Enhancing blood–brain barrier maturity in vitro using pericyte–astrocyte co-culture strategies on chip
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
BBB Maturity Enhancement Background and Objectives
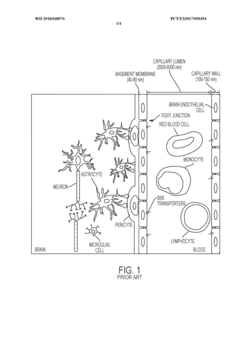
The blood-brain barrier (BBB) represents one of the most critical physiological interfaces in the human body, regulating the exchange of substances between the bloodstream and the central nervous system. Since its discovery in the late 19th century by Paul Ehrlich, understanding of the BBB has evolved significantly, revealing its complex cellular architecture comprising endothelial cells, pericytes, astrocytes, and basement membrane components. This intricate structure forms a highly selective semipermeable border that maintains brain homeostasis while protecting neural tissue from potentially harmful substances.
Recent technological advancements have enabled researchers to develop in vitro BBB models that attempt to recapitulate the physiological characteristics of the native barrier. However, achieving appropriate BBB maturity in these models remains a significant challenge. Traditional 2D cell culture systems fail to reproduce the three-dimensional cellular interactions and physiological fluid dynamics present in vivo, resulting in models with suboptimal barrier properties and limited translational value.
The emergence of organ-on-chip technology has created new opportunities to address these limitations by providing microenvironments that better mimic in vivo conditions. These platforms enable the integration of multiple cell types in defined spatial arrangements with controlled fluid flow, potentially enhancing BBB maturity. Particularly promising is the co-culture of pericytes and astrocytes with endothelial cells, as these supporting cells play crucial roles in inducing and maintaining BBB characteristics through complex cell-cell interactions and secreted factors.
The primary objective of this technical research is to explore and evaluate strategies for enhancing BBB maturity in vitro through pericyte-astrocyte co-culture approaches on microfluidic chip platforms. Specifically, we aim to identify optimal cellular compositions, spatial arrangements, and culture conditions that promote the development of BBB models with physiologically relevant barrier properties, including appropriate tight junction formation, transporter expression, and functional responses.
Additionally, this research seeks to establish quantifiable metrics for assessing BBB maturity in these models, enabling standardized evaluation and comparison across different experimental approaches. By developing more physiologically relevant BBB models, we anticipate significant advancements in neuropharmacology, particularly in drug screening for neurological disorders, understanding BBB dysfunction in pathological conditions, and developing targeted delivery strategies for CNS therapeutics.
The evolution of BBB modeling technology is trending toward increasingly complex systems that incorporate multiple cell types and physiological stimuli. This research aligns with this trajectory by focusing on the critical contributions of pericytes and astrocytes to BBB maturity, with the ultimate goal of creating more predictive preclinical models for neurological drug development and disease modeling.
Recent technological advancements have enabled researchers to develop in vitro BBB models that attempt to recapitulate the physiological characteristics of the native barrier. However, achieving appropriate BBB maturity in these models remains a significant challenge. Traditional 2D cell culture systems fail to reproduce the three-dimensional cellular interactions and physiological fluid dynamics present in vivo, resulting in models with suboptimal barrier properties and limited translational value.
The emergence of organ-on-chip technology has created new opportunities to address these limitations by providing microenvironments that better mimic in vivo conditions. These platforms enable the integration of multiple cell types in defined spatial arrangements with controlled fluid flow, potentially enhancing BBB maturity. Particularly promising is the co-culture of pericytes and astrocytes with endothelial cells, as these supporting cells play crucial roles in inducing and maintaining BBB characteristics through complex cell-cell interactions and secreted factors.
The primary objective of this technical research is to explore and evaluate strategies for enhancing BBB maturity in vitro through pericyte-astrocyte co-culture approaches on microfluidic chip platforms. Specifically, we aim to identify optimal cellular compositions, spatial arrangements, and culture conditions that promote the development of BBB models with physiologically relevant barrier properties, including appropriate tight junction formation, transporter expression, and functional responses.
Additionally, this research seeks to establish quantifiable metrics for assessing BBB maturity in these models, enabling standardized evaluation and comparison across different experimental approaches. By developing more physiologically relevant BBB models, we anticipate significant advancements in neuropharmacology, particularly in drug screening for neurological disorders, understanding BBB dysfunction in pathological conditions, and developing targeted delivery strategies for CNS therapeutics.
The evolution of BBB modeling technology is trending toward increasingly complex systems that incorporate multiple cell types and physiological stimuli. This research aligns with this trajectory by focusing on the critical contributions of pericytes and astrocytes to BBB maturity, with the ultimate goal of creating more predictive preclinical models for neurological drug development and disease modeling.
Market Analysis for BBB Models
The global market for blood-brain barrier (BBB) models is experiencing significant growth, driven by increasing research in neurodegenerative diseases and the need for more effective drug delivery systems targeting the central nervous system. Current market valuation stands at approximately 1.2 billion USD in 2023, with projections indicating a compound annual growth rate of 12.5% through 2030, potentially reaching 2.7 billion USD by the end of the decade.
The pharmaceutical industry represents the largest segment of demand, accounting for nearly 60% of the market share. This dominance stems from the critical need to develop CNS-targeting therapeutics with improved BBB penetration capabilities. Currently, less than 2% of small molecule drugs and virtually no large molecule therapeutics can effectively cross the BBB, creating substantial market pressure for improved in vitro models that accurately predict drug permeability.
Academic research institutions constitute the second-largest market segment at 25%, followed by biotechnology companies at 15%. Geographically, North America leads with 45% market share, followed by Europe (30%), Asia-Pacific (20%), and rest of the world (5%). The Asia-Pacific region, particularly China and South Korea, demonstrates the fastest growth trajectory with increasing investments in neuroscience research infrastructure.
Microfluidic BBB models, especially those incorporating co-culture systems like the pericyte-astrocyte platforms, represent the fastest-growing segment within this market. Traditional transwell systems still dominate with approximately 55% market share, but their growth has plateaued as researchers increasingly recognize their limitations in replicating physiological conditions.
Key market drivers include rising prevalence of neurological disorders, increasing R&D investments in neuropharmaceuticals, and growing recognition of the limitations of animal models in predicting human BBB responses. The global burden of neurological disorders is expected to increase substantially over the next decade due to aging populations, creating urgent demand for effective CNS therapeutics and corresponding testing platforms.
Market challenges include high development costs for advanced BBB models, technical complexity in achieving physiologically relevant barrier properties, and regulatory uncertainties regarding validation standards. Despite these challenges, the convergence of microfluidics, tissue engineering, and stem cell technologies is creating unprecedented opportunities for market expansion.
Industry analysts identify organ-on-chip BBB models with integrated sensor systems as having the highest growth potential, with projected annual growth rates exceeding 20% through 2028. Companies offering comprehensive solutions that combine advanced BBB models with analytical services are positioned to capture premium market segments.
The pharmaceutical industry represents the largest segment of demand, accounting for nearly 60% of the market share. This dominance stems from the critical need to develop CNS-targeting therapeutics with improved BBB penetration capabilities. Currently, less than 2% of small molecule drugs and virtually no large molecule therapeutics can effectively cross the BBB, creating substantial market pressure for improved in vitro models that accurately predict drug permeability.
Academic research institutions constitute the second-largest market segment at 25%, followed by biotechnology companies at 15%. Geographically, North America leads with 45% market share, followed by Europe (30%), Asia-Pacific (20%), and rest of the world (5%). The Asia-Pacific region, particularly China and South Korea, demonstrates the fastest growth trajectory with increasing investments in neuroscience research infrastructure.
Microfluidic BBB models, especially those incorporating co-culture systems like the pericyte-astrocyte platforms, represent the fastest-growing segment within this market. Traditional transwell systems still dominate with approximately 55% market share, but their growth has plateaued as researchers increasingly recognize their limitations in replicating physiological conditions.
Key market drivers include rising prevalence of neurological disorders, increasing R&D investments in neuropharmaceuticals, and growing recognition of the limitations of animal models in predicting human BBB responses. The global burden of neurological disorders is expected to increase substantially over the next decade due to aging populations, creating urgent demand for effective CNS therapeutics and corresponding testing platforms.
Market challenges include high development costs for advanced BBB models, technical complexity in achieving physiologically relevant barrier properties, and regulatory uncertainties regarding validation standards. Despite these challenges, the convergence of microfluidics, tissue engineering, and stem cell technologies is creating unprecedented opportunities for market expansion.
Industry analysts identify organ-on-chip BBB models with integrated sensor systems as having the highest growth potential, with projected annual growth rates exceeding 20% through 2028. Companies offering comprehensive solutions that combine advanced BBB models with analytical services are positioned to capture premium market segments.
Current Challenges in BBB In Vitro Models
Despite significant advancements in blood-brain barrier (BBB) in vitro modeling, current models still face substantial challenges in accurately replicating the complex physiological environment of the human BBB. Traditional 2D cell culture systems fail to capture the three-dimensional architecture and dynamic interactions present in vivo, resulting in models with limited predictive value for drug development and disease research.
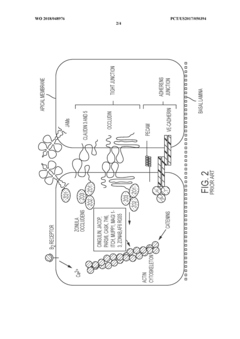
A primary limitation of existing models is the inadequate representation of cellular complexity. While endothelial cells are commonly incorporated, many models lack the critical supporting cells—particularly pericytes and astrocytes—that significantly contribute to BBB maturity and function. The absence of these cellular components results in incomplete barrier properties, including suboptimal tight junction formation and inadequate expression of transporters and metabolic enzymes.
Static culture conditions represent another significant challenge, as they fail to replicate the shear stress experienced by brain endothelial cells in vivo. This mechanical stimulus is crucial for proper cell alignment, tight junction formation, and expression of key BBB markers. Without physiologically relevant fluid dynamics, in vitro models often exhibit artificially high permeability and altered cellular responses to stimuli.
The selection of appropriate cell sources further complicates BBB modeling. Human primary cells, while physiologically relevant, suffer from limited availability, batch-to-batch variability, and restricted proliferation capacity. Immortalized cell lines offer improved accessibility but frequently demonstrate altered phenotypes and compromised barrier properties. Stem cell-derived models show promise but require complex differentiation protocols and still struggle to achieve full physiological maturity.
Reproducibility and standardization remain persistent challenges across BBB models. Variations in culture conditions, cell sources, and analytical methods make cross-laboratory comparisons difficult and hinder the establishment of reliable benchmarks for model validation. This lack of standardization impedes the broader adoption of BBB models in pharmaceutical research and regulatory contexts.
Technical limitations in monitoring and measuring BBB functionality present additional obstacles. Current methods for assessing barrier integrity, such as transendothelial electrical resistance (TEER) measurements and permeability assays, often provide incomplete information about the complex barrier properties. More sophisticated analytical techniques are needed to comprehensively characterize BBB models and validate their physiological relevance.
The translation gap between in vitro findings and in vivo outcomes represents perhaps the most significant challenge. Many BBB models demonstrate poor predictive value for clinical outcomes, particularly regarding drug penetration and efficacy. This disconnect highlights the need for more sophisticated models that better recapitulate the physiological complexity of the human BBB, especially when studying disease states or evaluating therapeutic interventions.
A primary limitation of existing models is the inadequate representation of cellular complexity. While endothelial cells are commonly incorporated, many models lack the critical supporting cells—particularly pericytes and astrocytes—that significantly contribute to BBB maturity and function. The absence of these cellular components results in incomplete barrier properties, including suboptimal tight junction formation and inadequate expression of transporters and metabolic enzymes.
Static culture conditions represent another significant challenge, as they fail to replicate the shear stress experienced by brain endothelial cells in vivo. This mechanical stimulus is crucial for proper cell alignment, tight junction formation, and expression of key BBB markers. Without physiologically relevant fluid dynamics, in vitro models often exhibit artificially high permeability and altered cellular responses to stimuli.
The selection of appropriate cell sources further complicates BBB modeling. Human primary cells, while physiologically relevant, suffer from limited availability, batch-to-batch variability, and restricted proliferation capacity. Immortalized cell lines offer improved accessibility but frequently demonstrate altered phenotypes and compromised barrier properties. Stem cell-derived models show promise but require complex differentiation protocols and still struggle to achieve full physiological maturity.
Reproducibility and standardization remain persistent challenges across BBB models. Variations in culture conditions, cell sources, and analytical methods make cross-laboratory comparisons difficult and hinder the establishment of reliable benchmarks for model validation. This lack of standardization impedes the broader adoption of BBB models in pharmaceutical research and regulatory contexts.
Technical limitations in monitoring and measuring BBB functionality present additional obstacles. Current methods for assessing barrier integrity, such as transendothelial electrical resistance (TEER) measurements and permeability assays, often provide incomplete information about the complex barrier properties. More sophisticated analytical techniques are needed to comprehensively characterize BBB models and validate their physiological relevance.
The translation gap between in vitro findings and in vivo outcomes represents perhaps the most significant challenge. Many BBB models demonstrate poor predictive value for clinical outcomes, particularly regarding drug penetration and efficacy. This disconnect highlights the need for more sophisticated models that better recapitulate the physiological complexity of the human BBB, especially when studying disease states or evaluating therapeutic interventions.
Current Pericyte-Astrocyte Co-culture Methodologies
01 Microfluidic BBB models
Microfluidic devices provide dynamic in vitro models of the blood-brain barrier that better mimic physiological conditions. These systems allow for controlled fluid flow, shear stress, and co-culture of multiple cell types in compartmentalized chambers. The dynamic environment improves tight junction formation and barrier integrity compared to static models, enabling more accurate assessment of drug permeability and transport mechanisms across the BBB.- Microfluidic BBB models: Microfluidic devices are used to create dynamic in vitro BBB models that better mimic the physiological conditions of the blood-brain barrier. These systems incorporate flow conditions and allow for the co-culture of multiple cell types, including endothelial cells, astrocytes, and pericytes. The dynamic nature of these models provides more accurate representation of BBB function, including permeability characteristics and response to stimuli, making them valuable for drug screening and disease modeling.
- 3D organoid and spheroid BBB models: Three-dimensional organoid and spheroid models represent advanced approaches to modeling the BBB in vitro. These models utilize stem cells or primary cells to self-organize into structures that recapitulate the cellular architecture and functionality of the neurovascular unit. The 3D configuration allows for more physiologically relevant cell-cell interactions and barrier properties compared to traditional 2D models, enabling more accurate assessment of drug penetration and toxicity studies.
- Cell-based transwell BBB models: Transwell systems represent a widely used approach for modeling the BBB in vitro. These models typically involve culturing brain endothelial cells on a semipermeable membrane, with or without supporting cells such as astrocytes and pericytes in the lower compartment. This setup allows for the measurement of compound permeability across the cellular barrier and assessment of tight junction formation. Various cell sources can be used, including primary cells, immortalized cell lines, and stem cell-derived cells, each with different levels of barrier maturity and physiological relevance.
- iPSC-derived BBB models: Induced pluripotent stem cell (iPSC) technology has enabled the development of more physiologically relevant BBB models. These models involve differentiating iPSCs into brain endothelial cells and other components of the neurovascular unit. The advantage of iPSC-derived models is their human origin and potential for patient-specific modeling of BBB function in health and disease. These models exhibit key BBB characteristics including tight junctions, transporter expression, and barrier properties, making them valuable for studying drug delivery across the BBB and neurological disorders.
- BBB model validation and maturity assessment: Methods for assessing the maturity and physiological relevance of in vitro BBB models are critical for their application in research and drug development. These include measurement of transendothelial electrical resistance (TEER), permeability to marker compounds, expression of tight junction proteins and transporters, and response to inflammatory stimuli. Advanced techniques such as transcriptomics, proteomics, and functional assays help determine how closely the models recapitulate the in vivo BBB. Standardized protocols and benchmarks are being developed to compare different BBB models and establish their suitability for specific applications.
02 3D cell culture BBB models
Three-dimensional cell culture systems provide more physiologically relevant BBB models compared to traditional 2D cultures. These models incorporate scaffolds, hydrogels, or spheroid formations that allow endothelial cells, astrocytes, and pericytes to organize in spatial arrangements similar to the in vivo BBB. The 3D architecture promotes improved cell-cell interactions, tight junction formation, and expression of transporters, resulting in barrier properties that more closely resemble the native BBB.Expand Specific Solutions03 iPSC-derived BBB models
Induced pluripotent stem cell (iPSC) technology enables the development of patient-specific BBB models. These models use human iPSCs differentiated into brain endothelial cells, astrocytes, and other BBB components. iPSC-derived BBB models exhibit key characteristics including tight junctions, transporter expression, and barrier function. They offer advantages for studying genetic factors in BBB development, disease modeling, and personalized medicine approaches for neurological disorders.Expand Specific Solutions04 BBB model validation and maturity assessment
Methods for evaluating the maturity and physiological relevance of in vitro BBB models include measuring transendothelial electrical resistance (TEER), permeability coefficients, expression of tight junction proteins, and transporter functionality. Advanced techniques such as impedance spectroscopy, fluorescent tracer assays, and molecular marker analysis provide comprehensive assessment of barrier integrity. These validation approaches are essential for determining the predictive value of BBB models for drug development and disease research.Expand Specific Solutions05 Disease-specific BBB models
Specialized in vitro BBB models have been developed to study specific neurological diseases and conditions that affect barrier function. These models incorporate disease-relevant features such as inflammatory mediators, pathogenic proteins, or genetic modifications to mimic conditions like Alzheimer's disease, stroke, multiple sclerosis, or brain tumors. Disease-specific BBB models enable investigation of pathological mechanisms and evaluation of therapeutic interventions targeting BBB dysfunction in neurological disorders.Expand Specific Solutions
Leading Organizations in BBB Research
The blood-brain barrier (BBB) in vitro modeling market is in an early growth phase, characterized by increasing research interest but limited commercial maturity. The market size is expanding as pharmaceutical companies seek better drug screening platforms, with projections suggesting significant growth potential in the next decade. Technologically, the field is transitioning from proof-of-concept to standardization, with leading players demonstrating varying levels of sophistication. Emulate, Inc. has established itself as a frontrunner with its organ-on-chip technology, while academic institutions like Massachusetts Institute of Technology and National University of Singapore contribute significant innovations. Pharmaceutical research centers including Cedars-Sinai and FUJIFILM are investing in BBB models to enhance drug development pipelines. The competitive landscape features collaboration between specialized biotech firms and research institutions, with Japanese and Chinese entities increasingly active in this space.
Emulate, Inc.
Technical Solution: Emulate has developed advanced Organ-on-Chip technology specifically designed to model the blood-brain barrier (BBB). Their BBB-Chip incorporates a co-culture system where human brain microvascular endothelial cells are grown on one side of a porous membrane, while primary human brain pericytes and astrocytes are cultured on the opposite side. This three-dimensional microenvironment mimics the in vivo cellular architecture and enables physiologically relevant cell-cell interactions. The chip features microfluidic channels that allow for continuous perfusion, creating shear stress conditions similar to those in brain capillaries. This dynamic culture environment has been shown to significantly enhance tight junction formation and expression of key BBB transporters compared to static models. Emulate's platform also incorporates real-time monitoring capabilities to assess barrier integrity through transendothelial electrical resistance (TEER) measurements and permeability assays, providing quantitative data on BBB maturation and functionality.
Strengths: The system allows for precise control of fluid dynamics and mechanical forces that promote BBB maturation. The platform is highly reproducible and enables high-throughput screening applications for drug development. Weaknesses: The technology requires specialized equipment and expertise to operate effectively. The cost per chip remains relatively high compared to traditional cell culture methods, limiting widespread adoption in academic research settings.
Wisconsin Alumni Research Foundation
Technical Solution: Wisconsin researchers have developed a sophisticated BBB-on-chip platform that features a unique vertical arrangement of the neurovascular unit components. Their system employs a multi-layered microfluidic device where brain endothelial cells are cultured on the upper surface of a porous membrane, while pericytes are attached to the underside of the same membrane, creating direct cellular contact through the pores. Astrocytes are cultured in a separate but adjacent compartment that allows for paracrine signaling through a hydrogel interface. This configuration closely mimics the anatomical arrangement of the BBB in vivo. A key innovation in their platform is the incorporation of a specialized medium circulation system that creates physiologically relevant shear stress conditions (4-12 dyne/cm²) while simultaneously allowing for different media compositions in the vascular and brain parenchymal compartments. Their research has demonstrated that this co-culture strategy significantly enhances the expression of tight junction proteins (ZO-1, claudin-5) and efflux transporters (P-gp, BCRP) compared to monoculture or static culture conditions. The platform also incorporates impedance spectroscopy capabilities for real-time monitoring of barrier integrity and allows for the introduction of immune cells to study neuroinflammatory processes.
Strengths: The vertical arrangement of cells creates a more anatomically correct model of the BBB compared to many other chip designs. The system allows for different media compositions in each compartment, enabling more precise control of the microenvironment on either side of the barrier. Weaknesses: The vertical stacking design makes direct microscopic visualization of cell-cell interactions more challenging than horizontal arrangements. The system requires more complex fabrication processes and has a steeper learning curve for new users compared to simpler designs.
Key Innovations in BBB Chip Technologies
Modeling blood-brain barrier in vitro
PatentWO2018048976A1
Innovation
- The development of synthetic human blood vessels with a hollow polymer wall and lumen, incorporating brain-derived microvascular endothelial cells, astrocytes, and pericytes, using a microfluidic method to create a more representative BBB model that allows for perfusion and shear stress simulation.
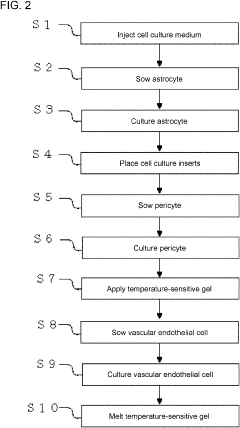
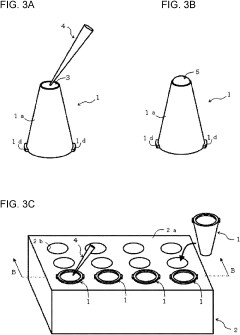
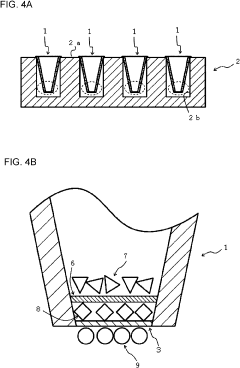
Cell culture method and cell culture device used with cell culture method
PatentPendingUS20230313149A1
Innovation
- A cell culture device with integrated upper and lower chambers separated by a porous membrane, allowing simultaneous seeding and culture of cells on both surfaces without sequential inversion, and using a temperature-sensitive gel to maintain the laminate structure of the cells.
Regulatory Considerations for BBB Models
The development of in vitro blood-brain barrier (BBB) models, particularly those utilizing pericyte-astrocyte co-culture strategies on chip, necessitates careful consideration of regulatory frameworks. These models must align with established guidelines to ensure their validity for drug development and therapeutic applications.
Regulatory bodies such as the FDA, EMA, and ICH have established specific requirements for preclinical testing models, including those representing the BBB. These agencies increasingly recognize the potential of organ-on-chip technologies to provide more physiologically relevant data than traditional in vitro systems. However, the regulatory landscape for these advanced models remains in development, creating both opportunities and challenges for researchers and developers.
Validation metrics represent a critical regulatory consideration for BBB models. Regulatory authorities require demonstration of barrier integrity through quantifiable parameters such as transendothelial electrical resistance (TEER), permeability coefficients, and expression of tight junction proteins. For pericyte-astrocyte co-culture models, additional validation metrics may include assessment of basement membrane formation, neurovascular unit functionality, and response to known BBB modulators.
Good Laboratory Practice (GLP) compliance presents another significant regulatory hurdle. BBB models intended for regulatory submission must be developed and tested under GLP conditions, with comprehensive documentation of protocols, materials, and quality control measures. This includes validation of the microfluidic platforms, cell sources, and analytical methods used in the model system.
Standardization efforts are underway to establish consensus protocols for BBB model development and characterization. Organizations such as the International Brain Barriers Society (IBBS) and the European Federation for Pharmaceutical Sciences (EUFEPS) are working to define minimum criteria for BBB model performance. These initiatives aim to facilitate regulatory acceptance by providing benchmarks against which new models can be evaluated.
The qualification process for novel methodologies represents a pathway for regulatory acceptance of innovative BBB models. Through the FDA's Biomarker Qualification Program or the EMA's Novel Methodologies Qualification, developers can seek formal recognition of their models as tools for specific contexts of use in drug development. This process typically requires extensive validation data demonstrating the model's predictive capacity for human BBB function.
Cross-comparison with established models and in vivo data remains essential for regulatory acceptance. Developers must demonstrate how their pericyte-astrocyte co-culture chip models correlate with gold standard approaches and human clinical outcomes. This comparative validation strengthens the regulatory case for adopting these advanced models in drug development pipelines.
Regulatory bodies such as the FDA, EMA, and ICH have established specific requirements for preclinical testing models, including those representing the BBB. These agencies increasingly recognize the potential of organ-on-chip technologies to provide more physiologically relevant data than traditional in vitro systems. However, the regulatory landscape for these advanced models remains in development, creating both opportunities and challenges for researchers and developers.
Validation metrics represent a critical regulatory consideration for BBB models. Regulatory authorities require demonstration of barrier integrity through quantifiable parameters such as transendothelial electrical resistance (TEER), permeability coefficients, and expression of tight junction proteins. For pericyte-astrocyte co-culture models, additional validation metrics may include assessment of basement membrane formation, neurovascular unit functionality, and response to known BBB modulators.
Good Laboratory Practice (GLP) compliance presents another significant regulatory hurdle. BBB models intended for regulatory submission must be developed and tested under GLP conditions, with comprehensive documentation of protocols, materials, and quality control measures. This includes validation of the microfluidic platforms, cell sources, and analytical methods used in the model system.
Standardization efforts are underway to establish consensus protocols for BBB model development and characterization. Organizations such as the International Brain Barriers Society (IBBS) and the European Federation for Pharmaceutical Sciences (EUFEPS) are working to define minimum criteria for BBB model performance. These initiatives aim to facilitate regulatory acceptance by providing benchmarks against which new models can be evaluated.
The qualification process for novel methodologies represents a pathway for regulatory acceptance of innovative BBB models. Through the FDA's Biomarker Qualification Program or the EMA's Novel Methodologies Qualification, developers can seek formal recognition of their models as tools for specific contexts of use in drug development. This process typically requires extensive validation data demonstrating the model's predictive capacity for human BBB function.
Cross-comparison with established models and in vivo data remains essential for regulatory acceptance. Developers must demonstrate how their pericyte-astrocyte co-culture chip models correlate with gold standard approaches and human clinical outcomes. This comparative validation strengthens the regulatory case for adopting these advanced models in drug development pipelines.
Translational Potential to Neurological Disease Research
The in vitro blood-brain barrier (BBB) model utilizing pericyte-astrocyte co-culture strategies on chip presents significant translational potential for neurological disease research. This advanced model bridges the gap between traditional cell culture systems and human pathophysiology, offering a more accurate representation of BBB dynamics in neurological disorders.
The enhanced BBB chip model enables precise investigation of disease mechanisms in conditions where BBB dysfunction plays a critical role, including Alzheimer's disease, Parkinson's disease, multiple sclerosis, and stroke. By replicating the complex cellular interactions between pericytes, astrocytes, and endothelial cells, researchers can observe how these relationships become dysregulated in pathological states, providing insights that were previously unattainable with simpler models.
Drug development for neurological conditions faces the persistent challenge of BBB penetration. The pericyte-astrocyte co-culture chip offers pharmaceutical companies a more predictive platform for screening potential therapeutic compounds, significantly reducing late-stage failures in clinical trials. This model can accurately assess both drug permeability across the BBB and potential neurotoxicity, streamlining the drug development pipeline for neurological disorders.
The chip system also presents opportunities for personalized medicine approaches. By incorporating patient-derived cells into the model, researchers can develop personalized BBB models that reflect individual disease characteristics. This capability allows for tailored therapeutic strategies based on patient-specific BBB properties and responses to treatment, advancing precision medicine for neurological disorders.
Inflammatory processes in neurological diseases can be effectively studied using this advanced BBB model. The co-culture system permits real-time monitoring of neuroinflammatory responses, including cytokine production, immune cell trafficking, and BBB integrity changes during inflammatory challenges. These insights are crucial for developing anti-inflammatory interventions for conditions like multiple sclerosis and neurodegenerative diseases.
Furthermore, the model serves as an ethical alternative to animal testing in neurological research. By providing a human-relevant platform that recapitulates key aspects of BBB physiology, it reduces reliance on animal models while potentially yielding more translatable results for human applications. This alignment with the 3Rs principle (replacement, reduction, refinement) represents a significant advancement in ethical neuroscience research practices.
The integration of this BBB model with other organ-on-chip systems creates potential for studying systemic effects on neurological health. Such multi-organ platforms could reveal how peripheral factors influence BBB function and neurological disease progression, offering a more holistic understanding of complex conditions like metabolic syndrome-associated cognitive decline or gut-brain axis disorders.
The enhanced BBB chip model enables precise investigation of disease mechanisms in conditions where BBB dysfunction plays a critical role, including Alzheimer's disease, Parkinson's disease, multiple sclerosis, and stroke. By replicating the complex cellular interactions between pericytes, astrocytes, and endothelial cells, researchers can observe how these relationships become dysregulated in pathological states, providing insights that were previously unattainable with simpler models.
Drug development for neurological conditions faces the persistent challenge of BBB penetration. The pericyte-astrocyte co-culture chip offers pharmaceutical companies a more predictive platform for screening potential therapeutic compounds, significantly reducing late-stage failures in clinical trials. This model can accurately assess both drug permeability across the BBB and potential neurotoxicity, streamlining the drug development pipeline for neurological disorders.
The chip system also presents opportunities for personalized medicine approaches. By incorporating patient-derived cells into the model, researchers can develop personalized BBB models that reflect individual disease characteristics. This capability allows for tailored therapeutic strategies based on patient-specific BBB properties and responses to treatment, advancing precision medicine for neurological disorders.
Inflammatory processes in neurological diseases can be effectively studied using this advanced BBB model. The co-culture system permits real-time monitoring of neuroinflammatory responses, including cytokine production, immune cell trafficking, and BBB integrity changes during inflammatory challenges. These insights are crucial for developing anti-inflammatory interventions for conditions like multiple sclerosis and neurodegenerative diseases.
Furthermore, the model serves as an ethical alternative to animal testing in neurological research. By providing a human-relevant platform that recapitulates key aspects of BBB physiology, it reduces reliance on animal models while potentially yielding more translatable results for human applications. This alignment with the 3Rs principle (replacement, reduction, refinement) represents a significant advancement in ethical neuroscience research practices.
The integration of this BBB model with other organ-on-chip systems creates potential for studying systemic effects on neurological health. Such multi-organ platforms could reveal how peripheral factors influence BBB function and neurological disease progression, offering a more holistic understanding of complex conditions like metabolic syndrome-associated cognitive decline or gut-brain axis disorders.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!