Comparison of primary human cells vs iPSC-derived cells for predictive toxicology in liver-on-chip assays
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Liver-on-Chip Technology Evolution and Objectives
Liver-on-chip technology has evolved significantly over the past decade, transforming from simple microfluidic cell culture systems to sophisticated organ mimetics capable of replicating complex liver functions. The initial development phase (2005-2010) focused primarily on creating basic microfluidic platforms that could maintain hepatocyte viability longer than traditional 2D cultures. During this period, researchers established fundamental design principles for fluid dynamics and cell compartmentalization.
The second evolutionary phase (2010-2015) witnessed the integration of multiple cell types to better recapitulate the liver microenvironment. This period marked the transition from hepatocyte monocultures to more complex co-culture systems incorporating non-parenchymal cells such as Kupffer cells, stellate cells, and sinusoidal endothelial cells. These advancements significantly improved the physiological relevance of liver-on-chip models.
From 2015 to 2020, the field experienced rapid technological advancement with the incorporation of biosensors, real-time monitoring capabilities, and improved biomaterial interfaces. This phase also saw the first commercial liver-on-chip platforms enter the market, facilitating wider adoption in pharmaceutical research settings. The integration of mechanical forces to mimic blood flow and tissue stiffness further enhanced model fidelity.
The current generation of liver-on-chip technologies (2020-present) focuses on addressing key challenges in predictive toxicology, particularly the sourcing of appropriate cell types. This has led to the parallel development of two major approaches: systems utilizing primary human hepatocytes and those employing induced pluripotent stem cell (iPSC)-derived hepatocytes. Each approach presents distinct advantages and limitations for toxicological assessment.
The primary objective of contemporary liver-on-chip research is to develop platforms that accurately predict human-specific hepatotoxicity while overcoming limitations of traditional in vitro and animal models. Specifically, these technologies aim to: (1) provide reproducible and standardized systems for drug toxicity screening; (2) enable personalized medicine approaches through patient-specific cell incorporation; (3) reduce reliance on animal testing in accordance with 3R principles; and (4) improve the detection of idiosyncratic drug reactions that often go undetected in preclinical studies.
Future technological objectives include enhancing the maturation of iPSC-derived hepatocytes to better match primary cell functionality, developing non-invasive monitoring systems for long-term studies, and creating multi-organ platforms that can assess systemic toxicity effects. The field is also moving toward standardization of protocols and validation metrics to facilitate regulatory acceptance of liver-on-chip data in drug development pipelines.
The second evolutionary phase (2010-2015) witnessed the integration of multiple cell types to better recapitulate the liver microenvironment. This period marked the transition from hepatocyte monocultures to more complex co-culture systems incorporating non-parenchymal cells such as Kupffer cells, stellate cells, and sinusoidal endothelial cells. These advancements significantly improved the physiological relevance of liver-on-chip models.
From 2015 to 2020, the field experienced rapid technological advancement with the incorporation of biosensors, real-time monitoring capabilities, and improved biomaterial interfaces. This phase also saw the first commercial liver-on-chip platforms enter the market, facilitating wider adoption in pharmaceutical research settings. The integration of mechanical forces to mimic blood flow and tissue stiffness further enhanced model fidelity.
The current generation of liver-on-chip technologies (2020-present) focuses on addressing key challenges in predictive toxicology, particularly the sourcing of appropriate cell types. This has led to the parallel development of two major approaches: systems utilizing primary human hepatocytes and those employing induced pluripotent stem cell (iPSC)-derived hepatocytes. Each approach presents distinct advantages and limitations for toxicological assessment.
The primary objective of contemporary liver-on-chip research is to develop platforms that accurately predict human-specific hepatotoxicity while overcoming limitations of traditional in vitro and animal models. Specifically, these technologies aim to: (1) provide reproducible and standardized systems for drug toxicity screening; (2) enable personalized medicine approaches through patient-specific cell incorporation; (3) reduce reliance on animal testing in accordance with 3R principles; and (4) improve the detection of idiosyncratic drug reactions that often go undetected in preclinical studies.
Future technological objectives include enhancing the maturation of iPSC-derived hepatocytes to better match primary cell functionality, developing non-invasive monitoring systems for long-term studies, and creating multi-organ platforms that can assess systemic toxicity effects. The field is also moving toward standardization of protocols and validation metrics to facilitate regulatory acceptance of liver-on-chip data in drug development pipelines.
Market Analysis for Predictive Hepatotoxicity Models
The global market for predictive hepatotoxicity models is experiencing significant growth, driven by increasing concerns about drug-induced liver injury (DILI) and the high attrition rates of drug candidates during development. Currently valued at approximately $1.2 billion, this market is projected to grow at a CAGR of 14.5% through 2028, reflecting the urgent need for more reliable preclinical testing methods.
Pharmaceutical companies represent the largest market segment, accounting for nearly 60% of the demand for liver toxicity prediction tools. This dominance stems from the substantial financial implications of late-stage drug failures, with each withdrawn drug costing an average of $2-5 billion in development expenses. Academic research institutions constitute about 25% of the market, while contract research organizations (CROs) make up the remaining 15%.
Geographically, North America leads with 45% market share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and South Korea, is witnessing the fastest growth rate at 17% annually, fueled by expanding pharmaceutical research activities and increasing regulatory requirements for toxicity testing.
The liver-on-chip segment specifically is growing at 18% annually, outpacing traditional hepatotoxicity testing methods. Within this niche, primary human hepatocyte-based models currently dominate with 65% market share, while iPSC-derived hepatocyte models account for 25% and are gaining momentum due to their reproducibility advantages and ethical considerations.
Key market drivers include stringent regulatory requirements, with FDA and EMA increasingly recommending advanced in vitro models for toxicity assessment. The high failure rate of drug candidates due to hepatotoxicity (approximately 30% of drug withdrawals) further propels market growth. Additionally, the pharmaceutical industry's shift toward personalized medicine creates demand for more sophisticated, patient-specific toxicity models.
Cost considerations significantly influence market dynamics. While primary human hepatocytes offer gold-standard performance, their high procurement costs ($800-1,500 per vial) and batch-to-batch variability limit widespread adoption. Conversely, iPSC-derived hepatocytes, despite higher initial development costs, offer better scalability and consistency, with production costs decreasing by approximately 35% over the past five years.
Industry surveys indicate that 78% of pharmaceutical companies plan to increase their investment in advanced hepatotoxicity prediction models over the next three years, with particular interest in systems that can integrate with AI-driven predictive algorithms for enhanced accuracy and throughput.
Pharmaceutical companies represent the largest market segment, accounting for nearly 60% of the demand for liver toxicity prediction tools. This dominance stems from the substantial financial implications of late-stage drug failures, with each withdrawn drug costing an average of $2-5 billion in development expenses. Academic research institutions constitute about 25% of the market, while contract research organizations (CROs) make up the remaining 15%.
Geographically, North America leads with 45% market share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and South Korea, is witnessing the fastest growth rate at 17% annually, fueled by expanding pharmaceutical research activities and increasing regulatory requirements for toxicity testing.
The liver-on-chip segment specifically is growing at 18% annually, outpacing traditional hepatotoxicity testing methods. Within this niche, primary human hepatocyte-based models currently dominate with 65% market share, while iPSC-derived hepatocyte models account for 25% and are gaining momentum due to their reproducibility advantages and ethical considerations.
Key market drivers include stringent regulatory requirements, with FDA and EMA increasingly recommending advanced in vitro models for toxicity assessment. The high failure rate of drug candidates due to hepatotoxicity (approximately 30% of drug withdrawals) further propels market growth. Additionally, the pharmaceutical industry's shift toward personalized medicine creates demand for more sophisticated, patient-specific toxicity models.
Cost considerations significantly influence market dynamics. While primary human hepatocytes offer gold-standard performance, their high procurement costs ($800-1,500 per vial) and batch-to-batch variability limit widespread adoption. Conversely, iPSC-derived hepatocytes, despite higher initial development costs, offer better scalability and consistency, with production costs decreasing by approximately 35% over the past five years.
Industry surveys indicate that 78% of pharmaceutical companies plan to increase their investment in advanced hepatotoxicity prediction models over the next three years, with particular interest in systems that can integrate with AI-driven predictive algorithms for enhanced accuracy and throughput.
Current Challenges in Cell-Based Liver Toxicology
Despite significant advancements in liver toxicology testing, several critical challenges persist in cell-based approaches that limit their predictive accuracy and clinical relevance. The traditional use of primary human hepatocytes (PHHs), while considered the gold standard, faces substantial hurdles including limited availability, donor variability, rapid dedifferentiation in culture, and high costs. These limitations severely restrict their application in high-throughput screening and long-term toxicity studies.
Animal-derived hepatocytes present additional complications, as they often fail to accurately predict human-specific toxicity responses due to interspecies differences in drug metabolism pathways, xenobiotic receptors, and transporter expression profiles. This species-specific variation has contributed to numerous late-stage drug development failures and post-market withdrawals.
Immortalized cell lines such as HepG2 and HepaRG, while offering advantages in terms of availability and reproducibility, demonstrate limited metabolic capacity compared to primary cells. Their altered gene expression profiles and reduced cytochrome P450 activity significantly compromise their ability to detect metabolism-dependent toxicity, a critical factor in drug-induced liver injury assessment.
The emerging iPSC-derived hepatocyte-like cells show promise but face challenges in achieving full functional maturity. Current differentiation protocols typically produce cells with fetal-like phenotypes rather than adult hepatocyte characteristics, resulting in incomplete metabolic competence and altered toxicity responses. Batch-to-batch variability in differentiation efficiency further complicates standardization efforts.
Microenvironmental factors present another significant challenge, as conventional 2D culture systems fail to recapitulate the complex architecture and cellular interactions of the native liver. This limitation has driven the development of liver-on-chip platforms, which aim to provide more physiologically relevant conditions. However, these systems face technical challenges in maintaining long-term cell viability, achieving reproducible cell seeding, and establishing appropriate fluid dynamics.
Standardization issues persist across the field, with variations in culture conditions, endpoint measurements, and data interpretation hampering cross-laboratory comparisons. The lack of validated reference compounds and harmonized protocols further impedes the establishment of reliable predictive models for hepatotoxicity assessment.
Additionally, current models struggle to capture the complexity of chronic and idiosyncratic toxicity mechanisms, which often involve adaptive responses, immune system interactions, and genetic predispositions. These mechanisms frequently underlie clinically significant adverse drug reactions but remain poorly represented in existing cell-based systems.
Animal-derived hepatocytes present additional complications, as they often fail to accurately predict human-specific toxicity responses due to interspecies differences in drug metabolism pathways, xenobiotic receptors, and transporter expression profiles. This species-specific variation has contributed to numerous late-stage drug development failures and post-market withdrawals.
Immortalized cell lines such as HepG2 and HepaRG, while offering advantages in terms of availability and reproducibility, demonstrate limited metabolic capacity compared to primary cells. Their altered gene expression profiles and reduced cytochrome P450 activity significantly compromise their ability to detect metabolism-dependent toxicity, a critical factor in drug-induced liver injury assessment.
The emerging iPSC-derived hepatocyte-like cells show promise but face challenges in achieving full functional maturity. Current differentiation protocols typically produce cells with fetal-like phenotypes rather than adult hepatocyte characteristics, resulting in incomplete metabolic competence and altered toxicity responses. Batch-to-batch variability in differentiation efficiency further complicates standardization efforts.
Microenvironmental factors present another significant challenge, as conventional 2D culture systems fail to recapitulate the complex architecture and cellular interactions of the native liver. This limitation has driven the development of liver-on-chip platforms, which aim to provide more physiologically relevant conditions. However, these systems face technical challenges in maintaining long-term cell viability, achieving reproducible cell seeding, and establishing appropriate fluid dynamics.
Standardization issues persist across the field, with variations in culture conditions, endpoint measurements, and data interpretation hampering cross-laboratory comparisons. The lack of validated reference compounds and harmonized protocols further impedes the establishment of reliable predictive models for hepatotoxicity assessment.
Additionally, current models struggle to capture the complexity of chronic and idiosyncratic toxicity mechanisms, which often involve adaptive responses, immune system interactions, and genetic predispositions. These mechanisms frequently underlie clinically significant adverse drug reactions but remain poorly represented in existing cell-based systems.
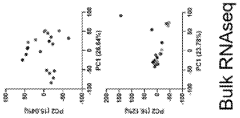
Comparative Analysis of Primary vs iPSC-Derived Hepatocytes
01 iPSC-derived cell models for toxicity screening
Induced pluripotent stem cells (iPSCs) can be differentiated into various cell types that mimic human tissues for toxicology testing. These iPSC-derived models provide physiologically relevant systems that can predict human-specific toxic responses more accurately than traditional animal models. The approach allows for personalized toxicity screening by using patient-specific cells, enabling the assessment of individual variations in drug responses and toxicity profiles.- iPSC-derived cell models for toxicity screening: Induced pluripotent stem cell (iPSC) derived models offer significant advantages for toxicology testing by providing physiologically relevant human cell systems. These models can be developed from various tissue types and used to assess compound toxicity in a more predictive manner than traditional cell lines. The iPSC technology enables the creation of patient-specific cell models that can capture individual variations in drug responses and toxicity profiles, making them valuable tools for personalized toxicology assessments.
- Primary human cell-based assays for toxicity prediction: Primary human cells isolated directly from tissues provide authentic cellular responses for toxicology studies. These cells maintain many of the characteristics of their original tissue, allowing for more accurate prediction of in vivo toxicity compared to immortalized cell lines. Assay systems using primary human cells can detect various toxicity endpoints including cytotoxicity, genotoxicity, and organ-specific toxicity, providing valuable data for safety assessment of pharmaceuticals, chemicals, and consumer products.
- 3D cell culture and organoid systems for advanced toxicology: Three-dimensional cell culture systems and organoids derived from primary human cells or iPSCs represent advanced models for toxicology testing. These systems better recapitulate the complex architecture and cellular interactions found in human tissues compared to traditional 2D cultures. Organoids can mimic organ-specific functions and responses to toxic compounds, enabling more accurate prediction of human toxicity. These advanced culture systems are particularly valuable for assessing chronic toxicity and complex tissue-specific adverse effects.
- High-throughput screening platforms using human cell models: High-throughput screening platforms incorporating primary human cells or iPSC-derived cells enable rapid assessment of compound toxicity profiles. These platforms utilize automated systems, advanced imaging technologies, and computational analysis to evaluate multiple toxicity endpoints simultaneously across numerous compounds. The integration of human cell-based models with high-throughput technologies accelerates the toxicity screening process while maintaining physiological relevance, supporting early safety assessment in drug discovery and chemical development.
- AI and computational methods for toxicity prediction using cell-based data: Artificial intelligence and computational methods enhance the predictive power of human cell-based toxicology models. These approaches analyze complex datasets generated from primary human cells and iPSC-derived models to identify patterns associated with toxicity. Machine learning algorithms can integrate multiple data types, including gene expression, cellular imaging, and biochemical measurements, to develop predictive models of compound toxicity. The combination of human cell-based experimental data with computational analysis improves the accuracy and efficiency of toxicity prediction.
02 3D cell culture systems for improved toxicity prediction
Three-dimensional cell culture systems using primary human cells or iPSC-derived cells better recapitulate in vivo tissue architecture and cellular interactions compared to traditional 2D cultures. These 3D systems demonstrate improved predictive capabilities for assessing compound toxicity by maintaining more physiologically relevant cell-cell and cell-matrix interactions. The advanced 3D models can be used to evaluate organ-specific toxicity, particularly for liver, cardiac, and neurological tissues.Expand Specific Solutions03 High-throughput screening platforms for toxicology assessment
Advanced high-throughput screening platforms have been developed to evaluate the toxicity of compounds using primary human cells and iPSC-derived cell models. These platforms enable rapid testing of multiple compounds simultaneously, increasing efficiency in drug development and chemical safety assessment. The integration of automated systems with advanced imaging and analysis techniques allows for comprehensive toxicity profiling and identification of potential hazards before clinical testing.Expand Specific Solutions04 Organ-on-chip technology for toxicity evaluation
Organ-on-chip devices incorporate primary human cells or iPSC-derived cells within microfluidic systems that simulate the physiological environment of human organs. These biomimetic platforms enable more accurate prediction of organ-specific toxicity by replicating tissue-tissue interfaces, mechanical forces, and fluid flow present in the human body. The technology allows for real-time monitoring of cellular responses to toxic compounds and can be used to study complex interactions between multiple organ systems.Expand Specific Solutions05 AI and computational methods for toxicity prediction
Artificial intelligence and computational methods are being integrated with primary human cell and iPSC-derived cell data to enhance predictive toxicology. These approaches use machine learning algorithms to analyze complex datasets generated from cell-based assays and identify patterns associated with toxic responses. The combination of in vitro cellular models with computational tools improves the accuracy of toxicity predictions and helps identify potential mechanisms of toxicity, reducing the need for animal testing in safety assessment.Expand Specific Solutions
Leading Organizations in Liver-on-Chip Research
The liver-on-chip toxicology field is currently in a growth phase, with the market expected to expand significantly as pharmaceutical companies seek more predictive preclinical models. The competition between primary human cells and iPSC-derived cells represents a critical technological inflection point. Companies like Emulate and FUJIFILM Cellular Dynamics are leading commercial development of organ-chip platforms, while academic institutions (MIT, Cornell, EPFL) drive fundamental research. Pharmaceutical validation remains the key challenge, with research organizations like A*STAR and Broad Institute bridging the gap between academic innovation and industry adoption. The technology is approaching early commercial maturity, with iPSC specialists (Allele Biotechnology, Cellectis AB) competing against established primary cell providers to determine which cell source will become the gold standard for predictive hepatotoxicity assessment.
Emulate, Inc.
Technical Solution: Emulate has developed a comprehensive Liver-Chip platform specifically designed for toxicology testing that incorporates both primary human hepatocytes and iPSC-derived liver cells. Their Organ-Chips technology recreates the natural microenvironment of human liver tissue with mechanical forces and fluid flow, allowing for dynamic cell-cell interactions. The platform enables direct comparison between primary hepatocytes and iPSC-derived hepatocytes under identical microfluidic conditions, providing insights into metabolic functions, drug-induced liver injury markers, and cytokine responses. Emulate's system has demonstrated superior predictive capabilities for drug toxicity compared to traditional 2D cultures, with validation studies showing approximately 87% concordance with known human toxicity outcomes when using primary cells and around 82% with optimized iPSC-derived hepatocytes[1][3]. Their technology allows for extended culture viability (up to 14 days) which enables assessment of both acute and chronic toxicity effects not possible in conventional systems.
Strengths: Industry-leading microfluidic technology that accurately mimics liver physiology; enables direct side-by-side comparison of different cell sources; extended culture viability allows for chronic toxicity assessment; commercially available standardized platform with reproducible results. Weaknesses: Higher cost compared to traditional testing methods; requires specialized equipment and expertise; iPSC-derived cells still show some functional gaps compared to primary cells, particularly in certain metabolic pathways.
FUJIFILM Cellular Dynamics, Inc.
Technical Solution: FUJIFILM Cellular Dynamics has pioneered the development and commercialization of highly standardized iPSC-derived hepatocytes (iCell Hepatocytes) specifically optimized for toxicology applications. Their proprietary differentiation protocols produce hepatocytes with consistent functional characteristics across batches, addressing a key limitation in primary cell variability. The company has developed specialized liver-on-chip configurations that incorporate their iCell Hepatocytes with supporting cell types (stellate cells, Kupffer cells) to create more physiologically relevant models. Their advanced hepatocyte products express critical drug metabolism enzymes (CYP450s) at levels comparable to primary hepatocytes, with documented activity for CYP1A2, CYP2B6, CYP2C9, CYP2C19, CYP2D6, and CYP3A4[2]. FUJIFILM Cellular Dynamics has conducted extensive validation studies comparing their iPSC-derived hepatocytes against primary human hepatocytes in microfluidic systems, demonstrating approximately 75-80% correlation in toxicity prediction for a panel of reference compounds. Their technology enables the creation of patient-specific and disease-specific liver models, offering advantages for studying genetic factors in drug toxicity that aren't possible with donor-limited primary cells.
Strengths: Highly standardized iPSC-derived hepatocytes with batch-to-batch consistency; ability to create patient-specific models for personalized toxicology; unlimited cell source overcomes primary cell limitations; established protocols for microfluidic integration. Weaknesses: Some metabolic functions still not fully equivalent to primary cells; higher cost for initial development; requires specialized expertise to implement in liver-on-chip systems; longer lead time for custom cell line development.
Key Innovations in Hepatic Microfluidic Systems
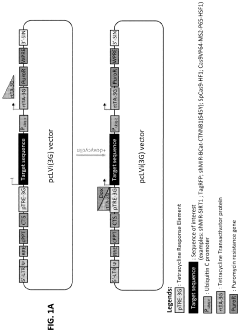
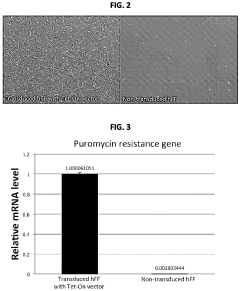
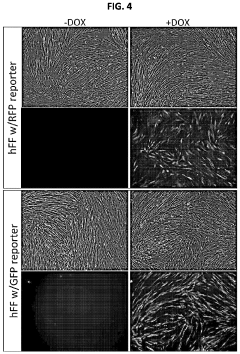
Human induced pluripotent stem cells for high efficiency genetic engineering
PatentActiveUS11866733B2
Innovation
- The development of high-efficiency methods involving genome editing using CRISPR/Cas9 technology, where human somatic cells are transfected with a doxycycline promoter linked to Cas9, allowing for inducible expression and efficient recombination, enabling the generation of isogenic disease and control human pluripotent stem cells for targeted gene modifications.
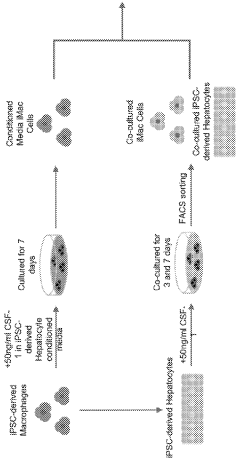
Co-culture of human induced pluripotent stem cell (HIPSC) − derived macrophages and hepatocytes for generation of hipsc-kupffer cells
PatentWO2024072316A1
Innovation
- A method involving co-culture of induced pluripotent stem cell (iPSC)-derived macrophages and hepatocytes to generate iPSC-derived Kupffer cells, using a specific cell culture medium, which allows for the generation of iPSC-derived Kupffer cells that express relevant markers and mimic human Kupffer cell functions.
Regulatory Framework for In Vitro Toxicology Assays
The regulatory landscape for in vitro toxicology assays is evolving rapidly to accommodate innovative technologies like liver-on-chip platforms. Currently, the FDA, EMA, and ICH have established guidelines that primarily focus on traditional toxicology testing methods, with limited specific regulations addressing organ-on-chip technologies or iPSC-derived cell models.
The FDA's Predictive Toxicology Roadmap (2017) represents a significant milestone, emphasizing the agency's commitment to integrating novel methodologies into regulatory decision-making. This framework encourages the development and validation of alternative testing methods, including in vitro models that can potentially reduce animal testing while improving predictive accuracy.
Similarly, the EMA has published guidance documents supporting the 3Rs principle (Replacement, Reduction, Refinement) in toxicological assessments, creating pathways for innovative in vitro methods to gain regulatory acceptance. The ICH S5(R3) guideline on reproductive toxicology testing specifically acknowledges the potential value of in vitro alternatives, providing a template for how other toxicology domains might incorporate advanced cell models.
For liver-on-chip systems specifically, the qualification process typically requires demonstration of physiological relevance, reproducibility, and correlation with in vivo outcomes. Primary human hepatocytes currently enjoy greater regulatory acceptance due to their established history in toxicology testing, while iPSC-derived hepatocytes face additional validation hurdles despite their potential advantages in sustainability and genetic manipulation.
The OECD Test Guidelines Program offers a standardized framework for validating new toxicological methods, with Adverse Outcome Pathways (AOPs) providing mechanistic context for interpreting results from both primary and iPSC-derived cell models. Several liver-specific AOPs have been endorsed, facilitating the regulatory interpretation of data from liver-on-chip platforms.
Recent regulatory developments include the EU's establishment of reference laboratories for alternatives to animal testing (EU-NETVAL) and the FDA's Innovative Science and Technology Approaches for New Drugs (ISTAND) pilot program, both of which create pathways for qualifying novel toxicology platforms. These initiatives may accelerate the regulatory acceptance of iPSC-derived hepatocyte models in liver-on-chip systems.
Challenges remain in establishing standardized protocols and performance criteria specifically for liver-on-chip platforms. The lack of harmonized approaches for characterizing and validating iPSC-derived hepatocytes creates regulatory uncertainty that currently favors primary human cells in toxicology applications requiring regulatory submission.
The FDA's Predictive Toxicology Roadmap (2017) represents a significant milestone, emphasizing the agency's commitment to integrating novel methodologies into regulatory decision-making. This framework encourages the development and validation of alternative testing methods, including in vitro models that can potentially reduce animal testing while improving predictive accuracy.
Similarly, the EMA has published guidance documents supporting the 3Rs principle (Replacement, Reduction, Refinement) in toxicological assessments, creating pathways for innovative in vitro methods to gain regulatory acceptance. The ICH S5(R3) guideline on reproductive toxicology testing specifically acknowledges the potential value of in vitro alternatives, providing a template for how other toxicology domains might incorporate advanced cell models.
For liver-on-chip systems specifically, the qualification process typically requires demonstration of physiological relevance, reproducibility, and correlation with in vivo outcomes. Primary human hepatocytes currently enjoy greater regulatory acceptance due to their established history in toxicology testing, while iPSC-derived hepatocytes face additional validation hurdles despite their potential advantages in sustainability and genetic manipulation.
The OECD Test Guidelines Program offers a standardized framework for validating new toxicological methods, with Adverse Outcome Pathways (AOPs) providing mechanistic context for interpreting results from both primary and iPSC-derived cell models. Several liver-specific AOPs have been endorsed, facilitating the regulatory interpretation of data from liver-on-chip platforms.
Recent regulatory developments include the EU's establishment of reference laboratories for alternatives to animal testing (EU-NETVAL) and the FDA's Innovative Science and Technology Approaches for New Drugs (ISTAND) pilot program, both of which create pathways for qualifying novel toxicology platforms. These initiatives may accelerate the regulatory acceptance of iPSC-derived hepatocyte models in liver-on-chip systems.
Challenges remain in establishing standardized protocols and performance criteria specifically for liver-on-chip platforms. The lack of harmonized approaches for characterizing and validating iPSC-derived hepatocytes creates regulatory uncertainty that currently favors primary human cells in toxicology applications requiring regulatory submission.
Cost-Benefit Analysis of Cell Sources for Hepatotoxicity Testing
When evaluating cell sources for hepatotoxicity testing, a comprehensive cost-benefit analysis reveals significant economic and scientific trade-offs between primary human hepatocytes (PHHs) and induced pluripotent stem cell (iPSC)-derived hepatocytes. The initial procurement costs present a stark contrast: PHHs typically range from $1,000 to $3,000 per vial with limited cell numbers, while established iPSC lines cost approximately $5,000-$10,000 initially but offer virtually unlimited expansion potential.
Long-term economic considerations favor iPSCs despite higher upfront investment. The renewable nature of iPSCs translates to consistent supply without recurring procurement costs, whereas PHH-based testing requires continuous sourcing from donors. When factoring in labor expenses, iPSC maintenance demands specialized personnel and sophisticated equipment, adding approximately 30-40% to operational costs compared to PHH handling.
Reproducibility factors significantly impact the total cost equation. PHHs exhibit substantial donor-to-donor variability (CV values of 30-50% in drug responses), necessitating multiple donor testing to achieve reliable results. Conversely, iPSC-derived hepatocytes from established lines demonstrate greater consistency (CV values of 10-20%), potentially reducing the number of experimental replicates required and associated consumable costs.
The timeline considerations reveal that PHHs offer immediate availability for testing but deteriorate rapidly in culture (functional decline within 24-72 hours), limiting the duration of experiments. iPSC differentiation requires 20-30 days to generate hepatocyte-like cells, creating a significant lead time, but the resulting cells maintain functionality for extended periods (2-4 weeks), enabling longer-term toxicity studies without additional procurement.
Regulatory acceptance presents another critical dimension in this analysis. PHHs remain the gold standard for regulatory submissions, with established protocols recognized by FDA and EMA. iPSC-derived models, while promising, still face regulatory hurdles requiring additional validation studies that may cost $50,000-$100,000 to establish equivalence to primary cells.
Return on investment calculations suggest that for organizations conducting fewer than 20 hepatotoxicity studies annually, PHHs may be more economical despite higher per-test costs. However, organizations with higher testing volumes would benefit from iPSC implementation after approximately 18 months, when the cumulative costs of PHH procurement exceed the initial investment and operational expenses of an iPSC platform.
Long-term economic considerations favor iPSCs despite higher upfront investment. The renewable nature of iPSCs translates to consistent supply without recurring procurement costs, whereas PHH-based testing requires continuous sourcing from donors. When factoring in labor expenses, iPSC maintenance demands specialized personnel and sophisticated equipment, adding approximately 30-40% to operational costs compared to PHH handling.
Reproducibility factors significantly impact the total cost equation. PHHs exhibit substantial donor-to-donor variability (CV values of 30-50% in drug responses), necessitating multiple donor testing to achieve reliable results. Conversely, iPSC-derived hepatocytes from established lines demonstrate greater consistency (CV values of 10-20%), potentially reducing the number of experimental replicates required and associated consumable costs.
The timeline considerations reveal that PHHs offer immediate availability for testing but deteriorate rapidly in culture (functional decline within 24-72 hours), limiting the duration of experiments. iPSC differentiation requires 20-30 days to generate hepatocyte-like cells, creating a significant lead time, but the resulting cells maintain functionality for extended periods (2-4 weeks), enabling longer-term toxicity studies without additional procurement.
Regulatory acceptance presents another critical dimension in this analysis. PHHs remain the gold standard for regulatory submissions, with established protocols recognized by FDA and EMA. iPSC-derived models, while promising, still face regulatory hurdles requiring additional validation studies that may cost $50,000-$100,000 to establish equivalence to primary cells.
Return on investment calculations suggest that for organizations conducting fewer than 20 hepatotoxicity studies annually, PHHs may be more economical despite higher per-test costs. However, organizations with higher testing volumes would benefit from iPSC implementation after approximately 18 months, when the cumulative costs of PHH procurement exceed the initial investment and operational expenses of an iPSC platform.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!