medicinal products. This initiative aims to develop globally recognized standards for characterization, quality control, and safety assessment of living therapeutic materials.
Post-market surveillance requirements for ELM-based drug delivery systems are particularly stringent, often requiring long-term patient monitoring and environmental impact assessments. Regulatory pathways are evolving to accommodate these innovative therapies, with several countries implementing adaptive licensing approaches that allow for controlled market entry with ongoing evidence generation.
The regulatory framework continues to evolve as scientific understanding advances, with increasing emphasis on standardized characterization methods and validated biocontainment strategies. Successful navigation of this complex regulatory landscape requires early and frequent engagement with regulatory authorities through mechanisms like the FDA's INTERACT program and the EMA's Innovation Task Force.
Bioethical Considerations and Safety Protocols
The development of Engineered Living Materials (ELMs) for on-demand drug delivery presents significant bioethical challenges that must be addressed through comprehensive safety protocols. These materials, which integrate living cells with non-living components, raise unique ethical considerations regarding their creation, deployment, and long-term impacts on human health and the environment.
Informed consent represents a foundational bioethical principle that must be rigorously applied in clinical applications of ELMs. Patients must receive complete information about the novel nature of these materials, including potential risks, benefits, and alternatives. The dynamic nature of living materials—capable of responding to environmental cues and potentially evolving over time—complicates traditional consent frameworks and necessitates ongoing communication with patients.
Environmental safety concerns are particularly acute with ELMs, as these materials contain engineered organisms that could potentially interact with natural ecosystems. Robust containment strategies must be developed to prevent unintended release, horizontal gene transfer, or ecological disruption. This includes physical containment mechanisms, genetic safeguards such as kill switches, and nutritional dependencies that limit survival outside controlled environments.
Regulatory frameworks for ELMs currently exist in a gray area between medical device, pharmaceutical, and genetically modified organism regulations. International harmonization of these frameworks is essential to ensure consistent safety standards while enabling scientific progress. Regulatory bodies must develop specialized guidelines that address the unique characteristics of living materials, including their ability to replicate, evolve, and respond to environmental stimuli.
Long-term monitoring protocols represent another critical component of ELM safety. Unlike conventional materials, ELMs may change over time, necessitating surveillance systems to track performance, detect mutations, and monitor potential adverse effects. This monitoring should extend beyond immediate clinical outcomes to include potential ecological impacts if containment is breached.
Equitable access to ELM technologies raises additional ethical considerations. As these advanced therapeutic platforms develop, ensuring they don't exacerbate existing healthcare disparities becomes paramount. Strategies to promote global access through technology transfer, tiered pricing models, and international research collaborations should be integrated into development pathways from early stages.
Dual-use concerns must also be addressed, as technologies developed for therapeutic drug delivery could potentially be repurposed for harmful applications. Research governance frameworks should include security reviews and responsible innovation principles to mitigate these risks while preserving beneficial applications.
Informed consent represents a foundational bioethical principle that must be rigorously applied in clinical applications of ELMs. Patients must receive complete information about the novel nature of these materials, including potential risks, benefits, and alternatives. The dynamic nature of living materials—capable of responding to environmental cues and potentially evolving over time—complicates traditional consent frameworks and necessitates ongoing communication with patients.
Environmental safety concerns are particularly acute with ELMs, as these materials contain engineered organisms that could potentially interact with natural ecosystems. Robust containment strategies must be developed to prevent unintended release, horizontal gene transfer, or ecological disruption. This includes physical containment mechanisms, genetic safeguards such as kill switches, and nutritional dependencies that limit survival outside controlled environments.
Regulatory frameworks for ELMs currently exist in a gray area between medical device, pharmaceutical, and genetically modified organism regulations. International harmonization of these frameworks is essential to ensure consistent safety standards while enabling scientific progress. Regulatory bodies must develop specialized guidelines that address the unique characteristics of living materials, including their ability to replicate, evolve, and respond to environmental stimuli.
Long-term monitoring protocols represent another critical component of ELM safety. Unlike conventional materials, ELMs may change over time, necessitating surveillance systems to track performance, detect mutations, and monitor potential adverse effects. This monitoring should extend beyond immediate clinical outcomes to include potential ecological impacts if containment is breached.
Equitable access to ELM technologies raises additional ethical considerations. As these advanced therapeutic platforms develop, ensuring they don't exacerbate existing healthcare disparities becomes paramount. Strategies to promote global access through technology transfer, tiered pricing models, and international research collaborations should be integrated into development pathways from early stages.
Dual-use concerns must also be addressed, as technologies developed for therapeutic drug delivery could potentially be repurposed for harmful applications. Research governance frameworks should include security reviews and responsible innovation principles to mitigate these risks while preserving beneficial applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!