Matrix remodeling dynamics: quantifying MMP activity within cancer-on-chip invasion assays
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Matrix Remodeling Technology Background and Objectives
Matrix remodeling, particularly the extracellular matrix (ECM), represents a critical biological process that has gained significant attention in cancer research over the past decades. The dynamic interplay between cancer cells and their surrounding matrix environment fundamentally influences tumor progression, invasion, and metastasis. Historically, matrix remodeling research evolved from basic histological observations to sophisticated molecular analyses, with significant breakthroughs occurring in the 1980s when matrix metalloproteinases (MMPs) were first identified as key enzymes in ECM degradation.
The technological evolution in this field has progressed from traditional 2D cell culture systems to more complex 3D models that better recapitulate the tumor microenvironment. Recent advancements in microfluidics and organ-on-chip technologies have revolutionized our ability to study these processes in controlled, physiologically relevant conditions. Cancer-on-chip platforms, in particular, have emerged as powerful tools for investigating tumor-matrix interactions in real-time.
Current research trends focus on quantitative assessment of matrix remodeling dynamics, with particular emphasis on measuring MMP activity within these sophisticated in vitro models. This shift from qualitative to quantitative analysis represents a critical advancement in understanding cancer invasion mechanisms at a molecular level. The integration of advanced imaging techniques, biosensors, and computational modeling has further enhanced our ability to capture and analyze these complex biological processes.
The primary objective of this technological domain is to develop robust, reproducible methods for quantifying MMP activity within cancer-on-chip invasion assays. This includes establishing standardized protocols for measuring enzyme kinetics, spatial distribution of proteolytic activity, and temporal dynamics of matrix degradation. Such quantitative approaches are essential for elucidating the mechanistic details of cancer invasion and for evaluating the efficacy of potential therapeutic interventions.
Additionally, this field aims to bridge the gap between in vitro observations and clinical relevance by creating increasingly sophisticated models that incorporate multiple cell types, physiological flow conditions, and patient-derived materials. The ultimate goal is to establish predictive platforms that can inform personalized treatment strategies by accurately recapitulating patient-specific tumor-matrix interactions.
As we advance toward precision medicine in oncology, quantitative assessment of matrix remodeling dynamics will play an increasingly important role in drug discovery, patient stratification, and therapeutic monitoring. The continued refinement of these technologies promises to yield valuable insights into the complex processes driving cancer progression and to identify novel targets for intervention.
The technological evolution in this field has progressed from traditional 2D cell culture systems to more complex 3D models that better recapitulate the tumor microenvironment. Recent advancements in microfluidics and organ-on-chip technologies have revolutionized our ability to study these processes in controlled, physiologically relevant conditions. Cancer-on-chip platforms, in particular, have emerged as powerful tools for investigating tumor-matrix interactions in real-time.
Current research trends focus on quantitative assessment of matrix remodeling dynamics, with particular emphasis on measuring MMP activity within these sophisticated in vitro models. This shift from qualitative to quantitative analysis represents a critical advancement in understanding cancer invasion mechanisms at a molecular level. The integration of advanced imaging techniques, biosensors, and computational modeling has further enhanced our ability to capture and analyze these complex biological processes.
The primary objective of this technological domain is to develop robust, reproducible methods for quantifying MMP activity within cancer-on-chip invasion assays. This includes establishing standardized protocols for measuring enzyme kinetics, spatial distribution of proteolytic activity, and temporal dynamics of matrix degradation. Such quantitative approaches are essential for elucidating the mechanistic details of cancer invasion and for evaluating the efficacy of potential therapeutic interventions.
Additionally, this field aims to bridge the gap between in vitro observations and clinical relevance by creating increasingly sophisticated models that incorporate multiple cell types, physiological flow conditions, and patient-derived materials. The ultimate goal is to establish predictive platforms that can inform personalized treatment strategies by accurately recapitulating patient-specific tumor-matrix interactions.
As we advance toward precision medicine in oncology, quantitative assessment of matrix remodeling dynamics will play an increasingly important role in drug discovery, patient stratification, and therapeutic monitoring. The continued refinement of these technologies promises to yield valuable insights into the complex processes driving cancer progression and to identify novel targets for intervention.
Market Analysis for Cancer-on-Chip Invasion Assays
The cancer-on-chip invasion assay market is experiencing significant growth, driven by increasing cancer research funding and the need for more physiologically relevant models. This segment represents a specialized niche within the broader organ-on-chip market, which was valued at approximately $103 million in 2022 and is projected to reach $594 million by 2030, growing at a CAGR of 28.1% during the forecast period.
Cancer-on-chip platforms specifically designed for invasion assays address critical needs in oncology research by enabling real-time visualization and quantification of matrix metalloproteinase (MMP) activity during cancer cell invasion. The demand for these systems stems primarily from academic research institutions, pharmaceutical companies, and biotechnology firms engaged in cancer drug discovery and development.
Geographically, North America dominates the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). The United States leads in adoption due to substantial research funding from organizations like the National Cancer Institute, which allocated over $7 billion to cancer research in 2022. China represents the fastest-growing market with increasing investments in cancer research infrastructure.
Key market drivers include the rising prevalence of cancer globally, with 19.3 million new cases reported in 2020 and projections reaching 28.4 million by 2040. The pharmaceutical industry's shift toward precision medicine approaches has intensified demand for advanced preclinical models that can accurately predict drug efficacy and resistance mechanisms.
The market faces certain constraints, including high system costs ranging from $30,000 to $100,000 per unit, technical complexity requiring specialized expertise, and regulatory uncertainties surrounding validation and standardization. These factors particularly limit adoption in smaller research institutions and emerging markets.
Customer segmentation reveals pharmaceutical companies as the largest market segment (45%), followed by academic research institutions (35%) and contract research organizations (15%). End-user preferences increasingly favor systems offering quantitative analysis capabilities, automation features, and compatibility with high-content imaging platforms.
Future market growth will be driven by technological advancements in microfluidics, biosensors for real-time MMP detection, and artificial intelligence integration for automated analysis. The development of standardized protocols and validation methods will further accelerate market expansion by addressing current adoption barriers and enabling broader implementation across research settings.
Cancer-on-chip platforms specifically designed for invasion assays address critical needs in oncology research by enabling real-time visualization and quantification of matrix metalloproteinase (MMP) activity during cancer cell invasion. The demand for these systems stems primarily from academic research institutions, pharmaceutical companies, and biotechnology firms engaged in cancer drug discovery and development.
Geographically, North America dominates the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). The United States leads in adoption due to substantial research funding from organizations like the National Cancer Institute, which allocated over $7 billion to cancer research in 2022. China represents the fastest-growing market with increasing investments in cancer research infrastructure.
Key market drivers include the rising prevalence of cancer globally, with 19.3 million new cases reported in 2020 and projections reaching 28.4 million by 2040. The pharmaceutical industry's shift toward precision medicine approaches has intensified demand for advanced preclinical models that can accurately predict drug efficacy and resistance mechanisms.
The market faces certain constraints, including high system costs ranging from $30,000 to $100,000 per unit, technical complexity requiring specialized expertise, and regulatory uncertainties surrounding validation and standardization. These factors particularly limit adoption in smaller research institutions and emerging markets.
Customer segmentation reveals pharmaceutical companies as the largest market segment (45%), followed by academic research institutions (35%) and contract research organizations (15%). End-user preferences increasingly favor systems offering quantitative analysis capabilities, automation features, and compatibility with high-content imaging platforms.
Future market growth will be driven by technological advancements in microfluidics, biosensors for real-time MMP detection, and artificial intelligence integration for automated analysis. The development of standardized protocols and validation methods will further accelerate market expansion by addressing current adoption barriers and enabling broader implementation across research settings.
Current MMP Activity Quantification Challenges
The quantification of matrix metalloproteinase (MMP) activity within cancer-on-chip invasion assays presents several significant challenges that impede accurate measurement and interpretation. Current methodologies suffer from limitations in spatial resolution, temporal dynamics capture, and physiological relevance, creating substantial barriers to translational research.
Traditional MMP activity assays such as zymography and ELISA-based methods provide only endpoint measurements, failing to capture the dynamic nature of MMP activity during cancer invasion processes. These techniques typically require sample destruction, preventing continuous monitoring of the same sample over time and losing valuable information about the temporal evolution of protease activity.
Fluorescence-based assays utilizing FRET (Förster Resonance Energy Transfer) substrates have improved temporal resolution but face challenges in maintaining stable signal-to-noise ratios in complex 3D microenvironments. The autofluorescence of extracellular matrix components and the presence of other biological materials in cancer-on-chip platforms often interfere with accurate signal detection, leading to potential misinterpretation of MMP activity levels.
Spatial heterogeneity represents another critical challenge, as current methods struggle to distinguish between localized MMP activity at the invasive front versus overall matrix degradation. This limitation is particularly problematic when studying the leading edge of invading cancer cells, where precise spatial information about protease activity is crucial for understanding invasion mechanisms.
The physiological relevance of existing quantification methods is questionable due to the artificial nature of many MMP substrates. These synthetic substrates may not accurately reflect the complex interactions between MMPs and native extracellular matrix components, potentially leading to results that poorly translate to in vivo conditions. Furthermore, the specificity of current assays for individual MMP types remains limited, making it difficult to distinguish between the activities of different MMP family members that may play distinct roles in cancer progression.
Technical challenges in integrating real-time MMP activity measurements with cancer-on-chip platforms further complicate quantification efforts. Many existing methods require specialized equipment or expertise not readily available in standard research laboratories, limiting widespread adoption and reproducibility of results across different research groups.
Standardization issues also plague the field, with variations in experimental conditions, substrate concentrations, and analysis methods making cross-study comparisons difficult. The lack of universally accepted quantification metrics and reference standards contributes to inconsistencies in reported MMP activity levels and their interpretation in the context of cancer invasion dynamics.
Traditional MMP activity assays such as zymography and ELISA-based methods provide only endpoint measurements, failing to capture the dynamic nature of MMP activity during cancer invasion processes. These techniques typically require sample destruction, preventing continuous monitoring of the same sample over time and losing valuable information about the temporal evolution of protease activity.
Fluorescence-based assays utilizing FRET (Förster Resonance Energy Transfer) substrates have improved temporal resolution but face challenges in maintaining stable signal-to-noise ratios in complex 3D microenvironments. The autofluorescence of extracellular matrix components and the presence of other biological materials in cancer-on-chip platforms often interfere with accurate signal detection, leading to potential misinterpretation of MMP activity levels.
Spatial heterogeneity represents another critical challenge, as current methods struggle to distinguish between localized MMP activity at the invasive front versus overall matrix degradation. This limitation is particularly problematic when studying the leading edge of invading cancer cells, where precise spatial information about protease activity is crucial for understanding invasion mechanisms.
The physiological relevance of existing quantification methods is questionable due to the artificial nature of many MMP substrates. These synthetic substrates may not accurately reflect the complex interactions between MMPs and native extracellular matrix components, potentially leading to results that poorly translate to in vivo conditions. Furthermore, the specificity of current assays for individual MMP types remains limited, making it difficult to distinguish between the activities of different MMP family members that may play distinct roles in cancer progression.
Technical challenges in integrating real-time MMP activity measurements with cancer-on-chip platforms further complicate quantification efforts. Many existing methods require specialized equipment or expertise not readily available in standard research laboratories, limiting widespread adoption and reproducibility of results across different research groups.
Standardization issues also plague the field, with variations in experimental conditions, substrate concentrations, and analysis methods making cross-study comparisons difficult. The lack of universally accepted quantification metrics and reference standards contributes to inconsistencies in reported MMP activity levels and their interpretation in the context of cancer invasion dynamics.
Current MMP Activity Quantification Solutions
01 Fluorescence-based MMP activity assays
Fluorescence-based methods provide sensitive quantification of matrix metalloproteinase (MMP) activity in real-time. These techniques utilize fluorogenic substrates that emit measurable signals upon cleavage by active MMPs, allowing for dynamic monitoring of enzyme kinetics. Such assays can be applied to study extracellular matrix remodeling in various physiological and pathological conditions, providing insights into the rate and extent of matrix degradation processes.- MMP activity assay methods for matrix remodeling: Various methods have been developed to quantify matrix metalloproteinase (MMP) activity in the context of extracellular matrix remodeling. These include fluorescence-based assays, zymography techniques, and ELISA-based detection systems that can measure specific MMP subtypes and their activity levels in biological samples. These methods allow researchers to monitor real-time changes in matrix degradation and remodeling dynamics in both in vitro and in vivo systems.
- Imaging techniques for matrix remodeling visualization: Advanced imaging technologies have been developed to visualize and quantify matrix remodeling dynamics. These include second harmonic generation microscopy, fluorescence resonance energy transfer (FRET)-based sensors, and other optical imaging methods that can detect structural changes in the extracellular matrix. These techniques provide spatial and temporal information about MMP activity and matrix reorganization, enabling researchers to study dynamic processes in tissue development, wound healing, and disease progression.
- Biomarkers for matrix remodeling assessment: Specific biomarkers have been identified that reflect MMP activity and matrix turnover. These include degradation products of collagens and other matrix proteins that can be detected in biological fluids. Measurement of these biomarkers provides insights into the rate and extent of matrix remodeling in various physiological and pathological conditions, allowing for non-invasive monitoring of tissue remodeling processes and potential therapeutic interventions.
- Computational models for matrix dynamics prediction: Mathematical and computational models have been developed to predict and analyze matrix remodeling dynamics based on MMP activity data. These models integrate experimental measurements with theoretical frameworks to simulate complex interactions between cells, MMPs, and matrix components. By incorporating parameters such as enzyme kinetics, diffusion rates, and mechanical properties, these models help researchers understand the spatiotemporal patterns of matrix remodeling and predict outcomes of interventions targeting MMP activity.
- Therapeutic modulation of MMP activity: Various approaches have been developed to modulate MMP activity for therapeutic purposes in conditions characterized by aberrant matrix remodeling. These include small molecule inhibitors, peptide-based regulators, and gene therapy strategies targeting specific MMPs or their regulators. Quantification of MMP activity before and after intervention provides critical information about treatment efficacy and helps optimize therapeutic strategies for conditions such as fibrosis, cancer, and inflammatory diseases where matrix remodeling plays a significant role.
02 In vivo imaging techniques for matrix remodeling
Advanced imaging technologies enable non-invasive visualization and quantification of MMP activity and matrix remodeling dynamics in living organisms. These methods incorporate specialized probes that interact with active MMPs or modified matrix components, generating detectable signals. Such approaches allow for longitudinal studies of tissue remodeling processes in disease models and can track the efficacy of therapeutic interventions targeting matrix metabolism.Expand Specific Solutions03 Computational models for matrix remodeling dynamics
Mathematical and computational models have been developed to simulate and predict MMP activity and matrix remodeling dynamics. These models integrate experimental data on enzyme kinetics, substrate availability, and inhibitor interactions to forecast remodeling outcomes under various conditions. Such computational approaches help researchers understand complex spatiotemporal patterns in matrix turnover and can guide experimental design for studying dynamic remodeling processes.Expand Specific Solutions04 Biomarkers for quantifying MMP-mediated matrix turnover
Specific biomarkers have been identified that reflect MMP activity and matrix turnover in tissues and biological fluids. These include matrix fragments generated by MMP cleavage, which bear unique neoepitopes that can be detected using specialized antibodies. Quantification of these biomarkers provides insights into the rate and extent of matrix degradation in various physiological and pathological states, enabling assessment of disease progression and treatment response.Expand Specific Solutions05 Cell-based systems for studying matrix remodeling dynamics
Engineered cell culture systems provide controlled environments for studying MMP activity and matrix remodeling dynamics. These include three-dimensional matrices embedded with cells that produce and remodel extracellular components, allowing researchers to observe and quantify dynamic interactions between cells and their surrounding matrix. Such systems can incorporate sensors or reporter molecules to track MMP activity in real-time, providing insights into the cellular mechanisms governing matrix homeostasis.Expand Specific Solutions
Key Players in Cancer-on-Chip Technology
The field of matrix remodeling dynamics in cancer-on-chip invasion assays is currently in an emerging growth phase, with market size expanding as cancer research increasingly adopts microfluidic technologies. The technical maturity varies across applications, with quantitative MMP activity measurement representing a particularly innovative frontier. Key players shaping this landscape include Dana-Farber Cancer Institute and Northwestern University, who lead academic research, while companies like Cellanyx Diagnostics and Traxxsson are developing commercial diagnostic applications. The Broad Institute and Scripps Research Institute contribute significant technological innovations, while pharmaceutical entities such as Daiichi Sankyo and Roche Diagnostics are investing in these platforms for drug development. This ecosystem reflects a collaborative environment where academic discoveries are increasingly translated into clinical and commercial applications.
Dana-Farber Cancer Institute, Inc.
Technical Solution: Dana-Farber has developed advanced microfluidic cancer-on-chip platforms specifically designed for quantifying matrix metalloproteinase (MMP) activity in real-time. Their approach integrates fluorogenic MMP substrates within 3D extracellular matrix (ECM) hydrogels that mimic the tumor microenvironment. When cancer cells invade through these matrices, they secrete MMPs that cleave the substrates, generating fluorescent signals proportional to enzymatic activity. This system employs high-resolution confocal microscopy with automated image analysis algorithms to spatiotemporally map MMP activity gradients around invading cells[1]. Dana-Farber's platform also incorporates multiple cell types (cancer cells, fibroblasts, immune cells) to recapitulate tumor-stroma interactions that regulate MMP expression and ECM remodeling dynamics.
Strengths: Exceptional sensitivity for detecting subtle changes in MMP activity; ability to correlate MMP activity with invasion metrics in the same assay; compatibility with patient-derived cells for personalized medicine applications. Weaknesses: Requires specialized imaging equipment and expertise; higher cost compared to conventional invasion assays; challenges in standardizing complex 3D microenvironments across experiments.
The Regents of the University of California
Technical Solution: The University of California has pioneered a comprehensive cancer-on-chip platform called "MMPSense" that combines microfluidic technology with advanced biosensing capabilities for quantifying MMP activity during cancer invasion. Their system features a central tumor chamber surrounded by precisely engineered ECM channels containing MMP-cleavable peptide sensors conjugated to quantum dots. As cancer cells invade, MMP activity is measured through ratiometric fluorescence imaging, providing quantitative data on protease activity with subcellular resolution[2]. The platform incorporates multiple parallel channels for simultaneous testing of different conditions, such as MMP inhibitors or varied matrix compositions. Additionally, their system enables continuous non-destructive monitoring through integrated microscopy, allowing for kinetic analysis of MMP activity throughout the invasion process[3]. The technology has been validated across multiple cancer types including breast, pancreatic, and glioblastoma models.
Strengths: High-throughput capability allowing multiple conditions to be tested simultaneously; exceptional spatial resolution for mapping MMP activity gradients; compatibility with live-cell imaging for longitudinal studies. Weaknesses: Complex fabrication process requiring specialized expertise; potential for photobleaching during extended imaging sessions; challenges in distinguishing between different MMP subtypes without additional molecular tools.
Core Technologies for Dynamic Matrix Assessment
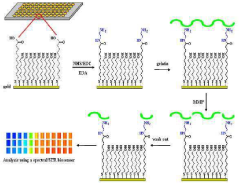
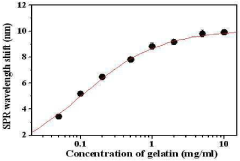
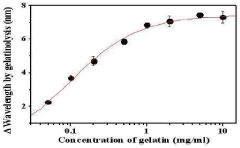
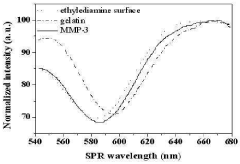
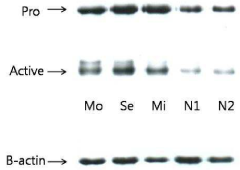
A chip for measuring a matrix metalloproteinase activity and a method using the same
PatentInactiveKR1020100105254A
Innovation
- A protein chip with an MMP substrate immobilized on a solid substrate is used to measure MMP activity by detecting the degradation of the substrate through SPR signal or fluorescence intensity changes.
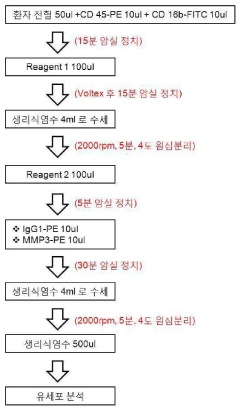
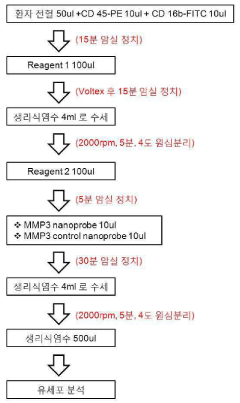
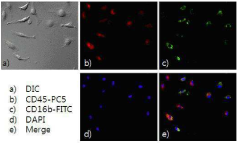
Method for quantifying active matrix metalloproteinase in peripheral blood cell using nanoprobe for diagnosing autoimmune diseases
PatentInactiveKR1020140119391A
Innovation
- A method using MMP-specific nanoprobe labeled peripheral blood cells with fluorophores, quenchers, and polymers to quantify activated MMPs via real-time flow cytometry, allowing for accurate measurement of MMPs in an activated state within cells, thereby overcoming the limitations of existing methods.
Regulatory Considerations for Oncology Diagnostics
The regulatory landscape for cancer diagnostics, particularly those involving matrix metalloproteinase (MMP) activity quantification in cancer-on-chip invasion assays, presents significant considerations for technology developers and clinical implementers. These innovative diagnostic approaches face a complex approval pathway through regulatory bodies such as the FDA in the United States and similar authorities worldwide.
For cancer-on-chip technologies measuring MMP activity, regulatory classification typically falls under in vitro diagnostic devices (IVDs), with potential designation as companion diagnostics if used to inform treatment decisions. The FDA's risk-based approach categorizes these technologies based on their intended use and potential risk to patients, with cancer diagnostics generally receiving higher scrutiny due to the critical nature of oncology decisions.
Premarket approval requirements for these technologies depend on their novelty and risk profile. Novel MMP activity quantification methods may require extensive clinical validation through the Premarket Approval (PMA) pathway, while more incremental innovations might qualify for the 510(k) clearance process if substantial equivalence to predicate devices can be demonstrated.
Analytical validation represents a critical regulatory hurdle, requiring developers to demonstrate precision, accuracy, sensitivity, and specificity of MMP activity measurements. This includes establishing reference ranges and determining clinical cut-off values that meaningfully distinguish between different cancer invasion potentials. The dynamic nature of matrix remodeling creates additional complexity in standardizing these measurements.
Clinical validation requirements present another significant challenge, necessitating evidence that quantified MMP activity correlates with clinical outcomes. This typically requires prospective clinical studies demonstrating the technology's ability to predict cancer progression, metastatic potential, or treatment response. The microfluidic nature of cancer-on-chip platforms adds complexity to validation protocols.
Quality system regulations mandate comprehensive documentation of design controls, manufacturing processes, and quality assurance procedures. For microfluidic devices measuring dynamic cellular processes, this includes validation of chip fabrication consistency, reagent stability, and software algorithms interpreting MMP activity data.
Laboratory developed test (LDT) considerations may apply if these assays are developed and used within a single laboratory. However, the FDA has signaled increased oversight of complex LDTs, particularly in oncology, potentially affecting the regulatory pathway for novel MMP quantification methods.
International harmonization efforts through organizations like the International Medical Device Regulators Forum (IMDRF) are working to standardize requirements across regions, potentially streamlining global market access for these innovative cancer diagnostic technologies.
For cancer-on-chip technologies measuring MMP activity, regulatory classification typically falls under in vitro diagnostic devices (IVDs), with potential designation as companion diagnostics if used to inform treatment decisions. The FDA's risk-based approach categorizes these technologies based on their intended use and potential risk to patients, with cancer diagnostics generally receiving higher scrutiny due to the critical nature of oncology decisions.
Premarket approval requirements for these technologies depend on their novelty and risk profile. Novel MMP activity quantification methods may require extensive clinical validation through the Premarket Approval (PMA) pathway, while more incremental innovations might qualify for the 510(k) clearance process if substantial equivalence to predicate devices can be demonstrated.
Analytical validation represents a critical regulatory hurdle, requiring developers to demonstrate precision, accuracy, sensitivity, and specificity of MMP activity measurements. This includes establishing reference ranges and determining clinical cut-off values that meaningfully distinguish between different cancer invasion potentials. The dynamic nature of matrix remodeling creates additional complexity in standardizing these measurements.
Clinical validation requirements present another significant challenge, necessitating evidence that quantified MMP activity correlates with clinical outcomes. This typically requires prospective clinical studies demonstrating the technology's ability to predict cancer progression, metastatic potential, or treatment response. The microfluidic nature of cancer-on-chip platforms adds complexity to validation protocols.
Quality system regulations mandate comprehensive documentation of design controls, manufacturing processes, and quality assurance procedures. For microfluidic devices measuring dynamic cellular processes, this includes validation of chip fabrication consistency, reagent stability, and software algorithms interpreting MMP activity data.
Laboratory developed test (LDT) considerations may apply if these assays are developed and used within a single laboratory. However, the FDA has signaled increased oversight of complex LDTs, particularly in oncology, potentially affecting the regulatory pathway for novel MMP quantification methods.
International harmonization efforts through organizations like the International Medical Device Regulators Forum (IMDRF) are working to standardize requirements across regions, potentially streamlining global market access for these innovative cancer diagnostic technologies.
Clinical Translation Pathways
The translation of matrix remodeling dynamics research into clinical applications represents a critical pathway for advancing cancer diagnostics and therapeutics. Cancer-on-chip platforms that quantify MMP activity offer significant potential for personalized medicine approaches, particularly in treatment selection and monitoring. These systems can be integrated into clinical workflows for patient-specific tumor profiling, enabling physicians to assess individual tumor invasiveness characteristics and predict metastatic potential with greater accuracy than conventional methods.
Regulatory pathways for these technologies are becoming increasingly defined, with the FDA and EMA establishing frameworks for qualifying biomarker technologies. The quantification of MMP activity within cancer-on-chip systems could potentially achieve companion diagnostic status, guiding the use of MMP inhibitors or other targeted therapies. This would require extensive validation studies demonstrating clinical utility and reproducibility across diverse patient populations.
Several clinical trials are currently exploring the correlation between quantified MMP activity in patient-derived samples and treatment outcomes. These studies aim to establish predictive algorithms that can guide therapeutic decision-making based on matrix remodeling dynamics. The integration of artificial intelligence with these quantification methods further enhances their clinical applicability by identifying subtle patterns in MMP activity that correlate with disease progression or treatment response.
Hospital implementation pathways are being developed through collaborations between academic research centers and clinical departments. These partnerships focus on establishing standardized protocols for sample processing, analysis, and interpretation that can be feasibly integrated into existing clinical laboratory infrastructures. Point-of-care adaptations of these technologies are also under development, potentially allowing for rapid assessment of tumor invasiveness during surgical procedures.
Economic considerations remain significant in the clinical translation process. Cost-effectiveness analyses suggest that while initial implementation costs are substantial, the potential reduction in ineffective treatments and improved patient outcomes could offset these expenses. Healthcare systems are increasingly exploring reimbursement models for advanced diagnostic technologies that demonstrate clear improvements in clinical decision-making.
The timeline for full clinical integration is estimated at 3-5 years, with initial applications likely focusing on high-mortality cancers where current prognostic tools are limited. Pilot programs in comprehensive cancer centers are establishing the foundation for broader implementation, creating evidence bases that will support wider adoption across healthcare systems globally.
Regulatory pathways for these technologies are becoming increasingly defined, with the FDA and EMA establishing frameworks for qualifying biomarker technologies. The quantification of MMP activity within cancer-on-chip systems could potentially achieve companion diagnostic status, guiding the use of MMP inhibitors or other targeted therapies. This would require extensive validation studies demonstrating clinical utility and reproducibility across diverse patient populations.
Several clinical trials are currently exploring the correlation between quantified MMP activity in patient-derived samples and treatment outcomes. These studies aim to establish predictive algorithms that can guide therapeutic decision-making based on matrix remodeling dynamics. The integration of artificial intelligence with these quantification methods further enhances their clinical applicability by identifying subtle patterns in MMP activity that correlate with disease progression or treatment response.
Hospital implementation pathways are being developed through collaborations between academic research centers and clinical departments. These partnerships focus on establishing standardized protocols for sample processing, analysis, and interpretation that can be feasibly integrated into existing clinical laboratory infrastructures. Point-of-care adaptations of these technologies are also under development, potentially allowing for rapid assessment of tumor invasiveness during surgical procedures.
Economic considerations remain significant in the clinical translation process. Cost-effectiveness analyses suggest that while initial implementation costs are substantial, the potential reduction in ineffective treatments and improved patient outcomes could offset these expenses. Healthcare systems are increasingly exploring reimbursement models for advanced diagnostic technologies that demonstrate clear improvements in clinical decision-making.
The timeline for full clinical integration is estimated at 3-5 years, with initial applications likely focusing on high-mortality cancers where current prognostic tools are limited. Pilot programs in comprehensive cancer centers are establishing the foundation for broader implementation, creating evidence bases that will support wider adoption across healthcare systems globally.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!