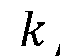3D-printed microchannel geometries to mimic organ-specific interstitial flow patterns
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
3D Bioprinting Evolution and Objectives
The evolution of 3D bioprinting technology has undergone significant transformation since its inception in the early 2000s. Initially limited to simple structures using rudimentary materials, the field has progressed to creating complex tissue-like constructs with multiple cell types and biomaterials. This technological advancement has been driven by innovations in printing techniques, biomaterial development, and cross-disciplinary collaboration between engineering, biology, and medicine.
The trajectory of 3D bioprinting has been marked by several key milestones. The first successful printing of cell-laden constructs in 2003 demonstrated the feasibility of incorporating living cells into printed structures. By 2010, researchers had developed more sophisticated bioinks that better mimicked the extracellular matrix, improving cell viability and functionality. The past decade has witnessed remarkable progress in printing resolution, speed, and the ability to create heterogeneous structures that more accurately represent native tissues.
Specifically regarding microchannel geometries for organ-specific interstitial flow patterns, the evolution has been particularly noteworthy. Early attempts focused on simple linear channels, but current technologies enable the creation of biomimetic vascular networks with hierarchical branching structures and varying diameters that replicate organ-specific flow dynamics. This progression has been essential for addressing one of the most significant challenges in tissue engineering: creating functional vasculature to support cell viability in thick tissue constructs.
The primary objective of developing 3D-printed microchannel geometries is to recreate the complex interstitial fluid dynamics found in native organs. This fluid movement is crucial for nutrient delivery, waste removal, and cell-to-cell communication within tissues. By accurately mimicking organ-specific flow patterns, researchers aim to create more physiologically relevant in vitro models for drug testing, disease modeling, and eventually, functional tissue replacements for transplantation.
Additional objectives include understanding how interstitial flow influences cellular behavior, including differentiation, migration, and matrix remodeling. There is growing recognition that fluid mechanical forces play a critical role in tissue development and homeostasis, making accurate replication of these forces essential for creating functional engineered tissues. Furthermore, researchers aim to develop standardized, reproducible methods for creating these complex microchannel architectures to facilitate broader adoption in both research and clinical applications.
Looking forward, the field is moving toward integrating multiple technologies, such as combining 3D bioprinting with microfluidics and organ-on-chip platforms, to create increasingly sophisticated tissue models with dynamic flow capabilities. The ultimate goal remains the development of fully functional, vascularized organ replacements, though significant challenges in scalability, resolution, and biological integration must still be overcome.
The trajectory of 3D bioprinting has been marked by several key milestones. The first successful printing of cell-laden constructs in 2003 demonstrated the feasibility of incorporating living cells into printed structures. By 2010, researchers had developed more sophisticated bioinks that better mimicked the extracellular matrix, improving cell viability and functionality. The past decade has witnessed remarkable progress in printing resolution, speed, and the ability to create heterogeneous structures that more accurately represent native tissues.
Specifically regarding microchannel geometries for organ-specific interstitial flow patterns, the evolution has been particularly noteworthy. Early attempts focused on simple linear channels, but current technologies enable the creation of biomimetic vascular networks with hierarchical branching structures and varying diameters that replicate organ-specific flow dynamics. This progression has been essential for addressing one of the most significant challenges in tissue engineering: creating functional vasculature to support cell viability in thick tissue constructs.
The primary objective of developing 3D-printed microchannel geometries is to recreate the complex interstitial fluid dynamics found in native organs. This fluid movement is crucial for nutrient delivery, waste removal, and cell-to-cell communication within tissues. By accurately mimicking organ-specific flow patterns, researchers aim to create more physiologically relevant in vitro models for drug testing, disease modeling, and eventually, functional tissue replacements for transplantation.
Additional objectives include understanding how interstitial flow influences cellular behavior, including differentiation, migration, and matrix remodeling. There is growing recognition that fluid mechanical forces play a critical role in tissue development and homeostasis, making accurate replication of these forces essential for creating functional engineered tissues. Furthermore, researchers aim to develop standardized, reproducible methods for creating these complex microchannel architectures to facilitate broader adoption in both research and clinical applications.
Looking forward, the field is moving toward integrating multiple technologies, such as combining 3D bioprinting with microfluidics and organ-on-chip platforms, to create increasingly sophisticated tissue models with dynamic flow capabilities. The ultimate goal remains the development of fully functional, vascularized organ replacements, though significant challenges in scalability, resolution, and biological integration must still be overcome.
Market Analysis for Organ-on-Chip Technologies
The organ-on-chip (OOC) market is experiencing rapid growth, driven by increasing demand for alternatives to animal testing and more physiologically relevant in vitro models. The global OOC market was valued at approximately $30 million in 2019 and is projected to reach $220 million by 2025, representing a compound annual growth rate (CAGR) of 39.9%. This exceptional growth rate positions OOC technology among the fastest-growing segments in the biomedical device industry.
Pharmaceutical companies constitute the largest market segment, accounting for nearly 38% of the total market share. These companies are increasingly adopting OOC platforms to reduce drug development costs and timelines while improving predictive accuracy in preclinical testing. Academic research institutions represent the second-largest market segment at 29%, followed by cosmetics companies at 18%, which are seeking alternatives to animal testing due to regulatory restrictions.
Regionally, North America dominates the market with approximately 42% share, followed by Europe at 31% and Asia-Pacific at 21%. The Asia-Pacific region is expected to witness the highest growth rate in the coming years due to increasing R&D investments and government initiatives supporting advanced biomedical technologies.
The specific segment of 3D-printed microchannel geometries for mimicking organ-specific interstitial flow patterns is emerging as a high-potential niche within the broader OOC market. This specialized technology addresses the critical need for more accurate replication of the microenvironment found in native tissues, particularly the complex fluid dynamics that influence cellular behavior and drug responses.
Market demand for these advanced microfluidic systems is being driven by several factors. First, there is growing recognition that traditional 2D cell cultures and animal models poorly predict human responses to drugs, resulting in high failure rates during clinical trials. Second, regulatory agencies worldwide are encouraging the development of alternative testing methods that reduce animal use while improving predictive accuracy.
Key customer segments for this technology include pharmaceutical R&D departments focused on drug discovery and toxicity testing, academic research laboratories studying disease mechanisms, and contract research organizations (CROs) providing specialized testing services. The technology's ability to recreate organ-specific flow conditions makes it particularly valuable for studying diseases affecting highly vascularized organs such as the liver, kidney, and brain, where interstitial flow patterns significantly impact cellular function.
Pharmaceutical companies constitute the largest market segment, accounting for nearly 38% of the total market share. These companies are increasingly adopting OOC platforms to reduce drug development costs and timelines while improving predictive accuracy in preclinical testing. Academic research institutions represent the second-largest market segment at 29%, followed by cosmetics companies at 18%, which are seeking alternatives to animal testing due to regulatory restrictions.
Regionally, North America dominates the market with approximately 42% share, followed by Europe at 31% and Asia-Pacific at 21%. The Asia-Pacific region is expected to witness the highest growth rate in the coming years due to increasing R&D investments and government initiatives supporting advanced biomedical technologies.
The specific segment of 3D-printed microchannel geometries for mimicking organ-specific interstitial flow patterns is emerging as a high-potential niche within the broader OOC market. This specialized technology addresses the critical need for more accurate replication of the microenvironment found in native tissues, particularly the complex fluid dynamics that influence cellular behavior and drug responses.
Market demand for these advanced microfluidic systems is being driven by several factors. First, there is growing recognition that traditional 2D cell cultures and animal models poorly predict human responses to drugs, resulting in high failure rates during clinical trials. Second, regulatory agencies worldwide are encouraging the development of alternative testing methods that reduce animal use while improving predictive accuracy.
Key customer segments for this technology include pharmaceutical R&D departments focused on drug discovery and toxicity testing, academic research laboratories studying disease mechanisms, and contract research organizations (CROs) providing specialized testing services. The technology's ability to recreate organ-specific flow conditions makes it particularly valuable for studying diseases affecting highly vascularized organs such as the liver, kidney, and brain, where interstitial flow patterns significantly impact cellular function.
Current Challenges in Microfluidic Channel Fabrication
Despite significant advancements in microfluidic technology, fabricating microchannels that accurately mimic organ-specific interstitial flow patterns remains challenging. Traditional fabrication methods like soft lithography and photolithography offer limited geometric complexity and struggle to replicate the intricate, three-dimensional architecture of biological tissues. These conventional approaches typically produce planar structures with rectangular cross-sections, which poorly represent the complex, tortuous pathways characteristic of interstitial spaces in organs.
Resolution limitations present another significant hurdle. Organ-specific interstitial spaces often contain microstructures at sub-micron scales, which many current fabrication techniques cannot reliably reproduce. Even advanced 3D printing technologies face difficulties in achieving the necessary resolution while maintaining structural integrity across larger tissue-scale constructs.
Material compatibility issues further complicate microfluidic channel fabrication. Biomimetic applications require materials that not only enable precise geometric control but also possess appropriate mechanical properties, surface chemistry, and biocompatibility. Many materials suitable for high-resolution fabrication lack the compliance and permeability characteristics of native tissues, creating a fundamental mismatch in mechanical behavior.
Multi-material integration represents another critical challenge. Organ microenvironments comprise heterogeneous structures with varying mechanical and biochemical properties. Current fabrication approaches struggle to seamlessly integrate multiple materials with distinct properties within a single microfluidic device, limiting the physiological relevance of engineered constructs.
Scalability and reproducibility concerns persist across fabrication platforms. Creating organ-specific microchannels with consistent properties at scale remains difficult, particularly when transitioning from prototype to production. Variations in channel dimensions, surface roughness, and material properties between batches can significantly impact fluid dynamics and cellular responses.
The dynamic nature of biological tissues presents perhaps the most formidable challenge. Natural interstitial spaces undergo continuous remodeling in response to physiological and pathological stimuli. Current fabrication approaches predominantly produce static structures that cannot adapt to changing conditions, failing to capture the dynamic aspects of tissue microenvironments.
Integration with sensing and actuation capabilities represents an emerging frontier. Advanced organ models require embedded functionality to monitor and modulate flow conditions, cellular responses, and tissue-level behaviors. Incorporating these capabilities while maintaining the fidelity of microchannel geometries demands innovative fabrication strategies that bridge microfluidics, electronics, and biomaterials science.
Resolution limitations present another significant hurdle. Organ-specific interstitial spaces often contain microstructures at sub-micron scales, which many current fabrication techniques cannot reliably reproduce. Even advanced 3D printing technologies face difficulties in achieving the necessary resolution while maintaining structural integrity across larger tissue-scale constructs.
Material compatibility issues further complicate microfluidic channel fabrication. Biomimetic applications require materials that not only enable precise geometric control but also possess appropriate mechanical properties, surface chemistry, and biocompatibility. Many materials suitable for high-resolution fabrication lack the compliance and permeability characteristics of native tissues, creating a fundamental mismatch in mechanical behavior.
Multi-material integration represents another critical challenge. Organ microenvironments comprise heterogeneous structures with varying mechanical and biochemical properties. Current fabrication approaches struggle to seamlessly integrate multiple materials with distinct properties within a single microfluidic device, limiting the physiological relevance of engineered constructs.
Scalability and reproducibility concerns persist across fabrication platforms. Creating organ-specific microchannels with consistent properties at scale remains difficult, particularly when transitioning from prototype to production. Variations in channel dimensions, surface roughness, and material properties between batches can significantly impact fluid dynamics and cellular responses.
The dynamic nature of biological tissues presents perhaps the most formidable challenge. Natural interstitial spaces undergo continuous remodeling in response to physiological and pathological stimuli. Current fabrication approaches predominantly produce static structures that cannot adapt to changing conditions, failing to capture the dynamic aspects of tissue microenvironments.
Integration with sensing and actuation capabilities represents an emerging frontier. Advanced organ models require embedded functionality to monitor and modulate flow conditions, cellular responses, and tissue-level behaviors. Incorporating these capabilities while maintaining the fidelity of microchannel geometries demands innovative fabrication strategies that bridge microfluidics, electronics, and biomaterials science.
Existing Approaches to Microchannel Geometry Design
01 3D printing techniques for microfluidic channel fabrication
Various 3D printing technologies can be used to fabricate microchannels with precise geometries for controlling interstitial flow patterns. These techniques include stereolithography, digital light processing, and fused deposition modeling, which allow for the creation of complex channel architectures with controlled dimensions and surface properties. The printed microchannels can be designed with specific cross-sectional profiles, branching patterns, and surface textures to achieve desired flow characteristics.- 3D printing techniques for microfluidic channel fabrication: Various 3D printing technologies can be used to fabricate microchannels with precise geometries for controlling interstitial flow patterns. These techniques allow for the creation of complex channel architectures with high resolution and reproducibility. The printing methods can include stereolithography, digital light processing, and fused deposition modeling, each offering different advantages in terms of resolution, material compatibility, and fabrication speed for microfluidic applications.
- Microchannel geometry optimization for flow control: The design and optimization of microchannel geometries significantly impact interstitial flow patterns. By manipulating channel dimensions, cross-sectional shapes, and network configurations, researchers can control flow velocity, pressure gradients, and shear stress distributions. Advanced computational modeling helps predict flow behavior in different geometries before fabrication, enabling iterative design improvements for specific applications such as tissue engineering or drug delivery systems.
- Simulation and visualization of interstitial flow patterns: Computational fluid dynamics and visualization techniques are essential for understanding interstitial flow patterns in 3D-printed microchannels. These methods allow researchers to simulate, analyze, and visualize complex flow behaviors within various microchannel geometries. Advanced imaging and rendering technologies enable real-time monitoring of flow patterns, helping to validate theoretical models and optimize channel designs for specific applications.
- Biomimetic microchannel designs for tissue engineering: Biomimetic approaches to microchannel design aim to replicate natural vascular networks and interstitial spaces found in biological tissues. These designs incorporate hierarchical branching structures, variable channel diameters, and physiologically relevant geometries to create more realistic flow patterns. Such biomimetic microchannels support cell growth, nutrient transport, and waste removal in engineered tissues, closely mimicking the natural microenvironment of cells.
- Smart materials and responsive microchannel systems: Integration of smart materials and responsive elements in 3D-printed microchannels enables dynamic control of interstitial flow patterns. These systems can adapt to environmental stimuli or external controls, allowing for on-demand adjustment of flow characteristics. Applications include programmable drug delivery systems, adaptive tissue culture platforms, and responsive microfluidic devices that can alter their flow properties based on specific triggers or requirements.
02 Computational modeling of flow patterns in microchannels
Computational fluid dynamics (CFD) and other simulation techniques are employed to predict and optimize interstitial flow patterns within 3D-printed microchannels. These models account for factors such as channel geometry, surface roughness, fluid properties, and boundary conditions to simulate flow behavior. The computational approaches enable the design of microchannel geometries that produce specific flow characteristics, such as laminar flow, mixing zones, or controlled pressure gradients, before physical fabrication.Expand Specific Solutions03 Biomimetic microchannel designs for tissue engineering
3D-printed microchannels can be designed to mimic natural vascular networks and interstitial spaces found in biological tissues. These biomimetic designs incorporate features such as hierarchical branching, variable channel diameters, and tortuous pathways that replicate physiological flow conditions. Such microchannel architectures are particularly valuable for tissue engineering applications, where they can facilitate nutrient delivery, waste removal, and cellular interactions similar to those in native tissues.Expand Specific Solutions04 Integration of sensors and monitoring systems in microfluidic devices
Advanced microfluidic devices incorporate integrated sensing and monitoring capabilities to analyze interstitial flow patterns in real-time. These systems may include optical sensors, pressure transducers, or electrical impedance measurements positioned at strategic locations within the microchannel network. The integration of these sensing elements with 3D-printed microchannels enables dynamic feedback on flow behavior, allowing for adjustment of flow parameters or validation of computational models.Expand Specific Solutions05 Multi-material printing for functional microchannel systems
Multi-material 3D printing approaches enable the fabrication of microchannels with regionally varying properties that influence interstitial flow patterns. By combining materials with different mechanical, chemical, or surface properties within a single print, researchers can create channels with hydrophilic/hydrophobic regions, elastic sections, or embedded functional elements. These heterogeneous structures can direct flow in specific patterns, create selective barriers, or respond dynamically to flow conditions.Expand Specific Solutions
Leading Organizations in 3D Bioprinting Research
The 3D-printed microchannel technology for organ-specific interstitial flow patterns is in an early growth phase, with an estimated market size of $2-3 billion and expanding at 15-20% annually. The competitive landscape features academic institutions leading fundamental research (MIT, Tsinghua University, King's College London) alongside specialized biotech companies developing commercial applications (BICO Group, Prellis Biologics, Aracari Biosciences). Technical maturity varies significantly: while basic microfluidic printing capabilities are established, organ-specific applications remain predominantly experimental. Major healthcare corporations like Siemens Healthineers are beginning to invest, signaling the technology's transition from research to clinical applications, though regulatory approval pathways remain challenging.
Systemic Bio LLC
Technical Solution: Systemic Bio has developed an advanced organ-on-chip platform that integrates 3D-printed microchannels with precise control over interstitial flow patterns. Their proprietary LambdaFlow technology enables the creation of microfluidic devices with physiologically relevant flow characteristics specific to different organ systems. The company utilizes multi-material printing approaches that combine rigid and flexible materials to create microchannels with regionally varying mechanical properties, mimicking the heterogeneity found in native tissues. Their system incorporates integrated sensors that provide real-time monitoring of flow rates, pressure, and other parameters critical for maintaining physiological conditions. Systemic Bio's platform is particularly notable for its ability to recreate the blood-brain barrier, with microchannels designed to replicate the unique tight junction characteristics and flow patterns of cerebral vasculature. The company has demonstrated successful application in drug development, with their liver-on-chip model showing improved predictive capabilities for hepatotoxicity compared to conventional 2D cultures.
Strengths: Integrated approach combining microchannel fabrication with comprehensive sensing and control systems; focus on physiologically relevant flow patterns specific to different organ systems; demonstrated applications in pharmaceutical testing. Weaknesses: Relatively new technology with limited long-term validation data; complex system integration may present reliability challenges; higher cost compared to traditional cell culture methods.
Aracari Biosciences, Inc.
Technical Solution: Aracari Biosciences specializes in creating vascularized tissue models using 3D-printed microchannels that accurately mimic organ-specific interstitial flow patterns. Their proprietary VascuNet technology enables the fabrication of hierarchical vascular networks with physiologically relevant branching patterns and dimensions. The company has developed tissue-specific formulations that recreate the unique extracellular matrix composition of different organs, influencing interstitial flow characteristics. Aracari's platform incorporates computational fluid dynamics modeling to design microchannel geometries that achieve target shear stress profiles and pressure gradients specific to different organ systems. Their system has been validated for multiple tissue types, including cardiac, hepatic, and neural tissues, demonstrating appropriate tissue-specific responses to flow conditions. The company has established partnerships with pharmaceutical companies to utilize their technology for drug screening applications, with published results showing improved predictive capabilities compared to conventional models.
Strengths: Specialized focus on vascularized tissue models with physiologically relevant flow characteristics; computational design approach that enables precise control over flow parameters; validated across multiple tissue types with pharmaceutical industry partnerships. Weaknesses: Limited throughput compared to traditional screening methods; requires specialized expertise to operate; higher cost per assay compared to conventional methods.
Key Innovations in Biomimetic Interstitial Flow Patterns
Triply periodic minimal surfaces for 3D printed organs and tissues
PatentWO2023081462A1
Innovation
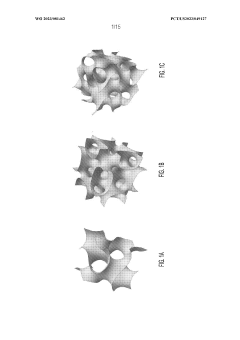
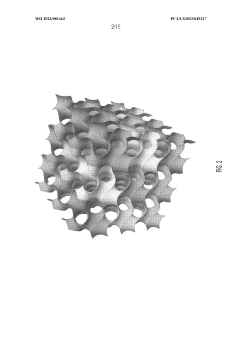
- The use of triply periodic minimal surfaces (TPMS) such as gyroid, Batwing, and Fischer S to create 3D scaffolds with embedded vascular networks that allow for gas exchange, where the vascular network is integrated within the scaffold walls, enabling efficient diffusion and mechanical support, and can be seeded with biological materials for enhanced functionality.
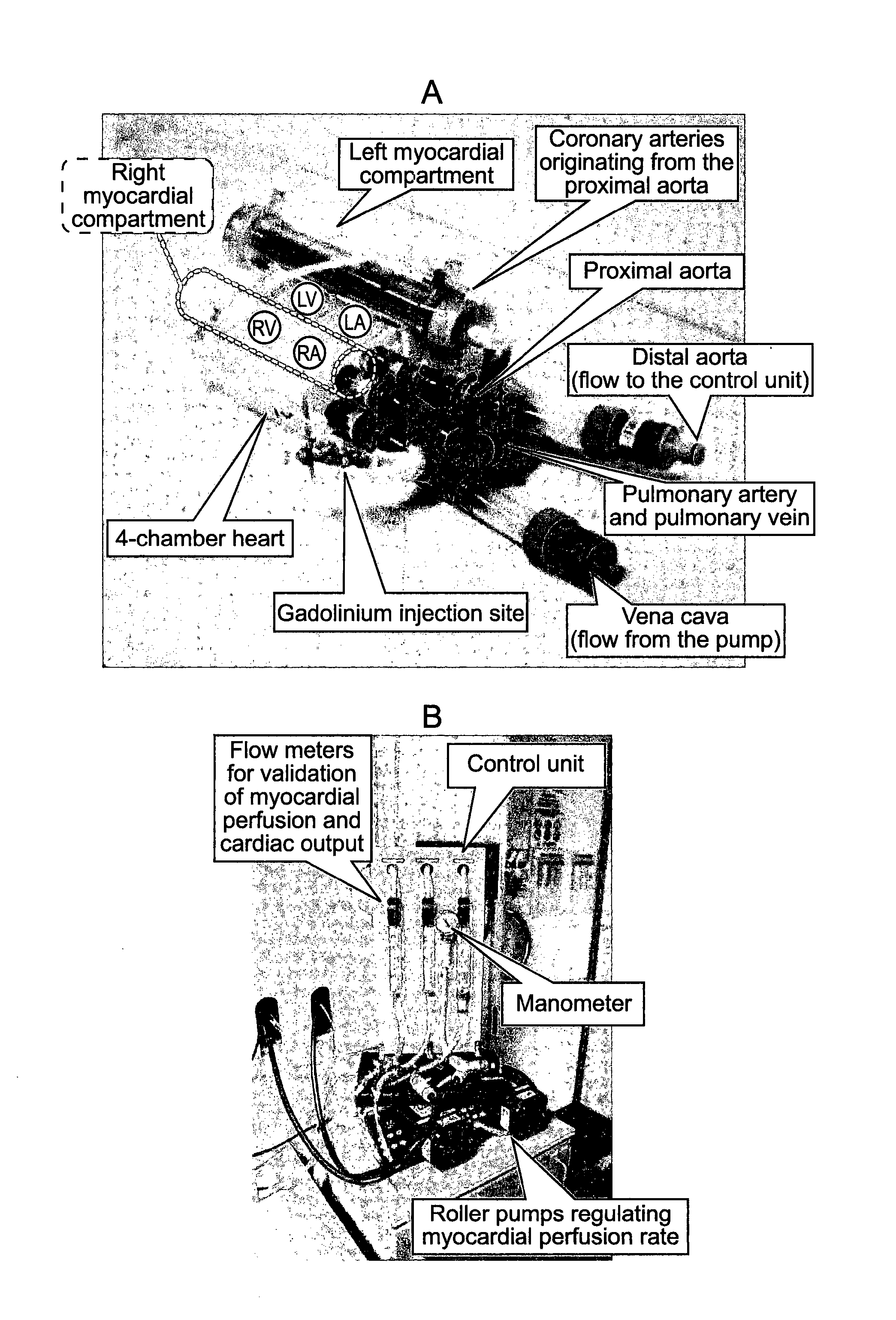
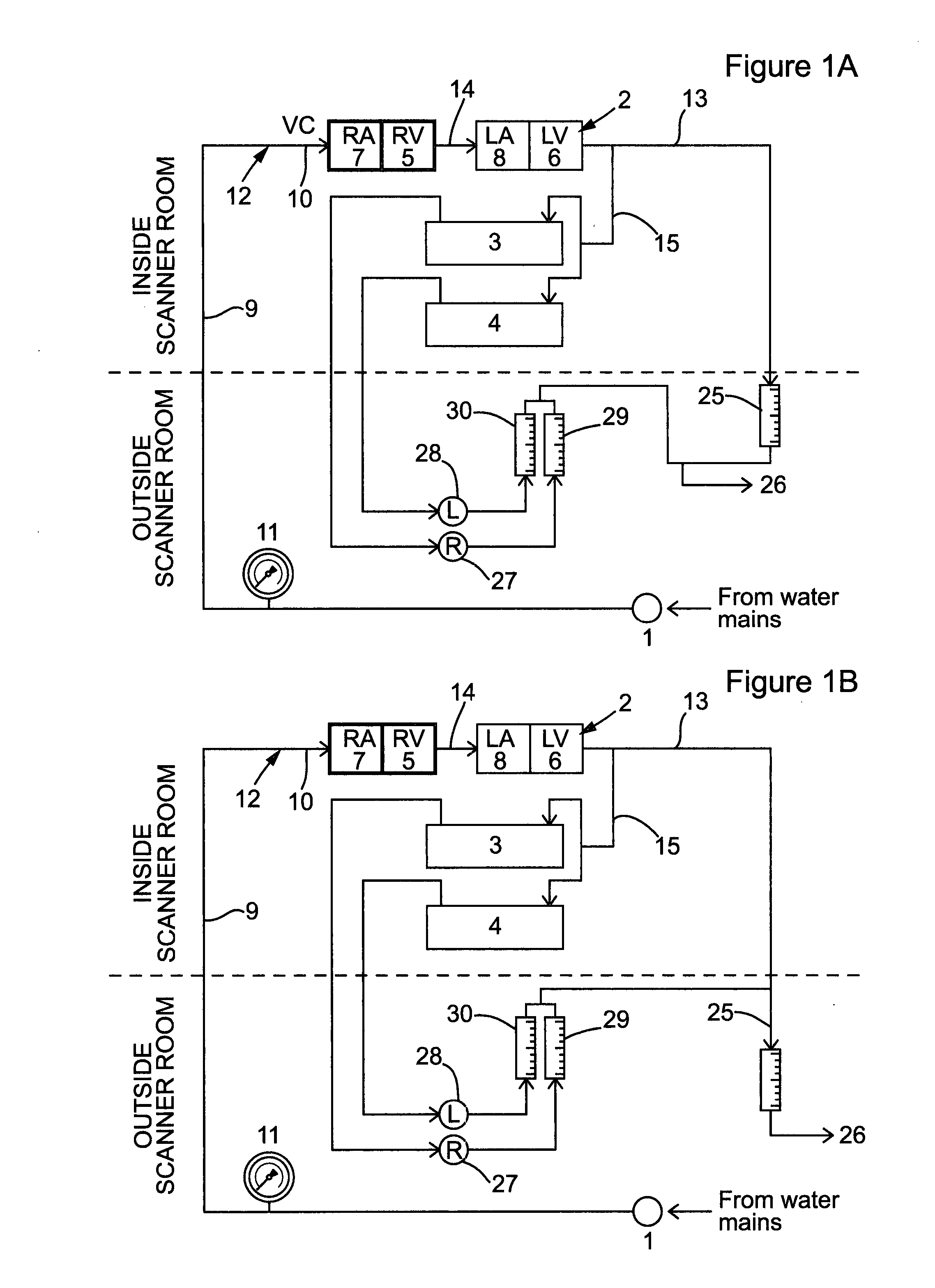
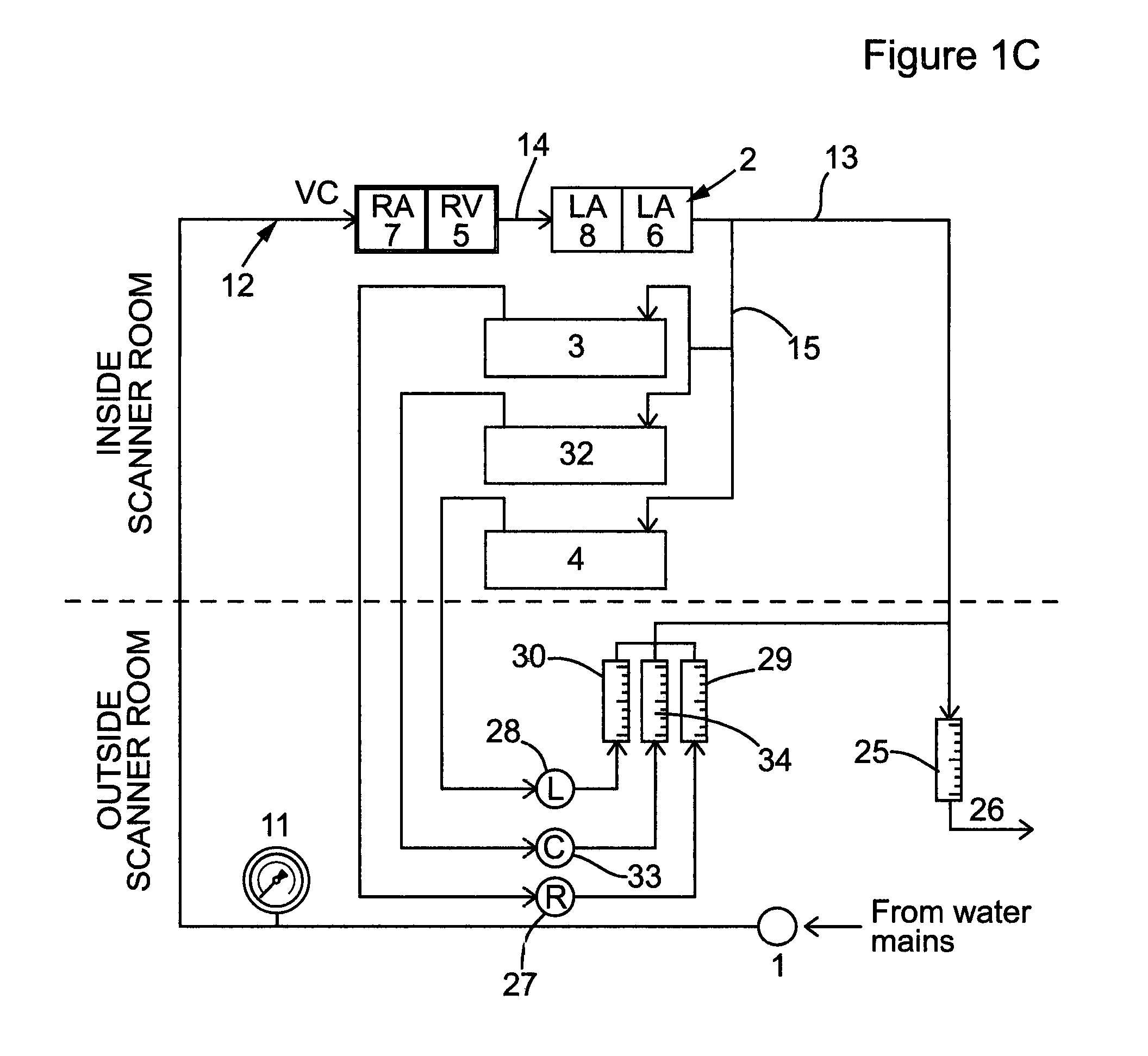
Perfusion phantom device
PatentActiveUS20160027340A1
Innovation
- A perfusion phantom device with multiple fluid channels mimicking blood flow through organs, allowing for controlled and reproducible simulation of first-pass perfusion, which can be used with MR or CT scanners to validate perfusion methodologies by replicating physiological features and allowing for the use of contrast agents.
Biomaterial Selection for Optimal Flow Characteristics
The selection of appropriate biomaterials is critical for creating 3D-printed microchannels that accurately mimic organ-specific interstitial flow patterns. Different organs exhibit unique flow characteristics, requiring tailored material properties to achieve physiological relevance. The biomaterial must balance mechanical stability, biocompatibility, and specific flow dynamics to create functional tissue models.
Hydrogels represent a primary class of materials suitable for this application due to their tunable properties and structural similarity to native extracellular matrices. Materials such as gelatin methacrylate (GelMA), poly(ethylene glycol) diacrylate (PEGDA), and alginate offer varying degrees of permeability and mechanical strength. GelMA provides excellent cell adhesion properties while maintaining appropriate flow characteristics, making it particularly suitable for vascularized tissue models. PEGDA offers superior mechanical stability and precise control over pore size, enabling accurate replication of pressure-flow relationships in organs like the liver and kidney.
Natural biomaterials including collagen and fibrin demonstrate inherent biocompatibility and promote cellular integration but present challenges in achieving consistent flow properties. These materials often require modification or blending with synthetic polymers to enhance printability and mechanical stability while preserving their biological advantages.
The rheological properties of selected biomaterials directly impact printing resolution and channel geometry fidelity. Materials must exhibit appropriate viscosity and shear-thinning behavior to facilitate extrusion while maintaining structural integrity post-printing. Crosslinking mechanisms, whether photo-initiated, chemical, or thermal, significantly influence the final mechanical properties and permeability of the printed structures.
Surface modification techniques can further optimize flow characteristics by altering hydrophilicity, charge distribution, and protein adsorption. Treatments such as plasma modification or chemical functionalization can adjust surface properties to match organ-specific endothelial or epithelial interfaces, thereby influencing fluid-wall interactions and flow patterns.
Degradation kinetics must be carefully considered, as material remodeling over time affects channel patency and flow stability. Ideally, degradation rates should match the timeline of tissue formation to maintain physiological flow conditions throughout the experimental duration. Materials with controllable degradation profiles, such as enzymatically degradable peptide-modified hydrogels, offer advantages for long-term studies.
Ultimately, the optimal biomaterial selection depends on the specific organ being modeled, with consideration for tissue-specific mechanical properties, cellular interactions, and unique flow patterns characteristic of each organ microenvironment.
Hydrogels represent a primary class of materials suitable for this application due to their tunable properties and structural similarity to native extracellular matrices. Materials such as gelatin methacrylate (GelMA), poly(ethylene glycol) diacrylate (PEGDA), and alginate offer varying degrees of permeability and mechanical strength. GelMA provides excellent cell adhesion properties while maintaining appropriate flow characteristics, making it particularly suitable for vascularized tissue models. PEGDA offers superior mechanical stability and precise control over pore size, enabling accurate replication of pressure-flow relationships in organs like the liver and kidney.
Natural biomaterials including collagen and fibrin demonstrate inherent biocompatibility and promote cellular integration but present challenges in achieving consistent flow properties. These materials often require modification or blending with synthetic polymers to enhance printability and mechanical stability while preserving their biological advantages.
The rheological properties of selected biomaterials directly impact printing resolution and channel geometry fidelity. Materials must exhibit appropriate viscosity and shear-thinning behavior to facilitate extrusion while maintaining structural integrity post-printing. Crosslinking mechanisms, whether photo-initiated, chemical, or thermal, significantly influence the final mechanical properties and permeability of the printed structures.
Surface modification techniques can further optimize flow characteristics by altering hydrophilicity, charge distribution, and protein adsorption. Treatments such as plasma modification or chemical functionalization can adjust surface properties to match organ-specific endothelial or epithelial interfaces, thereby influencing fluid-wall interactions and flow patterns.
Degradation kinetics must be carefully considered, as material remodeling over time affects channel patency and flow stability. Ideally, degradation rates should match the timeline of tissue formation to maintain physiological flow conditions throughout the experimental duration. Materials with controllable degradation profiles, such as enzymatically degradable peptide-modified hydrogels, offer advantages for long-term studies.
Ultimately, the optimal biomaterial selection depends on the specific organ being modeled, with consideration for tissue-specific mechanical properties, cellular interactions, and unique flow patterns characteristic of each organ microenvironment.
Validation Methods for Physiological Flow Accuracy
Validation of physiological accuracy in 3D-printed microchannels requires rigorous methodologies to ensure that the fabricated structures truly replicate organ-specific interstitial flow patterns. The validation process typically begins with computational fluid dynamics (CFD) simulations that predict flow behavior within the designed geometries. These simulations establish theoretical benchmarks against which experimental results can be compared, incorporating parameters such as pressure gradients, flow rates, and shear stress distributions specific to the target organ environment.
Experimental validation follows, employing multiple complementary techniques to assess flow characteristics. Particle image velocimetry (PIV) serves as a primary method, utilizing fluorescent tracer particles to visualize and quantify flow patterns within transparent microchannels. High-speed imaging captures particle movement, allowing for detailed velocity field mapping and identification of potential flow anomalies. This technique provides spatial resolution down to micrometers, essential for validating the complex geometries that characterize organ-specific interstitial spaces.
Micro-particle tracking velocimetry (μPTV) offers complementary data by tracking individual particles through the microchannel network, providing insights into local flow behaviors and potential preferential pathways. These optical methods are often supplemented with pressure sensors integrated at strategic points within the microchannel system to monitor pressure differentials that drive interstitial flows in vivo.
Biological validation represents a critical step beyond physical measurements. This involves culturing relevant cell types within the microchannels and assessing cellular responses to flow conditions. Markers of mechanotransduction, such as cytoskeletal reorganization and expression of flow-sensitive genes, provide functional validation that the engineered flow patterns elicit physiologically relevant cellular behaviors. Comparison with ex vivo tissue samples further confirms biological fidelity.
Multi-modal imaging techniques, including micro-computed tomography (micro-CT) with contrast agents, enable non-destructive assessment of flow distribution throughout complex 3D-printed architectures. These methods verify that flow reaches all intended regions of the microchannel network, particularly important for replicating the heterogeneous perfusion patterns characteristic of many organs.
Validation protocols must also incorporate time-dependent analyses to account for pulsatile flows present in certain organ systems. Synchronization of imaging with controlled pulsatile pumps allows for phase-resolved flow characterization, capturing dynamic aspects of interstitial fluid movement that static measurements would miss.
Ultimately, comprehensive validation requires establishing quantitative metrics and acceptance criteria based on published physiological data for the specific organ being modeled. Statistical methods comparing experimental measurements with in vivo reference values provide objective assessment of physiological accuracy, guiding iterative refinement of microchannel designs to achieve increasingly faithful replication of organ-specific interstitial flow patterns.
Experimental validation follows, employing multiple complementary techniques to assess flow characteristics. Particle image velocimetry (PIV) serves as a primary method, utilizing fluorescent tracer particles to visualize and quantify flow patterns within transparent microchannels. High-speed imaging captures particle movement, allowing for detailed velocity field mapping and identification of potential flow anomalies. This technique provides spatial resolution down to micrometers, essential for validating the complex geometries that characterize organ-specific interstitial spaces.
Micro-particle tracking velocimetry (μPTV) offers complementary data by tracking individual particles through the microchannel network, providing insights into local flow behaviors and potential preferential pathways. These optical methods are often supplemented with pressure sensors integrated at strategic points within the microchannel system to monitor pressure differentials that drive interstitial flows in vivo.
Biological validation represents a critical step beyond physical measurements. This involves culturing relevant cell types within the microchannels and assessing cellular responses to flow conditions. Markers of mechanotransduction, such as cytoskeletal reorganization and expression of flow-sensitive genes, provide functional validation that the engineered flow patterns elicit physiologically relevant cellular behaviors. Comparison with ex vivo tissue samples further confirms biological fidelity.
Multi-modal imaging techniques, including micro-computed tomography (micro-CT) with contrast agents, enable non-destructive assessment of flow distribution throughout complex 3D-printed architectures. These methods verify that flow reaches all intended regions of the microchannel network, particularly important for replicating the heterogeneous perfusion patterns characteristic of many organs.
Validation protocols must also incorporate time-dependent analyses to account for pulsatile flows present in certain organ systems. Synchronization of imaging with controlled pulsatile pumps allows for phase-resolved flow characterization, capturing dynamic aspects of interstitial fluid movement that static measurements would miss.
Ultimately, comprehensive validation requires establishing quantitative metrics and acceptance criteria based on published physiological data for the specific organ being modeled. Statistical methods comparing experimental measurements with in vivo reference values provide objective assessment of physiological accuracy, guiding iterative refinement of microchannel designs to achieve increasingly faithful replication of organ-specific interstitial flow patterns.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!