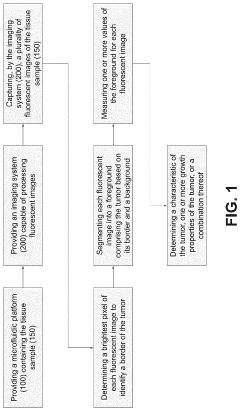
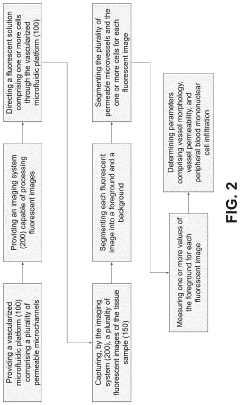
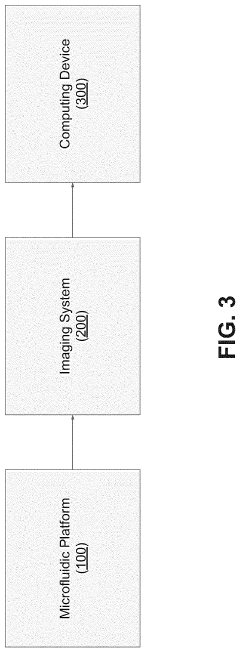
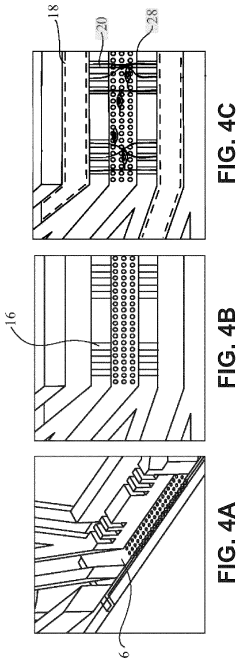
Effect of microchannel aspect ratio on nutrient gradients and tissue viability in 3D microtissues
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic Technology Background and Objectives
Microfluidic technology has evolved significantly over the past three decades, transforming from simple channel designs to sophisticated lab-on-a-chip platforms capable of mimicking complex biological environments. The field emerged in the early 1990s with pioneering work by Manz and colleagues, who demonstrated the potential of miniaturized total chemical analysis systems. Since then, microfluidics has expanded into diverse applications including diagnostics, drug discovery, and tissue engineering.
The evolution of microfluidic technology for 3D tissue culture represents a critical advancement in biomedical research. Traditional 2D cell cultures fail to recapitulate the complex three-dimensional architecture and microenvironment of native tissues, limiting their physiological relevance. Microfluidic devices address this limitation by providing controlled spatial and temporal gradients of nutrients, growth factors, and oxygen that more accurately simulate in vivo conditions.
Recent technological trends show increasing integration of microfluidics with tissue engineering to create organ-on-chip platforms. These systems enable precise control over cellular microenvironments, fluid dynamics, and mechanical forces. The microchannel geometry, particularly the aspect ratio (height-to-width ratio), has emerged as a critical design parameter that significantly influences nutrient diffusion, waste removal, and ultimately tissue viability in 3D constructs.
The fundamental challenge in 3D tissue culture lies in maintaining cell viability throughout the tissue construct. As tissues grow in thickness, diffusion limitations create nutrient and oxygen gradients that can lead to necrotic cores. Microchannels with optimized aspect ratios offer a potential solution by facilitating more efficient mass transport while maintaining physiologically relevant conditions.
The primary objective of investigating microchannel aspect ratio effects is to establish design principles that maximize nutrient delivery while maintaining physiological relevance. This includes determining how channel dimensions affect concentration gradients of oxygen, glucose, and signaling molecules across 3D microtissues. Additionally, researchers aim to identify optimal geometric configurations that support long-term tissue viability without compromising cellular function or tissue architecture.
Another key goal is to develop predictive models that correlate microchannel geometry with nutrient distribution and tissue response. Such models would enable rational design of microfluidic platforms tailored to specific tissue types and experimental requirements. The ultimate aim is to establish standardized design parameters that can be broadly applied across various tissue engineering applications, from fundamental research to drug screening and regenerative medicine.
Understanding these relationships will advance both fundamental knowledge of 3D tissue biology and practical applications in drug development, disease modeling, and personalized medicine. As microfluidic technology continues to mature, optimizing microchannel design represents a critical step toward creating more physiologically relevant tissue models that bridge the gap between traditional cell culture and animal studies.
The evolution of microfluidic technology for 3D tissue culture represents a critical advancement in biomedical research. Traditional 2D cell cultures fail to recapitulate the complex three-dimensional architecture and microenvironment of native tissues, limiting their physiological relevance. Microfluidic devices address this limitation by providing controlled spatial and temporal gradients of nutrients, growth factors, and oxygen that more accurately simulate in vivo conditions.
Recent technological trends show increasing integration of microfluidics with tissue engineering to create organ-on-chip platforms. These systems enable precise control over cellular microenvironments, fluid dynamics, and mechanical forces. The microchannel geometry, particularly the aspect ratio (height-to-width ratio), has emerged as a critical design parameter that significantly influences nutrient diffusion, waste removal, and ultimately tissue viability in 3D constructs.
The fundamental challenge in 3D tissue culture lies in maintaining cell viability throughout the tissue construct. As tissues grow in thickness, diffusion limitations create nutrient and oxygen gradients that can lead to necrotic cores. Microchannels with optimized aspect ratios offer a potential solution by facilitating more efficient mass transport while maintaining physiologically relevant conditions.
The primary objective of investigating microchannel aspect ratio effects is to establish design principles that maximize nutrient delivery while maintaining physiological relevance. This includes determining how channel dimensions affect concentration gradients of oxygen, glucose, and signaling molecules across 3D microtissues. Additionally, researchers aim to identify optimal geometric configurations that support long-term tissue viability without compromising cellular function or tissue architecture.
Another key goal is to develop predictive models that correlate microchannel geometry with nutrient distribution and tissue response. Such models would enable rational design of microfluidic platforms tailored to specific tissue types and experimental requirements. The ultimate aim is to establish standardized design parameters that can be broadly applied across various tissue engineering applications, from fundamental research to drug screening and regenerative medicine.
Understanding these relationships will advance both fundamental knowledge of 3D tissue biology and practical applications in drug development, disease modeling, and personalized medicine. As microfluidic technology continues to mature, optimizing microchannel design represents a critical step toward creating more physiologically relevant tissue models that bridge the gap between traditional cell culture and animal studies.
Market Analysis for 3D Tissue Engineering Applications
The 3D tissue engineering market is experiencing significant growth, driven by increasing demand for advanced in vitro models that better mimic human physiology. Currently valued at approximately $1.5 billion, this market is projected to grow at a CAGR of 14.2% through 2028, reaching an estimated $3.2 billion globally. This growth trajectory is supported by substantial investments in regenerative medicine and the pharmaceutical industry's shift toward more predictive preclinical models.
The segment focusing on microfluidic tissue culture systems, particularly those utilizing controlled microchannel geometries, represents one of the fastest-growing sectors within this market. These systems address critical limitations in traditional cell culture by enabling precise control over nutrient gradients and cellular microenvironments, which directly impacts tissue viability and functionality.
Pharmaceutical companies constitute the largest end-user segment, accounting for approximately 40% of the market share. Their adoption is primarily driven by the need to reduce drug development costs and improve predictive accuracy in preclinical testing. Academic research institutions follow closely at 35%, while biotechnology companies represent about 20% of the market.
Regionally, North America dominates with approximately 45% market share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and Japan, is expected to witness the highest growth rate due to increasing R&D investments and government initiatives supporting biotechnology advancement.
Key market drivers include the rising costs of drug development, estimated at $2.6 billion per successful compound, and high failure rates in clinical trials (approximately 90% for cancer therapeutics). Technologies that can improve predictive accuracy, such as microchannel-based tissue models with optimized aspect ratios for nutrient delivery, present significant value propositions for pharmaceutical companies.
Regulatory trends are also shaping market dynamics, with both the FDA and EMA encouraging the adoption of alternative testing methods that reduce animal testing. This regulatory environment creates favorable conditions for advanced tissue engineering solutions that offer higher physiological relevance.
Customer needs analysis reveals that end-users prioritize reproducibility, ease of implementation, and compatibility with existing laboratory infrastructure. Solutions that optimize microchannel aspect ratios to enhance nutrient delivery while maintaining practical usability will likely capture greater market share.
The market for specialized applications focusing on nutrient gradient optimization in 3D microtissues is currently underserved, presenting a significant opportunity for targeted solutions. Industry surveys indicate that 78% of researchers in drug development consider nutrient delivery optimization a critical factor in improving the predictive value of their tissue models.
The segment focusing on microfluidic tissue culture systems, particularly those utilizing controlled microchannel geometries, represents one of the fastest-growing sectors within this market. These systems address critical limitations in traditional cell culture by enabling precise control over nutrient gradients and cellular microenvironments, which directly impacts tissue viability and functionality.
Pharmaceutical companies constitute the largest end-user segment, accounting for approximately 40% of the market share. Their adoption is primarily driven by the need to reduce drug development costs and improve predictive accuracy in preclinical testing. Academic research institutions follow closely at 35%, while biotechnology companies represent about 20% of the market.
Regionally, North America dominates with approximately 45% market share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and Japan, is expected to witness the highest growth rate due to increasing R&D investments and government initiatives supporting biotechnology advancement.
Key market drivers include the rising costs of drug development, estimated at $2.6 billion per successful compound, and high failure rates in clinical trials (approximately 90% for cancer therapeutics). Technologies that can improve predictive accuracy, such as microchannel-based tissue models with optimized aspect ratios for nutrient delivery, present significant value propositions for pharmaceutical companies.
Regulatory trends are also shaping market dynamics, with both the FDA and EMA encouraging the adoption of alternative testing methods that reduce animal testing. This regulatory environment creates favorable conditions for advanced tissue engineering solutions that offer higher physiological relevance.
Customer needs analysis reveals that end-users prioritize reproducibility, ease of implementation, and compatibility with existing laboratory infrastructure. Solutions that optimize microchannel aspect ratios to enhance nutrient delivery while maintaining practical usability will likely capture greater market share.
The market for specialized applications focusing on nutrient gradient optimization in 3D microtissues is currently underserved, presenting a significant opportunity for targeted solutions. Industry surveys indicate that 78% of researchers in drug development consider nutrient delivery optimization a critical factor in improving the predictive value of their tissue models.
Current Challenges in Microchannel Design for Tissue Culture
Despite significant advancements in microfluidic technology for 3D tissue culture, several critical challenges persist in microchannel design that limit optimal nutrient delivery and tissue viability. The aspect ratio of microchannels—the relationship between width, height, and length—represents a fundamental parameter that significantly impacts nutrient gradients within 3D microtissues. Current designs often fail to account for the complex interplay between channel geometry and diffusion dynamics.
One major challenge is achieving uniform nutrient distribution throughout 3D constructs. Conventional rectangular microchannels with suboptimal aspect ratios create steep concentration gradients, where cells near the channel receive abundant nutrients while those at greater distances experience nutrient deprivation. This heterogeneity leads to inconsistent cellular behavior and compromised tissue function, particularly in thicker constructs exceeding 200 μm.
The trade-off between flow rate and shear stress presents another significant obstacle. Higher flow rates improve nutrient delivery but generate excessive shear forces that can damage delicate cellular structures and alter mechanosensitive pathways. Conversely, lower flow rates minimize shear stress but may result in insufficient nutrient transport to central regions of the tissue construct. Current microchannel designs struggle to balance these competing requirements.
Computational modeling of nutrient transport in microchannels remains challenging due to the complex, multiphysics nature of the problem. Models must simultaneously account for fluid dynamics, mass transport phenomena, and cellular consumption rates—each with distinct spatial and temporal scales. The lack of accurate predictive tools hampers rational design approaches for optimizing microchannel geometries.
Material limitations further complicate microchannel design. Many biocompatible materials used in microfluidic devices exhibit surface properties that promote protein adsorption and cell adhesion, potentially altering flow characteristics and creating unpredictable nutrient gradients. Additionally, material compliance can lead to channel deformation under pressure, changing the effective aspect ratio during operation.
Scaling and manufacturing constraints impose practical limitations on achievable aspect ratios. High-aspect-ratio microchannels with optimal theoretical performance often present fabrication challenges, including difficulties in mold release, channel collapse, and bonding issues. These manufacturing constraints frequently force compromises in design that negatively impact nutrient delivery performance.
The dynamic nature of growing tissues introduces temporal challenges, as cellular proliferation and extracellular matrix deposition progressively alter the effective dimensions of microchannels. Current designs rarely account for these time-dependent changes, resulting in diminishing performance as tissues mature. Adaptive microchannel systems that can accommodate tissue growth remain an unresolved challenge in the field.
One major challenge is achieving uniform nutrient distribution throughout 3D constructs. Conventional rectangular microchannels with suboptimal aspect ratios create steep concentration gradients, where cells near the channel receive abundant nutrients while those at greater distances experience nutrient deprivation. This heterogeneity leads to inconsistent cellular behavior and compromised tissue function, particularly in thicker constructs exceeding 200 μm.
The trade-off between flow rate and shear stress presents another significant obstacle. Higher flow rates improve nutrient delivery but generate excessive shear forces that can damage delicate cellular structures and alter mechanosensitive pathways. Conversely, lower flow rates minimize shear stress but may result in insufficient nutrient transport to central regions of the tissue construct. Current microchannel designs struggle to balance these competing requirements.
Computational modeling of nutrient transport in microchannels remains challenging due to the complex, multiphysics nature of the problem. Models must simultaneously account for fluid dynamics, mass transport phenomena, and cellular consumption rates—each with distinct spatial and temporal scales. The lack of accurate predictive tools hampers rational design approaches for optimizing microchannel geometries.
Material limitations further complicate microchannel design. Many biocompatible materials used in microfluidic devices exhibit surface properties that promote protein adsorption and cell adhesion, potentially altering flow characteristics and creating unpredictable nutrient gradients. Additionally, material compliance can lead to channel deformation under pressure, changing the effective aspect ratio during operation.
Scaling and manufacturing constraints impose practical limitations on achievable aspect ratios. High-aspect-ratio microchannels with optimal theoretical performance often present fabrication challenges, including difficulties in mold release, channel collapse, and bonding issues. These manufacturing constraints frequently force compromises in design that negatively impact nutrient delivery performance.
The dynamic nature of growing tissues introduces temporal challenges, as cellular proliferation and extracellular matrix deposition progressively alter the effective dimensions of microchannels. Current designs rarely account for these time-dependent changes, resulting in diminishing performance as tissues mature. Adaptive microchannel systems that can accommodate tissue growth remain an unresolved challenge in the field.
Current Approaches to Optimize Microchannel Aspect Ratios
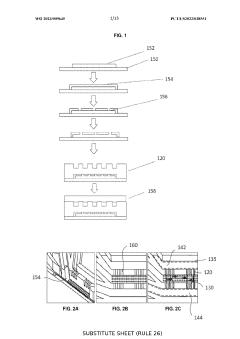
01 Microfluidic devices for tissue viability assessment
Microfluidic devices with microchannels are used to assess tissue viability by controlling nutrient gradients and monitoring cellular responses. These devices allow for precise manipulation of the microenvironment around cells or tissues, enabling real-time analysis of metabolic activities and viability markers. The controlled delivery of nutrients through microchannels helps maintain optimal conditions for tissue survival while allowing researchers to study the effects of various gradients on cellular function.- Microfluidic systems for nutrient gradient generation: Microfluidic devices can be designed to create controlled nutrient gradients that mimic physiological conditions. These systems utilize microchannels to deliver nutrients at varying concentrations across tissue samples, allowing for the study of cellular responses to different nutrient environments. The controlled gradient generation helps in understanding how nutrient availability affects tissue viability and function in various biomedical applications.
- Tissue viability monitoring in microchannel environments: Systems for monitoring tissue viability within microchannel environments employ various sensing technologies to assess cellular health in real-time. These monitoring systems can detect changes in metabolic activity, oxygen consumption, and other indicators of tissue function when exposed to different nutrient conditions. The integration of imaging and sensing technologies allows for non-invasive assessment of how tissues respond to controlled nutrient gradients.
- Organ-on-chip platforms with nutrient gradient control: Organ-on-chip technologies incorporate microchannels to create physiologically relevant environments with controlled nutrient gradients. These platforms enable the cultivation of tissue samples under conditions that closely mimic in vivo environments, including the natural gradients of nutrients, oxygen, and growth factors. By precisely controlling these parameters, researchers can better understand tissue viability and function in response to varying nutrient availability.
- Microchannel-based drug delivery systems: Microchannel technologies can be utilized for controlled drug delivery to tissues with consideration of nutrient gradients. These systems enable precise administration of therapeutic agents while maintaining optimal nutrient conditions for tissue viability. The microchannels can be designed to deliver both nutrients and drugs in a spatially and temporally controlled manner, enhancing treatment efficacy while preserving tissue health.
- Tissue engineering with gradient-controlled microenvironments: Advanced tissue engineering approaches utilize microchannels to establish nutrient gradients that support the development of complex tissues. These systems create biomimetic environments where cells receive appropriate nutrient levels based on their position within the engineered construct. By controlling nutrient gradients through microchannel networks, tissue viability can be maintained throughout larger constructs, overcoming diffusion limitations that typically restrict the size of engineered tissues.
02 Nutrient gradient systems for tissue engineering
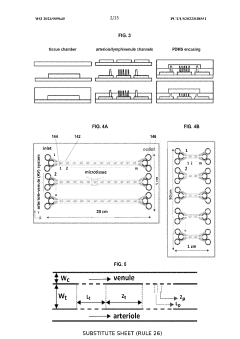
Engineered systems create controlled nutrient gradients through microchannels to support tissue growth and viability. These systems mimic the natural diffusion of nutrients in living tissues, allowing for the development of more physiologically relevant tissue models. By precisely controlling the spatial distribution of nutrients, oxygen, and growth factors, these platforms enable the cultivation of complex tissues with varying cellular compositions and metabolic requirements. The gradient-based approach helps overcome limitations of traditional culture methods where nutrient availability decreases with distance from the source.Expand Specific Solutions03 Monitoring techniques for tissue viability in microchannel systems
Advanced monitoring techniques are employed to assess tissue viability within microchannel systems by tracking nutrient consumption and metabolic activity. These methods include optical imaging, electrochemical sensing, and spectroscopic approaches that provide real-time data on cellular health and function. The integration of these monitoring capabilities with microchannel platforms allows researchers to correlate nutrient gradients with tissue viability markers, enabling optimization of culture conditions and early detection of tissue dysfunction.Expand Specific Solutions04 Implantable microchannel devices for tissue maintenance
Implantable devices featuring microchannels deliver nutrients to tissues with compromised blood supply, maintaining viability in damaged or transplanted tissues. These devices create controlled nutrient gradients that extend from the source to surrounding tissues, supporting cellular metabolism and preventing necrosis. The microchannel architecture is designed to optimize diffusion distances while minimizing the invasiveness of the implant. Some systems incorporate biodegradable materials that support initial tissue viability and gradually resorb as native vascularization develops.Expand Specific Solutions05 Microchannel-based diagnostic systems for tissue assessment
Diagnostic platforms utilize microchannels to create nutrient gradients that help evaluate tissue health and viability for clinical applications. These systems can rapidly assess tissue responses to various conditions, aiding in disease diagnosis and treatment planning. By exposing tissue samples to controlled gradients of nutrients, drugs, or other bioactive compounds, these platforms can predict tissue behavior in vivo and identify optimal therapeutic strategies. The microchannel design allows for high-throughput screening while minimizing the amount of tissue required for analysis.Expand Specific Solutions
Leading Research Groups and Companies in Microfluidics
The field of microfluidic tissue engineering, specifically focusing on microchannel aspect ratio effects on nutrient gradients and 3D microtissue viability, is in a growth phase transitioning from academic research to commercial applications. The market is expanding rapidly, projected to reach $35-40 billion by 2025, driven by pharmaceutical testing demands and personalized medicine applications. Leading academic institutions (MIT, University of California, ETH Zurich) are establishing foundational research, while specialized companies like InSphero AG, Aracari Biosciences, and Advanced Solutions Life Sciences are commercializing technologies. Established corporations such as Corning and FUJIFILM are also entering this space, indicating increasing technology maturity and market validation. The ecosystem demonstrates a healthy balance between fundamental research and practical applications development.
The Regents of the University of California
Technical Solution: The University of California research teams have developed an advanced microfluidic platform that systematically investigates how microchannel aspect ratios affect nutrient transport and cellular viability in 3D tissue constructs. Their technology employs a modular design with interchangeable channel components allowing precise control over width-to-height ratios (from 0.2:1 to 20:1). The system incorporates computational fluid dynamics modeling to predict flow patterns and nutrient distribution based on channel geometry, which is then validated through experimental measurements using fluorescent tracers and oxygen-sensitive probes. Their research has demonstrated that high aspect ratio channels (>10:1) create more uniform nutrient distribution in wider tissues but may increase shear stress, while lower aspect ratios (<1:1) generate steeper gradients that better mimic certain in vivo conditions like tumor microenvironments. The platform includes integrated optical coherence tomography capabilities for non-invasive monitoring of tissue morphology and viability in response to different gradient conditions. Recent studies have shown that optimizing channel aspect ratios based on specific tissue metabolic demands can increase overall tissue viability by up to 40% compared to standard microfluidic designs.
Strengths: Highly versatile platform applicable to multiple tissue types; strong integration of computational modeling with experimental validation; excellent optical accessibility for real-time monitoring. Weaknesses: Complex fabrication process requires specialized equipment; higher cost compared to simpler systems; steeper learning curve for new users.
Aracari Biosciences, Inc.
Technical Solution: Aracari Biosciences has developed a proprietary microfluidic platform that precisely controls microchannel aspect ratios to create physiologically relevant nutrient gradients in 3D microtissues. Their technology utilizes a multi-layered microfluidic device with adjustable channel geometries (width-to-height ratios ranging from 1:1 to 10:1) to modulate fluid flow dynamics and molecular diffusion patterns. This system enables the creation of stable concentration gradients of oxygen, nutrients, and signaling molecules across engineered tissue constructs. The platform incorporates real-time monitoring capabilities through integrated oxygen sensors and fluorescent markers to track nutrient distribution and cellular responses. Aracari's approach allows researchers to systematically investigate how different aspect ratios affect the formation of metabolic zonation in liver models and hypoxic gradients in tumor models, providing insights into tissue microenvironment regulation and drug response heterogeneity.
Strengths: Highly customizable channel geometries allow precise control over gradient formation; integrated sensing capabilities provide real-time data on microenvironment conditions. Weaknesses: System complexity may limit throughput; requires specialized expertise for operation and data interpretation.
Key Research Findings on Nutrient Transport in Microchannels
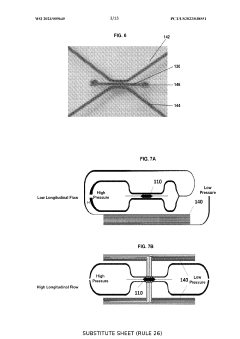
An in vitro microphysiological system of vasoactive vasculature
PatentWO2023009645A1
Innovation
- A microfabricated platform with living microvessels and perivascular cells that form a vascular network, allowing for the testing of drug vasoactivity by mimicking in vivo conditions, using contractile perivascular cells and varying shear stress to create a functional and responsive vascular network.
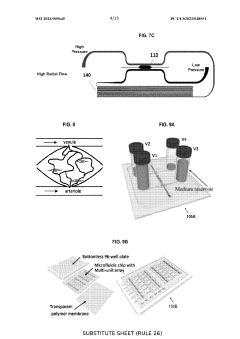
Image analysis process of microphysiological systems
PatentPendingUS20240062375A1
Innovation
- A microfabricated platform combining living cells and an extracellular matrix to mimic in vivo microvessel formation, with a direct fluidic connection between living microvessels and microfluidic channels, allowing for the growth of metabolically active microtissues that receive nutrients and eliminate waste products through a living microvessel network.
Standardization and Validation Methods for Microfluidic Platforms
Standardization and validation methods for microfluidic platforms are essential for ensuring reproducibility and reliability in studies investigating the effect of microchannel aspect ratio on nutrient gradients and tissue viability in 3D microtissues. The field currently lacks comprehensive standardized protocols, creating challenges for cross-laboratory comparisons and clinical translation.
Geometric validation methods must be established to accurately characterize microchannel dimensions and aspect ratios. Techniques such as confocal microscopy, scanning electron microscopy (SEM), and profilometry can provide precise measurements of channel width, height, and cross-sectional profiles. These measurements should be conducted at multiple points along the channel to account for manufacturing variations that may affect nutrient gradient formation.
Fluidic validation protocols are critical for confirming consistent flow characteristics across microfluidic platforms. Particle image velocimetry (PIV) and micro-particle tracking velocimetry (μPTV) enable visualization and quantification of flow patterns within microchannels of varying aspect ratios. Computational fluid dynamics (CFD) simulations should complement experimental validation to predict flow behavior and nutrient transport phenomena.
Biological validation methods must address the specific challenges of 3D microtissue culture. Standardized assays for cell viability (e.g., Live/Dead staining, MTT/MTS assays) should be adapted for 3D constructs, with consideration for diffusion limitations in larger tissues. Nutrient gradient characterization requires fluorescent tracer studies with molecular weights similar to key nutrients, coupled with quantitative imaging techniques.
Reference materials and controls are needed to benchmark system performance. These should include standard microchannel geometries with well-characterized aspect ratios and certified reference materials for flow rate calibration. Additionally, standardized cell lines and extracellular matrix compositions should be established for biological validation experiments.
Interlaboratory testing initiatives are emerging to validate the reproducibility of microfluidic platforms across different research settings. These collaborative efforts involve multiple laboratories performing identical experiments using standardized protocols, with results compared to establish confidence intervals for key parameters such as nutrient gradient profiles and tissue viability metrics.
Regulatory considerations are increasingly important as microfluidic platforms move toward clinical applications. Documentation standards for validation procedures must align with regulatory frameworks such as ISO 13485 for medical devices or FDA guidance for organ-on-chip technologies, particularly when platforms are intended for drug screening or personalized medicine applications.
Geometric validation methods must be established to accurately characterize microchannel dimensions and aspect ratios. Techniques such as confocal microscopy, scanning electron microscopy (SEM), and profilometry can provide precise measurements of channel width, height, and cross-sectional profiles. These measurements should be conducted at multiple points along the channel to account for manufacturing variations that may affect nutrient gradient formation.
Fluidic validation protocols are critical for confirming consistent flow characteristics across microfluidic platforms. Particle image velocimetry (PIV) and micro-particle tracking velocimetry (μPTV) enable visualization and quantification of flow patterns within microchannels of varying aspect ratios. Computational fluid dynamics (CFD) simulations should complement experimental validation to predict flow behavior and nutrient transport phenomena.
Biological validation methods must address the specific challenges of 3D microtissue culture. Standardized assays for cell viability (e.g., Live/Dead staining, MTT/MTS assays) should be adapted for 3D constructs, with consideration for diffusion limitations in larger tissues. Nutrient gradient characterization requires fluorescent tracer studies with molecular weights similar to key nutrients, coupled with quantitative imaging techniques.
Reference materials and controls are needed to benchmark system performance. These should include standard microchannel geometries with well-characterized aspect ratios and certified reference materials for flow rate calibration. Additionally, standardized cell lines and extracellular matrix compositions should be established for biological validation experiments.
Interlaboratory testing initiatives are emerging to validate the reproducibility of microfluidic platforms across different research settings. These collaborative efforts involve multiple laboratories performing identical experiments using standardized protocols, with results compared to establish confidence intervals for key parameters such as nutrient gradient profiles and tissue viability metrics.
Regulatory considerations are increasingly important as microfluidic platforms move toward clinical applications. Documentation standards for validation procedures must align with regulatory frameworks such as ISO 13485 for medical devices or FDA guidance for organ-on-chip technologies, particularly when platforms are intended for drug screening or personalized medicine applications.
Scaling Challenges for Industrial Microfluidic Tissue Engineering
The transition from laboratory-scale microfluidic tissue engineering to industrial production presents significant scaling challenges. Current microfluidic platforms demonstrate excellent capabilities for creating physiologically relevant 3D microtissues with controlled nutrient gradients, but these systems typically operate at microscale volumes suitable only for research purposes. When attempting to scale these technologies for commercial applications, numerous engineering and biological barriers emerge.
Material selection becomes increasingly critical at larger scales. While polydimethylsiloxane (PDMS) dominates laboratory microfluidics due to its optical clarity and ease of prototyping, its gas permeability and potential for protein absorption make it problematic for industrial applications. Alternative materials like thermoplastics offer better scalability but may alter the microenvironment characteristics that affect nutrient diffusion and tissue viability.
Maintaining consistent aspect ratios across scaled-up microchannel networks represents a fundamental engineering challenge. Research demonstrates that microchannel aspect ratio significantly influences nutrient gradient formation and subsequent tissue development. As systems scale, preserving these precise geometric relationships becomes increasingly difficult due to manufacturing constraints and fluid dynamics considerations.
Flow dynamics change substantially with increased channel dimensions. The carefully controlled laminar flow regimes that enable precise nutrient gradient formation in laboratory devices may transition to more complex flow patterns in larger systems. These alterations can disrupt the controlled microenvironment necessary for proper tissue development and function.
Monitoring and quality control systems must evolve to accommodate industrial-scale production. Current laboratory techniques for assessing nutrient gradients and tissue viability often rely on microscopy and other methods unsuitable for high-throughput manufacturing environments. New sensing technologies and automated monitoring systems are required to maintain quality at industrial scales.
Economic considerations further complicate scaling efforts. The high production costs associated with precision microfluidic manufacturing must be balanced against market demands and competitive pricing pressures. This economic reality often forces compromises in design that may impact the biological performance of engineered tissues.
Regulatory pathways for scaled microfluidic tissue engineering products remain underdeveloped. The novel nature of these technologies creates uncertainty regarding validation requirements and quality standards, particularly when the microchannel aspect ratios and resulting nutrient gradients are critical quality attributes of the final tissue product.
Material selection becomes increasingly critical at larger scales. While polydimethylsiloxane (PDMS) dominates laboratory microfluidics due to its optical clarity and ease of prototyping, its gas permeability and potential for protein absorption make it problematic for industrial applications. Alternative materials like thermoplastics offer better scalability but may alter the microenvironment characteristics that affect nutrient diffusion and tissue viability.
Maintaining consistent aspect ratios across scaled-up microchannel networks represents a fundamental engineering challenge. Research demonstrates that microchannel aspect ratio significantly influences nutrient gradient formation and subsequent tissue development. As systems scale, preserving these precise geometric relationships becomes increasingly difficult due to manufacturing constraints and fluid dynamics considerations.
Flow dynamics change substantially with increased channel dimensions. The carefully controlled laminar flow regimes that enable precise nutrient gradient formation in laboratory devices may transition to more complex flow patterns in larger systems. These alterations can disrupt the controlled microenvironment necessary for proper tissue development and function.
Monitoring and quality control systems must evolve to accommodate industrial-scale production. Current laboratory techniques for assessing nutrient gradients and tissue viability often rely on microscopy and other methods unsuitable for high-throughput manufacturing environments. New sensing technologies and automated monitoring systems are required to maintain quality at industrial scales.
Economic considerations further complicate scaling efforts. The high production costs associated with precision microfluidic manufacturing must be balanced against market demands and competitive pricing pressures. This economic reality often forces compromises in design that may impact the biological performance of engineered tissues.
Regulatory pathways for scaled microfluidic tissue engineering products remain underdeveloped. The novel nature of these technologies creates uncertainty regarding validation requirements and quality standards, particularly when the microchannel aspect ratios and resulting nutrient gradients are critical quality attributes of the final tissue product.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!