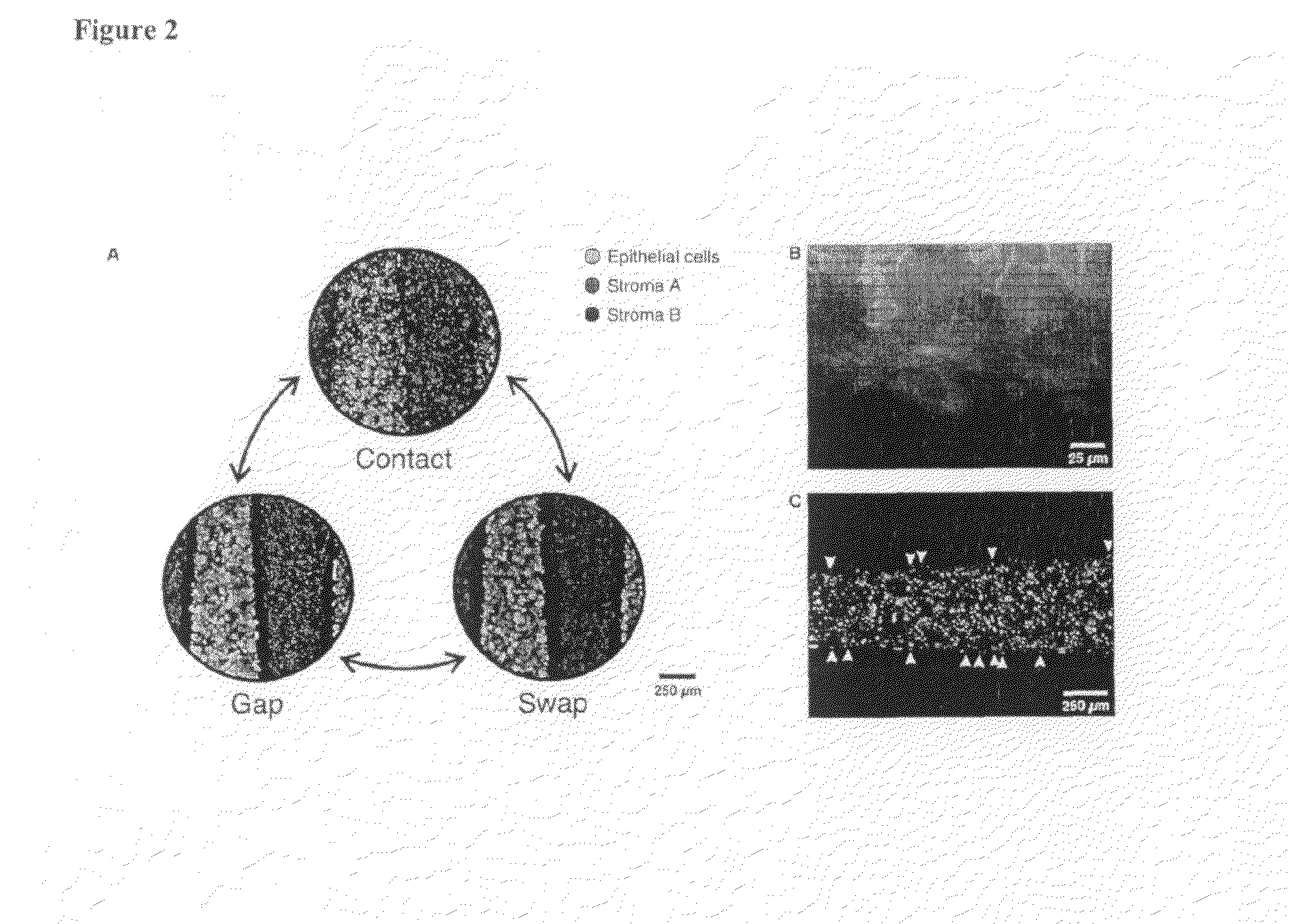
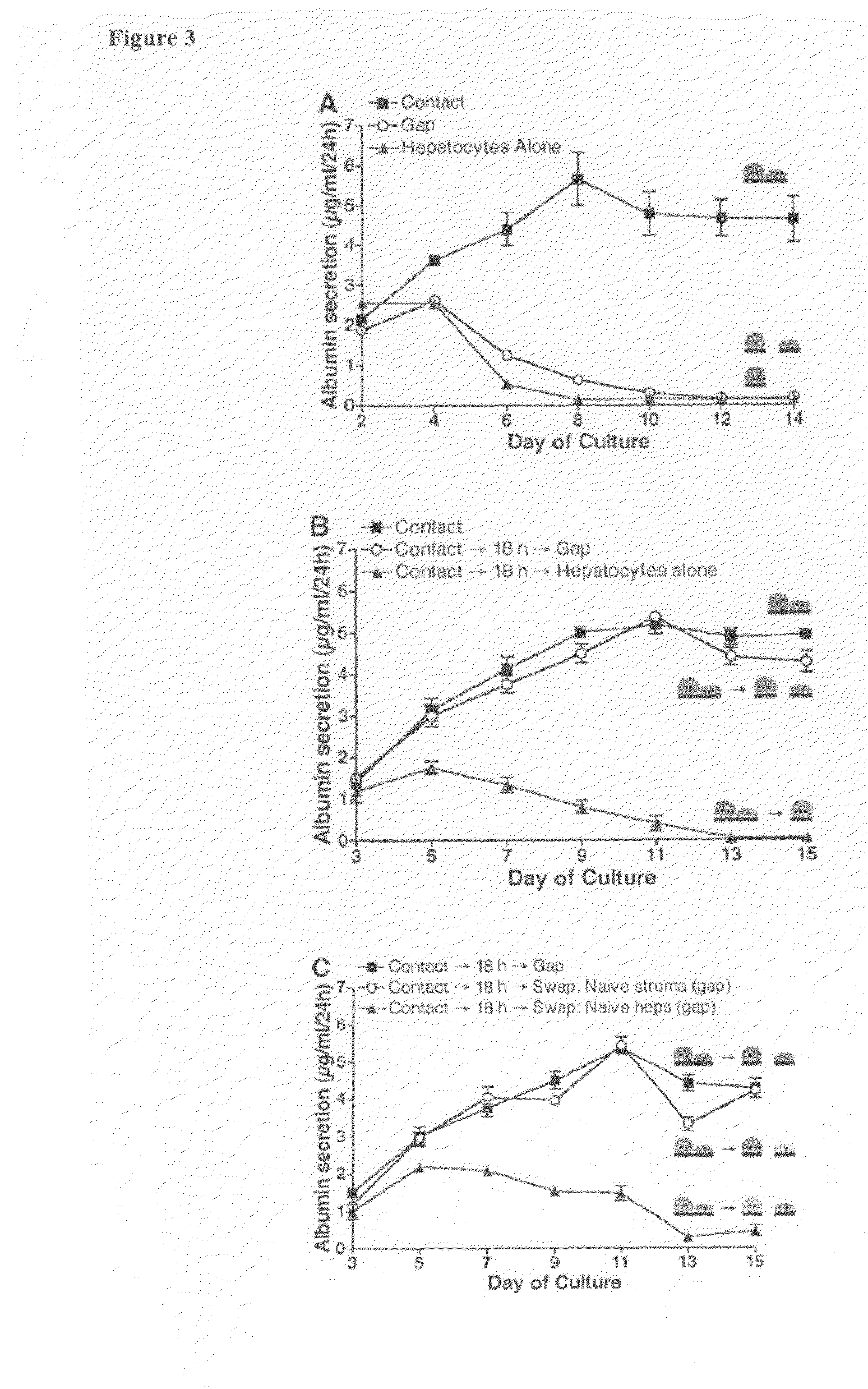
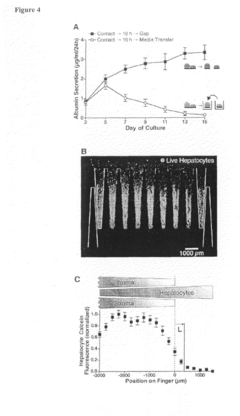
Surface engineering of microchannels to control cell attachment and barrier formation kinetics
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microchannel Surface Engineering Background and Objectives
Surface engineering of microchannels represents a critical frontier in biomedical engineering, emerging from the convergence of microfluidics, materials science, and cell biology. This interdisciplinary field has evolved significantly over the past two decades, transitioning from simple channel fabrication to sophisticated surface modification techniques that can precisely control cellular interactions at the micro-scale level.
The historical trajectory of microchannel surface engineering began with basic PDMS (polydimethylsiloxane) fabrication methods in the early 2000s, which offered limited control over surface properties. Subsequent advancements introduced various chemical modification strategies, including plasma treatment, chemical vapor deposition, and layer-by-layer assembly techniques, enabling greater customization of surface characteristics.
Recent technological developments have focused on biomimetic approaches that replicate the complex extracellular matrix environment, incorporating specific proteins, peptides, and growth factors to guide cellular behavior within microchannels. These innovations have been particularly valuable in organ-on-chip platforms and tissue barrier models, where controlled cell attachment and barrier formation are essential for physiological relevance.
The primary objective of microchannel surface engineering is to develop reproducible, scalable methods for precisely controlling the spatial and temporal aspects of cell attachment and subsequent barrier formation. This control is crucial for creating physiologically relevant in vitro models that accurately recapitulate tissue interfaces and barriers found in the human body, such as the blood-brain barrier, lung alveolar interfaces, and intestinal epithelial barriers.
Current research aims to address several key challenges, including achieving spatial selectivity in surface modification, maintaining long-term stability of engineered surfaces, and developing dynamic surfaces that can respond to external stimuli or cellular signals. These capabilities would enable more sophisticated tissue models with improved predictive value for drug development and disease modeling applications.
The field is trending toward integration of multiple surface engineering strategies to create multifunctional microchannels capable of supporting complex cellular architectures and physiological processes. Emerging approaches include gradient surface modifications, photopatterning of bioactive molecules, and stimuli-responsive coatings that can dynamically alter surface properties in response to specific triggers.
The ultimate goal is to establish standardized, reproducible methodologies for engineering microchannel surfaces that can reliably control cell attachment kinetics, spatial organization, and barrier formation processes, thereby enabling more accurate modeling of human physiology and pathology in microfluidic devices.
The historical trajectory of microchannel surface engineering began with basic PDMS (polydimethylsiloxane) fabrication methods in the early 2000s, which offered limited control over surface properties. Subsequent advancements introduced various chemical modification strategies, including plasma treatment, chemical vapor deposition, and layer-by-layer assembly techniques, enabling greater customization of surface characteristics.
Recent technological developments have focused on biomimetic approaches that replicate the complex extracellular matrix environment, incorporating specific proteins, peptides, and growth factors to guide cellular behavior within microchannels. These innovations have been particularly valuable in organ-on-chip platforms and tissue barrier models, where controlled cell attachment and barrier formation are essential for physiological relevance.
The primary objective of microchannel surface engineering is to develop reproducible, scalable methods for precisely controlling the spatial and temporal aspects of cell attachment and subsequent barrier formation. This control is crucial for creating physiologically relevant in vitro models that accurately recapitulate tissue interfaces and barriers found in the human body, such as the blood-brain barrier, lung alveolar interfaces, and intestinal epithelial barriers.
Current research aims to address several key challenges, including achieving spatial selectivity in surface modification, maintaining long-term stability of engineered surfaces, and developing dynamic surfaces that can respond to external stimuli or cellular signals. These capabilities would enable more sophisticated tissue models with improved predictive value for drug development and disease modeling applications.
The field is trending toward integration of multiple surface engineering strategies to create multifunctional microchannels capable of supporting complex cellular architectures and physiological processes. Emerging approaches include gradient surface modifications, photopatterning of bioactive molecules, and stimuli-responsive coatings that can dynamically alter surface properties in response to specific triggers.
The ultimate goal is to establish standardized, reproducible methodologies for engineering microchannel surfaces that can reliably control cell attachment kinetics, spatial organization, and barrier formation processes, thereby enabling more accurate modeling of human physiology and pathology in microfluidic devices.
Market Analysis for Cell-Based Microfluidic Systems
The global market for cell-based microfluidic systems has experienced significant growth in recent years, driven by increasing applications in drug discovery, personalized medicine, and tissue engineering. The market size for microfluidic devices was valued at approximately $13.5 billion in 2022 and is projected to reach $30.7 billion by 2027, growing at a CAGR of 17.8%. Within this broader market, cell-based microfluidic systems represent a rapidly expanding segment with particular emphasis on engineered microchannel surfaces.
Surface engineering of microchannels for controlled cell attachment has emerged as a critical factor in market differentiation. End-users across pharmaceutical companies, academic research institutions, and biotechnology firms are increasingly demanding microfluidic platforms that can precisely control cellular behavior and barrier formation kinetics. This demand stems from the need for more physiologically relevant in vitro models that can better predict in vivo responses, thereby reducing drug development costs and accelerating time-to-market.
The healthcare segment dominates the application landscape, accounting for 45% of the market share. Within healthcare, organ-on-chip devices that rely on precisely engineered microchannel surfaces to mimic tissue barriers have shown a remarkable 25% annual growth rate. Blood-brain barrier models, lung-on-chip, and gut-on-chip systems represent the fastest-growing applications, with combined revenues exceeding $1.2 billion in 2022.
Regional analysis reveals North America as the leading market with 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is experiencing the highest growth rate at 22% annually, driven by increasing R&D investments in China, Japan, and South Korea. Notably, China has doubled its funding for microfluidic research over the past five years, with particular emphasis on surface modification technologies.
Customer segmentation shows that pharmaceutical companies constitute the largest end-user group (42%), followed by academic research institutions (31%) and contract research organizations (18%). The pharmaceutical sector's dominance is attributed to the increasing adoption of microfluidic systems in drug screening and toxicity testing, where controlled cell attachment is crucial for developing predictive models.
Market challenges include high initial investment costs, technical complexity in surface engineering, and regulatory uncertainties. The average cost of implementing advanced microfluidic systems with engineered surfaces ranges from $50,000 to $200,000, creating a significant barrier for smaller research institutions. Additionally, standardization issues and reproducibility concerns have limited wider adoption, though recent technological advances are gradually addressing these limitations.
Surface engineering of microchannels for controlled cell attachment has emerged as a critical factor in market differentiation. End-users across pharmaceutical companies, academic research institutions, and biotechnology firms are increasingly demanding microfluidic platforms that can precisely control cellular behavior and barrier formation kinetics. This demand stems from the need for more physiologically relevant in vitro models that can better predict in vivo responses, thereby reducing drug development costs and accelerating time-to-market.
The healthcare segment dominates the application landscape, accounting for 45% of the market share. Within healthcare, organ-on-chip devices that rely on precisely engineered microchannel surfaces to mimic tissue barriers have shown a remarkable 25% annual growth rate. Blood-brain barrier models, lung-on-chip, and gut-on-chip systems represent the fastest-growing applications, with combined revenues exceeding $1.2 billion in 2022.
Regional analysis reveals North America as the leading market with 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is experiencing the highest growth rate at 22% annually, driven by increasing R&D investments in China, Japan, and South Korea. Notably, China has doubled its funding for microfluidic research over the past five years, with particular emphasis on surface modification technologies.
Customer segmentation shows that pharmaceutical companies constitute the largest end-user group (42%), followed by academic research institutions (31%) and contract research organizations (18%). The pharmaceutical sector's dominance is attributed to the increasing adoption of microfluidic systems in drug screening and toxicity testing, where controlled cell attachment is crucial for developing predictive models.
Market challenges include high initial investment costs, technical complexity in surface engineering, and regulatory uncertainties. The average cost of implementing advanced microfluidic systems with engineered surfaces ranges from $50,000 to $200,000, creating a significant barrier for smaller research institutions. Additionally, standardization issues and reproducibility concerns have limited wider adoption, though recent technological advances are gradually addressing these limitations.
Current Challenges in Microchannel Surface Modification
Despite significant advancements in microchannel surface engineering, researchers and industry professionals continue to face substantial challenges in developing optimal surface modifications for controlling cell attachment and barrier formation kinetics. One of the primary obstacles is achieving uniform surface modification across the entire microchannel network, particularly in complex geometries with high aspect ratios or intricate designs. Conventional surface treatment methods often result in heterogeneous coating distribution, creating inconsistent cell attachment patterns that compromise experimental reproducibility and device performance.
Material compatibility presents another significant hurdle, as surface modification techniques must maintain the optical transparency and mechanical properties of the base substrate while introducing the desired biochemical functionalities. Many current approaches involve harsh chemical treatments or plasma processing that can degrade the bulk properties of polymeric materials commonly used in microfluidic devices, such as PDMS, PMMA, or COC.
The stability of surface modifications under flow conditions remains problematic, with many coatings exhibiting degradation or delamination when exposed to continuous perfusion or physiological fluids. This temporal instability severely limits the long-term utility of microfluidic devices for extended cell culture applications, particularly for barrier tissue models that require weeks of maturation to achieve physiologically relevant functionality.
Scalability and manufacturing integration pose considerable challenges for transitioning from laboratory prototypes to commercial production. Many effective surface modification techniques rely on specialized equipment or time-consuming protocols that are difficult to incorporate into high-throughput manufacturing processes, creating a significant barrier to widespread adoption and commercialization.
The biological complexity of cell-surface interactions further complicates microchannel engineering efforts. Different cell types exhibit varying adhesion preferences and barrier formation characteristics, necessitating customized surface chemistries that can selectively promote or inhibit attachment of specific cell populations. Current approaches often lack this level of specificity, resulting in suboptimal cell patterning and barrier formation.
Characterization and quality control of modified surfaces within enclosed microchannels present methodological challenges, as many analytical techniques are not compatible with the confined geometry of microfluidic devices. This limitation hinders the systematic optimization of surface properties and makes it difficult to establish standardized protocols for reproducible device fabrication.
Regulatory considerations add another layer of complexity, particularly for biomedical applications, as novel surface modification materials must demonstrate biocompatibility and safety for potential clinical translation. Many promising surface engineering approaches utilize components that lack established safety profiles, creating uncertainty in their pathway to approval for human applications.
Material compatibility presents another significant hurdle, as surface modification techniques must maintain the optical transparency and mechanical properties of the base substrate while introducing the desired biochemical functionalities. Many current approaches involve harsh chemical treatments or plasma processing that can degrade the bulk properties of polymeric materials commonly used in microfluidic devices, such as PDMS, PMMA, or COC.
The stability of surface modifications under flow conditions remains problematic, with many coatings exhibiting degradation or delamination when exposed to continuous perfusion or physiological fluids. This temporal instability severely limits the long-term utility of microfluidic devices for extended cell culture applications, particularly for barrier tissue models that require weeks of maturation to achieve physiologically relevant functionality.
Scalability and manufacturing integration pose considerable challenges for transitioning from laboratory prototypes to commercial production. Many effective surface modification techniques rely on specialized equipment or time-consuming protocols that are difficult to incorporate into high-throughput manufacturing processes, creating a significant barrier to widespread adoption and commercialization.
The biological complexity of cell-surface interactions further complicates microchannel engineering efforts. Different cell types exhibit varying adhesion preferences and barrier formation characteristics, necessitating customized surface chemistries that can selectively promote or inhibit attachment of specific cell populations. Current approaches often lack this level of specificity, resulting in suboptimal cell patterning and barrier formation.
Characterization and quality control of modified surfaces within enclosed microchannels present methodological challenges, as many analytical techniques are not compatible with the confined geometry of microfluidic devices. This limitation hinders the systematic optimization of surface properties and makes it difficult to establish standardized protocols for reproducible device fabrication.
Regulatory considerations add another layer of complexity, particularly for biomedical applications, as novel surface modification materials must demonstrate biocompatibility and safety for potential clinical translation. Many promising surface engineering approaches utilize components that lack established safety profiles, creating uncertainty in their pathway to approval for human applications.
Current Approaches to Control Cell Attachment in Microchannels
01 Microchannel surface modification for enhanced cell adhesion
Surface engineering techniques can be applied to microchannels to enhance cell attachment and proliferation. These modifications include chemical treatments, plasma processing, or coating with biocompatible materials that promote cellular adhesion. The engineered surfaces provide optimal conditions for cells to attach, spread, and form functional barriers more efficiently than unmodified surfaces. These techniques are particularly important in tissue engineering and organ-on-chip applications where stable cell attachment is crucial.- Microchannel surface modifications for enhanced cell adhesion: Surface engineering techniques can be applied to microchannels to promote cell attachment through chemical or physical modifications. These include plasma treatment, coating with extracellular matrix proteins, or creating specific surface topographies that enhance cellular adhesion. Such modifications can significantly improve the initial attachment of cells to microchannel surfaces, which is crucial for establishing stable cell barriers in microfluidic devices used for biological studies.
- Microfluidic devices for studying barrier formation kinetics: Specialized microfluidic platforms can be designed to monitor and analyze the kinetics of cellular barrier formation in real-time. These devices typically incorporate transparent materials for microscopic observation, integrated sensors for measuring transepithelial electrical resistance, and controlled flow conditions. By precisely controlling the microenvironment, researchers can study how various factors affect the rate and quality of barrier formation, which is essential for modeling physiological barriers such as the blood-brain barrier or intestinal epithelium.
- 3D microchannel architectures for tissue barrier models: Three-dimensional microchannel structures can better mimic the in vivo environment for cell growth and barrier formation. These architectures often incorporate multiple layers, varying channel geometries, and biomimetic features that promote physiologically relevant cell organization. The 3D structure allows for more complex cell-cell interactions, improved barrier integrity, and more accurate modeling of tissue interfaces, making them valuable tools for drug permeability studies and disease modeling.
- Flow dynamics influence on cell attachment and barrier formation: The fluid flow characteristics within microchannels significantly impact cell attachment patterns and subsequent barrier formation. Controlled shear stress can enhance cellular alignment, promote tight junction formation, and improve barrier function. Microfluidic systems can be engineered with specific flow profiles to optimize these parameters, allowing for the development of more physiologically relevant barrier models that reflect the dynamic conditions cells experience in vivo.
- Real-time monitoring systems for barrier integrity assessment: Advanced monitoring systems can be integrated into microchannel platforms to assess barrier formation kinetics in real-time. These include impedance-based measurements, fluorescent tracer diffusion assays, and optical imaging techniques that provide quantitative data on barrier development and integrity. Such systems enable researchers to track the temporal progression of barrier formation, identify critical phases in the process, and evaluate the impact of various compounds or conditions on barrier function without disrupting the cellular environment.
02 Microfluidic systems for studying barrier formation kinetics
Specialized microfluidic devices with engineered microchannels allow for real-time monitoring of cell barrier formation kinetics. These systems enable precise control of flow conditions, nutrient delivery, and environmental parameters while simultaneously allowing for imaging and measurement of barrier integrity development over time. The microfluidic approach provides advantages over traditional static culture methods by mimicking physiological conditions and enabling dynamic assessment of barrier function establishment.Expand Specific Solutions03 Topographical features influencing cell attachment patterns
The physical topography of microchannel surfaces significantly impacts cell attachment patterns and subsequent barrier formation. Features such as grooves, ridges, pillars, or controlled roughness can guide cell orientation, migration, and intercellular junction formation. By engineering specific micro and nano-scale topographical patterns, cellular alignment and barrier integrity can be enhanced, leading to more physiologically relevant tissue models with improved barrier properties.Expand Specific Solutions04 Biomolecular coatings for accelerated barrier formation
Application of specific biomolecular coatings to microchannel surfaces can accelerate the kinetics of barrier formation. These coatings include extracellular matrix proteins, growth factors, peptides, and other bioactive molecules that promote cell adhesion, proliferation, and differentiation. The strategic selection and patterning of these biomolecules can significantly reduce the time required for cells to establish functional barriers while improving barrier quality and stability.Expand Specific Solutions05 Dynamic monitoring systems for barrier formation assessment
Advanced monitoring systems integrated with microchannels enable real-time assessment of barrier formation kinetics. These systems utilize techniques such as transepithelial/transendothelial electrical resistance (TEER) measurements, fluorescent marker permeability assays, and impedance sensing to quantify barrier integrity development. The continuous monitoring capabilities allow for precise characterization of the temporal dynamics of barrier formation and the effects of various stimuli on barrier function.Expand Specific Solutions
Leading Organizations in Microfluidic Surface Engineering
Surface engineering of microchannels for cell attachment and barrier formation is currently in a growth phase, with increasing market adoption across biomedical and tissue engineering sectors. The global market is expanding rapidly, estimated to reach significant value as organ-on-chip and microfluidic technologies gain traction. Leading academic institutions like MIT, UC system, and Shanghai Jiao Tong University are advancing fundamental research, while companies such as Fraunhofer-Gesellschaft, FUJIFILM, and DexCom are developing commercial applications. The technology shows varying maturity levels - basic surface modification techniques are well-established, but advanced functionalization for specific cell behaviors remains in development. Research collaborations between institutions like The Regents of the University of California and industry partners are accelerating progress toward clinical and commercial applications.
The Regents of the University of California
Technical Solution: The University of California has developed a comprehensive microchannel surface engineering platform called "BiomimetiX" that focuses on recreating native tissue microenvironments. Their approach utilizes a combination of surface chemistry modifications and controlled topographical features to regulate cell attachment kinetics. A key innovation is their development of "spatiotemporal release matrices" that can deliver adhesion molecules and growth factors with precise timing to orchestrate barrier formation[2]. UC researchers have pioneered the use of zwitterionic polymer coatings that can be selectively activated to transition from cell-repellent to cell-adhesive states, allowing unprecedented control over where and when cells attach within microchannels[4]. Their technology incorporates microfluidic gradient generators to establish defined concentration profiles of extracellular matrix components across channel surfaces. This has proven particularly effective for creating polarized epithelial and endothelial barriers with physiologically relevant structure and function[6]. Recent advancements include integration with oxygen-sensing capabilities to monitor metabolic activity during barrier formation.
Strengths: Exceptional biomimetic capabilities; highly versatile platform adaptable to multiple tissue types; excellent integration with sensing technologies for real-time monitoring of barrier function. Weaknesses: Complex fabrication process requires specialized expertise; higher production costs compared to simpler systems; some components have limited shelf-life requiring careful storage.
The Georgia Tech Research Corp.
Technical Solution: Georgia Tech Research Corporation has developed an innovative platform for microchannel surface engineering called "Programmable Microtexture Array" (PMA). This technology utilizes precisely controlled topographical features combined with chemical surface modifications to guide cell attachment and barrier formation. Their approach incorporates photopolymerizable hydrogels with tunable mechanical properties that can be patterned within microchannels to create regions with differential cell adhesion properties[1]. A key innovation is their development of "gradient-generating microstructures" that establish controlled concentration profiles of cell adhesion molecules across the microchannel surface, enabling directed cell migration and organized tissue formation[3]. Georgia Tech has also pioneered the integration of electrically conductive polymers into microchannel surfaces, allowing electrical stimulation to modulate cell behavior and barrier formation kinetics. Their technology has demonstrated particular success in creating blood-brain barrier and lung alveolar barrier models with physiologically relevant transport properties and barrier function[5].
Strengths: Excellent integration of both physical and chemical surface modification strategies; highly customizable platform adaptable to various tissue types; strong capabilities for creating complex co-culture systems. Weaknesses: More complex fabrication process compared to simpler systems; requires specialized equipment for production; higher initial setup costs though potentially more cost-effective for long-term applications.
Key Innovations in Barrier Formation Kinetics Control
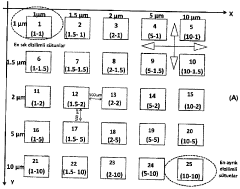
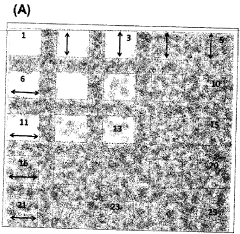
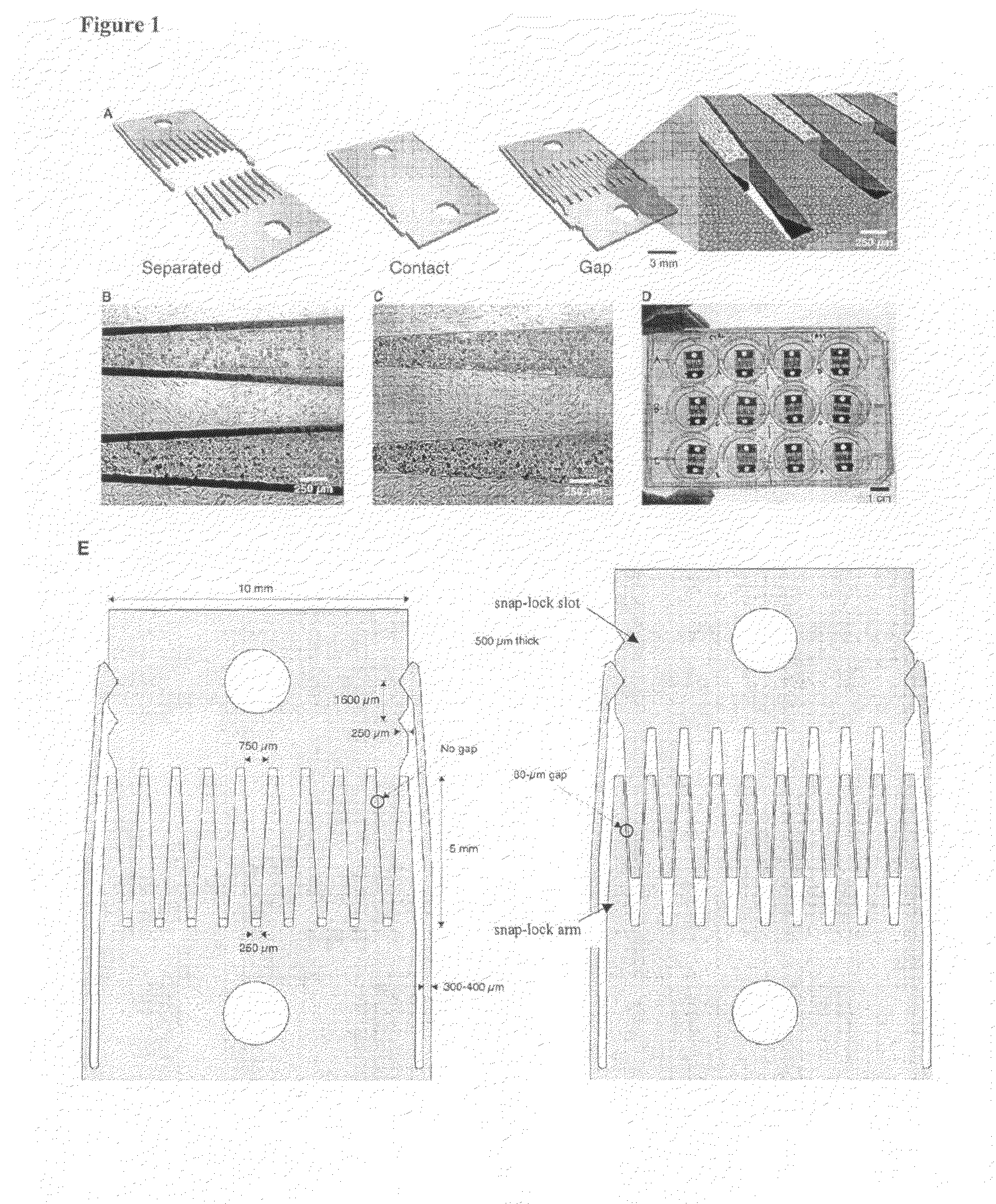
a grid containing areas covered with systematically distributed micro-nanocolumns to control cell attachment.
PatentInactiveTR201414485T2
Innovation
- A test platform with systematically varying micro- and nano-chute-based topographies, where columns are spaced to inhibit or promote cell adhesion, allowing for adjustable cell retention through surface coating and material hardness, facilitating cell-specific responses without chemical cues.
Micromechanical devices for control of cell-cell interaction, and methods of use thereof
PatentInactiveUS20100167330A1
Innovation
- The development of micromachined substrates with moving parts allows for dynamic regulation of cell-cell interactions through precise mechanical control of tissue composition and spatial organization, enabling reversible manipulation of cell positioning and separation with micron-scale precision, allowing for the investigation of intercellular communication and microenvironmental regulation.
Scalability and Manufacturing Considerations
The scalability of surface engineering techniques for microchannels represents a critical factor in transitioning from laboratory prototypes to commercial applications. Current manufacturing methods for surface-modified microchannels include photolithography, soft lithography, microcontact printing, and plasma treatment. Each method presents distinct advantages and limitations when considering large-scale production. Photolithography offers high precision but involves expensive equipment and complex processes, limiting its scalability for mass production. Conversely, soft lithography techniques provide better cost-effectiveness but may face challenges in maintaining consistent quality across large production volumes.
Material selection significantly impacts manufacturing scalability. While PDMS (polydimethylsiloxane) remains popular for prototyping due to its optical transparency and biocompatibility, its absorption properties and manufacturing limitations make it less suitable for large-scale production. Alternative materials such as thermoplastics (polystyrene, polycarbonate) offer better manufacturing compatibility through injection molding techniques, though they may require additional surface treatments to achieve desired cell attachment properties.
Quality control represents another crucial consideration in scaling surface-engineered microchannels. Maintaining consistent surface properties across thousands of devices requires robust quality assurance protocols. Advanced characterization techniques including atomic force microscopy, X-ray photoelectron spectroscopy, and high-throughput cell adhesion assays must be integrated into production workflows to ensure uniformity of surface properties that control cell attachment kinetics.
Cost-effectiveness remains paramount for commercial viability. Current estimates suggest that surface modification adds approximately 15-30% to manufacturing costs, depending on the complexity of the patterning and the specific biomolecules involved. Economies of scale can potentially reduce these costs, particularly through automation of surface functionalization processes and optimization of biomolecule usage.
Regulatory considerations also impact manufacturing strategies, especially for medical applications. Surface-engineered microchannels intended for clinical applications must comply with stringent quality standards, requiring validated manufacturing processes with minimal batch-to-batch variation. Documentation of manufacturing protocols, material sourcing, and quality control measures becomes essential for regulatory approval.
Future manufacturing innovations that may enhance scalability include roll-to-roll processing for continuous production of surface-modified films, automated microfluidic handling systems for precise biomolecule deposition, and 3D printing technologies capable of incorporating surface modification during the fabrication process. These emerging technologies could significantly reduce production costs while maintaining the precise control over cell attachment and barrier formation kinetics required for effective microchannel functionality.
Material selection significantly impacts manufacturing scalability. While PDMS (polydimethylsiloxane) remains popular for prototyping due to its optical transparency and biocompatibility, its absorption properties and manufacturing limitations make it less suitable for large-scale production. Alternative materials such as thermoplastics (polystyrene, polycarbonate) offer better manufacturing compatibility through injection molding techniques, though they may require additional surface treatments to achieve desired cell attachment properties.
Quality control represents another crucial consideration in scaling surface-engineered microchannels. Maintaining consistent surface properties across thousands of devices requires robust quality assurance protocols. Advanced characterization techniques including atomic force microscopy, X-ray photoelectron spectroscopy, and high-throughput cell adhesion assays must be integrated into production workflows to ensure uniformity of surface properties that control cell attachment kinetics.
Cost-effectiveness remains paramount for commercial viability. Current estimates suggest that surface modification adds approximately 15-30% to manufacturing costs, depending on the complexity of the patterning and the specific biomolecules involved. Economies of scale can potentially reduce these costs, particularly through automation of surface functionalization processes and optimization of biomolecule usage.
Regulatory considerations also impact manufacturing strategies, especially for medical applications. Surface-engineered microchannels intended for clinical applications must comply with stringent quality standards, requiring validated manufacturing processes with minimal batch-to-batch variation. Documentation of manufacturing protocols, material sourcing, and quality control measures becomes essential for regulatory approval.
Future manufacturing innovations that may enhance scalability include roll-to-roll processing for continuous production of surface-modified films, automated microfluidic handling systems for precise biomolecule deposition, and 3D printing technologies capable of incorporating surface modification during the fabrication process. These emerging technologies could significantly reduce production costs while maintaining the precise control over cell attachment and barrier formation kinetics required for effective microchannel functionality.
Biocompatibility and Regulatory Compliance
Biocompatibility is a critical consideration in the surface engineering of microchannels for cell attachment and barrier formation applications. The materials used in microchannel fabrication must not elicit adverse biological responses when in contact with cells and tissues. Common biocompatible materials include polydimethylsiloxane (PDMS), poly(methyl methacrylate) (PMMA), and various hydrogels that have demonstrated minimal cytotoxicity in laboratory and clinical settings.
Surface modifications to enhance biocompatibility often involve coating microchannels with extracellular matrix proteins such as collagen, fibronectin, or laminin. These proteins promote cell adhesion and proliferation while maintaining cellular function. Additionally, anti-fouling coatings like polyethylene glycol (PEG) can be strategically applied to prevent non-specific protein adsorption and cell attachment in regions where barrier integrity is essential.
Regulatory compliance presents significant challenges for microchannel-based devices intended for clinical applications. In the United States, the Food and Drug Administration (FDA) classifies these devices based on their intended use and associated risks. Most cell-based microfluidic devices fall under Class II or III, requiring premarket notification (510(k)) or premarket approval (PMA) pathways. The European Union's Medical Device Regulation (MDR) similarly imposes stringent requirements for safety and performance.
ISO 10993 standards provide a framework for evaluating biocompatibility, requiring manufacturers to assess cytotoxicity, sensitization, irritation, and systemic toxicity. For microchannels designed to control cell attachment, particular attention must be paid to ISO 10993-5 (cytotoxicity) and ISO 10993-23 (irritation). Documentation of material selection rationale, manufacturing processes, and quality control measures is essential for regulatory submissions.
Long-term stability of surface modifications presents both technical and regulatory challenges. Manufacturers must demonstrate that engineered surfaces maintain their properties throughout the device's intended shelf life and usage period. Accelerated aging studies and real-time stability testing are typically required to satisfy regulatory expectations regarding the durability of surface treatments that control cell attachment kinetics.
Risk management according to ISO 14971 must address potential hazards specific to surface-engineered microchannels, including leaching of surface modification agents, unexpected cellular responses, and barrier integrity failures. Manufacturers must implement appropriate mitigation strategies and demonstrate their effectiveness through validation studies that simulate intended use conditions.
Surface modifications to enhance biocompatibility often involve coating microchannels with extracellular matrix proteins such as collagen, fibronectin, or laminin. These proteins promote cell adhesion and proliferation while maintaining cellular function. Additionally, anti-fouling coatings like polyethylene glycol (PEG) can be strategically applied to prevent non-specific protein adsorption and cell attachment in regions where barrier integrity is essential.
Regulatory compliance presents significant challenges for microchannel-based devices intended for clinical applications. In the United States, the Food and Drug Administration (FDA) classifies these devices based on their intended use and associated risks. Most cell-based microfluidic devices fall under Class II or III, requiring premarket notification (510(k)) or premarket approval (PMA) pathways. The European Union's Medical Device Regulation (MDR) similarly imposes stringent requirements for safety and performance.
ISO 10993 standards provide a framework for evaluating biocompatibility, requiring manufacturers to assess cytotoxicity, sensitization, irritation, and systemic toxicity. For microchannels designed to control cell attachment, particular attention must be paid to ISO 10993-5 (cytotoxicity) and ISO 10993-23 (irritation). Documentation of material selection rationale, manufacturing processes, and quality control measures is essential for regulatory submissions.
Long-term stability of surface modifications presents both technical and regulatory challenges. Manufacturers must demonstrate that engineered surfaces maintain their properties throughout the device's intended shelf life and usage period. Accelerated aging studies and real-time stability testing are typically required to satisfy regulatory expectations regarding the durability of surface treatments that control cell attachment kinetics.
Risk management according to ISO 14971 must address potential hazards specific to surface-engineered microchannels, including leaching of surface modification agents, unexpected cellular responses, and barrier integrity failures. Manufacturers must implement appropriate mitigation strategies and demonstrate their effectiveness through validation studies that simulate intended use conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!