Incorporating dynamic PK/PD modeling into multi-organ chips to predict human systemic exposure
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PK/PD Modeling Evolution and Research Objectives
Pharmacokinetic/Pharmacodynamic (PK/PD) modeling has evolved significantly over the past decades, transforming from simple compartmental models to sophisticated integrated systems that can predict drug behavior in complex biological environments. The journey began in the 1950s with rudimentary one-compartment models that provided basic insights into drug absorption, distribution, and elimination. By the 1970s, multi-compartment models emerged, offering more nuanced representations of drug disposition across different physiological spaces.
The 1980s and 1990s witnessed a paradigm shift with the development of physiologically-based pharmacokinetic (PBPK) models, which incorporated anatomical and physiological parameters to better predict drug concentrations in specific tissues. This era also saw the integration of pharmacodynamic components, allowing researchers to link drug concentrations with biological responses and therapeutic outcomes.
Recent advancements have been driven by computational capabilities and systems biology approaches, enabling the creation of mechanistic models that account for molecular-level interactions and complex biological pathways. The emergence of population PK/PD modeling has further enhanced our ability to account for inter-individual variability in drug responses, a critical factor in personalized medicine.
Despite these advances, traditional PK/PD models face limitations in accurately predicting human systemic exposure, particularly for complex biological products and novel therapeutic modalities. These models often rely on animal data that may not translate well to humans or on simplified in vitro systems that fail to capture the complexity of multi-organ interactions.
Multi-organ chips (MOCs) represent a revolutionary platform that can bridge this gap by providing a more physiologically relevant environment for drug testing. These microfluidic devices contain multiple interconnected chambers housing different tissue types, allowing for the study of organ-organ interactions and systemic effects that are impossible to observe in traditional cell cultures.
The integration of dynamic PK/PD modeling with MOC technology presents an unprecedented opportunity to create predictive frameworks that more accurately reflect human physiology. This convergence aims to overcome the limitations of both standalone computational models and static in vitro systems, potentially revolutionizing drug development and toxicology assessment.
Our research objectives focus on developing robust methodologies for incorporating dynamic PK/PD modeling into multi-organ chip platforms. We aim to establish standardized protocols for data collection, model parameterization, and validation that can be widely adopted across the pharmaceutical industry. Additionally, we seek to explore how these integrated systems can be optimized for different drug classes and therapeutic areas, ultimately creating a more efficient and predictive drug development paradigm.
The 1980s and 1990s witnessed a paradigm shift with the development of physiologically-based pharmacokinetic (PBPK) models, which incorporated anatomical and physiological parameters to better predict drug concentrations in specific tissues. This era also saw the integration of pharmacodynamic components, allowing researchers to link drug concentrations with biological responses and therapeutic outcomes.
Recent advancements have been driven by computational capabilities and systems biology approaches, enabling the creation of mechanistic models that account for molecular-level interactions and complex biological pathways. The emergence of population PK/PD modeling has further enhanced our ability to account for inter-individual variability in drug responses, a critical factor in personalized medicine.
Despite these advances, traditional PK/PD models face limitations in accurately predicting human systemic exposure, particularly for complex biological products and novel therapeutic modalities. These models often rely on animal data that may not translate well to humans or on simplified in vitro systems that fail to capture the complexity of multi-organ interactions.
Multi-organ chips (MOCs) represent a revolutionary platform that can bridge this gap by providing a more physiologically relevant environment for drug testing. These microfluidic devices contain multiple interconnected chambers housing different tissue types, allowing for the study of organ-organ interactions and systemic effects that are impossible to observe in traditional cell cultures.
The integration of dynamic PK/PD modeling with MOC technology presents an unprecedented opportunity to create predictive frameworks that more accurately reflect human physiology. This convergence aims to overcome the limitations of both standalone computational models and static in vitro systems, potentially revolutionizing drug development and toxicology assessment.
Our research objectives focus on developing robust methodologies for incorporating dynamic PK/PD modeling into multi-organ chip platforms. We aim to establish standardized protocols for data collection, model parameterization, and validation that can be widely adopted across the pharmaceutical industry. Additionally, we seek to explore how these integrated systems can be optimized for different drug classes and therapeutic areas, ultimately creating a more efficient and predictive drug development paradigm.
Market Analysis for Predictive Drug Testing Platforms
The predictive drug testing platform market is experiencing significant growth, driven by the increasing costs and failure rates in traditional drug development processes. The global market for in vitro toxicity testing was valued at approximately $8.7 billion in 2022 and is projected to grow at a CAGR of 9.8% through 2030. Within this broader market, organ-on-chip technology represents one of the fastest-growing segments, with a market value of $112 million in 2022 and expected to reach $1.2 billion by 2030.
The integration of dynamic PK/PD modeling with multi-organ chips addresses critical market needs across pharmaceutical and biotechnology sectors. Currently, pharmaceutical companies spend an average of $2.6 billion to bring a single drug to market, with only about 12% of drugs entering clinical trials receiving final approval. This inefficiency creates strong market demand for more predictive preclinical testing platforms that can better simulate human physiological responses.
Key market segments for these integrated platforms include large pharmaceutical companies seeking to reduce late-stage clinical failures, biotechnology firms requiring efficient screening methods for novel compounds, contract research organizations (CROs) offering specialized testing services, and academic research institutions advancing the fundamental science of drug behavior in human systems.
Geographically, North America dominates the market with approximately 42% share, followed by Europe at 28% and Asia-Pacific at 22%, with the latter showing the fastest growth rate. This distribution reflects the concentration of pharmaceutical R&D expenditure and regulatory pressures for reducing animal testing in these regions.
Customer needs analysis reveals three primary market drivers: regulatory pressure to reduce animal testing (particularly in Europe following Directive 2010/63/EU), economic pressure to reduce drug development costs, and scientific demand for more physiologically relevant human data. The integration of PK/PD modeling with multi-organ chips directly addresses these needs by providing translatable human-relevant data earlier in the development process.
Market adoption barriers include high initial investment costs for platform implementation, technical complexity requiring specialized expertise, and regulatory uncertainty regarding validation and qualification of these novel testing methods. Despite these challenges, market surveys indicate that 78% of pharmaceutical R&D executives consider predictive in vitro platforms a strategic priority for their organizations over the next five years.
The market shows particular growth potential in applications for complex drug modalities such as biologics, gene therapies, and combination products, where traditional testing methods have proven especially inadequate for predicting human responses.
The integration of dynamic PK/PD modeling with multi-organ chips addresses critical market needs across pharmaceutical and biotechnology sectors. Currently, pharmaceutical companies spend an average of $2.6 billion to bring a single drug to market, with only about 12% of drugs entering clinical trials receiving final approval. This inefficiency creates strong market demand for more predictive preclinical testing platforms that can better simulate human physiological responses.
Key market segments for these integrated platforms include large pharmaceutical companies seeking to reduce late-stage clinical failures, biotechnology firms requiring efficient screening methods for novel compounds, contract research organizations (CROs) offering specialized testing services, and academic research institutions advancing the fundamental science of drug behavior in human systems.
Geographically, North America dominates the market with approximately 42% share, followed by Europe at 28% and Asia-Pacific at 22%, with the latter showing the fastest growth rate. This distribution reflects the concentration of pharmaceutical R&D expenditure and regulatory pressures for reducing animal testing in these regions.
Customer needs analysis reveals three primary market drivers: regulatory pressure to reduce animal testing (particularly in Europe following Directive 2010/63/EU), economic pressure to reduce drug development costs, and scientific demand for more physiologically relevant human data. The integration of PK/PD modeling with multi-organ chips directly addresses these needs by providing translatable human-relevant data earlier in the development process.
Market adoption barriers include high initial investment costs for platform implementation, technical complexity requiring specialized expertise, and regulatory uncertainty regarding validation and qualification of these novel testing methods. Despite these challenges, market surveys indicate that 78% of pharmaceutical R&D executives consider predictive in vitro platforms a strategic priority for their organizations over the next five years.
The market shows particular growth potential in applications for complex drug modalities such as biologics, gene therapies, and combination products, where traditional testing methods have proven especially inadequate for predicting human responses.
Multi-Organ Chips: Current Capabilities and Limitations
Multi-organ chips (MOCs) represent a significant advancement in microphysiological systems, offering platforms that integrate multiple tissue types to simulate organ interactions within the human body. Current MOC technologies vary in complexity, ranging from simple two-organ systems to more sophisticated platforms incorporating four or more organ types. These systems typically utilize microfluidic channels connecting separate tissue compartments, allowing for controlled fluid exchange that mimics blood circulation.
The primary capability of state-of-the-art MOCs lies in their ability to maintain functional tissue cultures for extended periods, typically 7-28 days, enabling observation of longer-term physiological responses. Advanced systems incorporate sensors for real-time monitoring of parameters such as oxygen consumption, pH, and specific biomarkers, providing dynamic readouts of tissue function and response.
Despite these advancements, significant limitations persist in current MOC technologies. Scaling remains a critical challenge, with most systems operating at scales that do not accurately represent the proportional organ sizes and blood flow distributions found in the human body. This scaling discrepancy creates fundamental barriers to accurate pharmacokinetic modeling.
Physiological fidelity presents another major limitation. While MOCs can maintain basic tissue functionality, they often fail to replicate the full complexity of native tissue architecture and cellular diversity. Many systems lack important physiological features such as immune components, innervation, and hormone regulation, which are crucial for accurate drug response prediction.
Technical challenges further constrain MOC applications. Bubble formation in microfluidic channels, protein adsorption to device materials, and maintaining stable long-term cultures without contamination remain persistent issues. Additionally, most current systems lack standardization in design, operation protocols, and readout parameters, complicating cross-platform comparisons and data integration.
From a pharmacokinetic perspective, current MOCs typically employ simplified fluid dynamics that inadequately capture the complexity of human systemic circulation. Most systems utilize constant flow rates rather than pulsatile flow, and many lack the ability to model variable blood flow distribution to different organs under changing physiological conditions.
Perhaps most critically for PK/PD applications, current MOCs generally lack integrated computational modeling capabilities that can translate on-chip observations to predicted human systemic exposure. The absence of real-time, dynamic PK/PD modeling frameworks that can account for inter-organ interactions represents a significant gap in current technology, limiting the predictive power of these platforms for drug development applications.
The primary capability of state-of-the-art MOCs lies in their ability to maintain functional tissue cultures for extended periods, typically 7-28 days, enabling observation of longer-term physiological responses. Advanced systems incorporate sensors for real-time monitoring of parameters such as oxygen consumption, pH, and specific biomarkers, providing dynamic readouts of tissue function and response.
Despite these advancements, significant limitations persist in current MOC technologies. Scaling remains a critical challenge, with most systems operating at scales that do not accurately represent the proportional organ sizes and blood flow distributions found in the human body. This scaling discrepancy creates fundamental barriers to accurate pharmacokinetic modeling.
Physiological fidelity presents another major limitation. While MOCs can maintain basic tissue functionality, they often fail to replicate the full complexity of native tissue architecture and cellular diversity. Many systems lack important physiological features such as immune components, innervation, and hormone regulation, which are crucial for accurate drug response prediction.
Technical challenges further constrain MOC applications. Bubble formation in microfluidic channels, protein adsorption to device materials, and maintaining stable long-term cultures without contamination remain persistent issues. Additionally, most current systems lack standardization in design, operation protocols, and readout parameters, complicating cross-platform comparisons and data integration.
From a pharmacokinetic perspective, current MOCs typically employ simplified fluid dynamics that inadequately capture the complexity of human systemic circulation. Most systems utilize constant flow rates rather than pulsatile flow, and many lack the ability to model variable blood flow distribution to different organs under changing physiological conditions.
Perhaps most critically for PK/PD applications, current MOCs generally lack integrated computational modeling capabilities that can translate on-chip observations to predicted human systemic exposure. The absence of real-time, dynamic PK/PD modeling frameworks that can account for inter-organ interactions represents a significant gap in current technology, limiting the predictive power of these platforms for drug development applications.
Existing Integration Methods for PK/PD Models with MOCs
01 Multi-organ chip platforms for PK/PD modeling
Multi-organ chip platforms provide advanced in vitro systems for pharmacokinetic and pharmacodynamic modeling by integrating multiple tissue types to simulate organ interactions. These platforms allow for the study of drug absorption, distribution, metabolism, and excretion across different organ systems simultaneously. The technology enables more accurate prediction of human systemic exposure compared to traditional single-organ models, as it accounts for the complex interplay between organs in drug processing.- Multi-organ chip platforms for PK/PD modeling: Multi-organ chip platforms provide advanced in vitro systems for modeling pharmacokinetics and pharmacodynamics across multiple organ systems simultaneously. These platforms integrate different tissue types to simulate the complex interactions between organs during drug metabolism and distribution. By recreating physiological connections between organ compartments, these systems enable more accurate prediction of human systemic exposure to drugs and chemicals than traditional single-organ models.
- Computational models for predicting human systemic exposure: Advanced computational models are developed to predict human systemic exposure to drugs and chemicals using data from multi-organ chip experiments. These models integrate physiologically-based pharmacokinetic (PBPK) principles with dynamic modeling approaches to simulate absorption, distribution, metabolism, and excretion processes. Machine learning algorithms and mathematical modeling techniques are employed to analyze complex datasets and improve prediction accuracy for human exposure scenarios.
- Integration of in vitro data with in silico modeling: The integration of experimental data from multi-organ chips with in silico modeling approaches enhances the predictive power of PK/PD models. This combined approach allows researchers to extrapolate from in vitro results to in vivo human exposure scenarios. By incorporating physiological parameters and scaling factors, these integrated models can account for species differences and provide more reliable predictions of drug behavior in humans without relying solely on animal testing.
- Real-time monitoring systems for dynamic PK/PD assessment: Real-time monitoring systems are implemented in multi-organ chips to continuously assess pharmacokinetic and pharmacodynamic parameters. These systems utilize biosensors, imaging technologies, and microfluidic sampling to track drug concentrations, metabolite formation, and biological responses across different organ compartments over time. The dynamic data collection enables more accurate modeling of temporal changes in drug distribution and effects, improving the prediction of human systemic exposure.
- Validation methods for multi-organ chip PK/PD models: Validation methods are essential for establishing the reliability of PK/PD predictions from multi-organ chip systems. These methods include comparison with clinical data, correlation with established in vivo models, and assessment of inter-laboratory reproducibility. Standardized protocols and reference compounds are used to evaluate the predictive performance of multi-organ chip platforms. Validation studies help determine the applicability domain of these models for human systemic exposure prediction and support their regulatory acceptance.
02 Computational methods for PK/PD prediction
Advanced computational algorithms and mathematical models are employed to predict pharmacokinetic and pharmacodynamic parameters from multi-organ chip data. These methods include machine learning approaches, differential equation-based models, and physiologically-based pharmacokinetic (PBPK) modeling techniques. The computational tools enable the integration of experimental data from organ-on-chip platforms with existing knowledge to generate accurate predictions of drug behavior in human systems.Expand Specific Solutions03 Real-time monitoring systems for dynamic modeling
Real-time monitoring systems integrated with multi-organ chips allow for continuous data collection on drug concentrations, metabolite formation, and physiological responses. These systems employ biosensors, imaging technologies, and microfluidic sampling methods to track dynamic changes in biological parameters. The continuous data streams enable dynamic PK/PD modeling that captures temporal variations in drug behavior and physiological responses, improving the accuracy of human systemic exposure predictions.Expand Specific Solutions04 Physiological parameter optimization for human exposure prediction
Methods for optimizing physiological parameters in multi-organ chip systems to better mimic human conditions are essential for accurate systemic exposure prediction. These approaches include adjusting flow rates, nutrient compositions, hormone levels, and cellular densities to match human physiological conditions. The optimization techniques ensure that the in vitro system closely resembles human physiology, thereby improving the translational value of PK/PD data obtained from multi-organ chips.Expand Specific Solutions05 Integration of disease models in PK/PD prediction
Incorporating disease models into multi-organ chip systems allows for the prediction of drug behavior in pathological conditions. These models simulate disease-specific alterations in organ function, metabolism, and drug response. By integrating disease models with PK/PD analysis, researchers can predict how pathological conditions affect drug disposition and efficacy, providing valuable insights for personalized medicine approaches and improving the prediction of human systemic exposure in diverse patient populations.Expand Specific Solutions
Leading Organizations in PK/PD and Organ-on-Chip Research
The field of dynamic PK/PD modeling in multi-organ chips is currently in an early growth phase, with market size estimated to reach significant expansion as pharmaceutical companies seek more predictive preclinical models. This technology sits at the intersection of microfluidics, tissue engineering, and computational modeling, with moderate technical maturity but rapidly evolving capabilities. Key players include academic institutions (MIT, Harvard, Soochow University) driving fundamental research, while specialized biotech companies (Javelin Biotech, Quris Technologies, EnLiSense) are commercializing platforms. Pharmaceutical corporations (GlaxoSmithKline, Philip Morris) are investing in the technology to reduce clinical trial failures. The competitive landscape shows increasing collaboration between technology developers and drug manufacturers to validate these systems against human clinical data.
Massachusetts Institute of Technology
Technical Solution: MIT has developed a sophisticated approach to incorporating dynamic PK/PD modeling into multi-organ chip systems through their PhysioMimetics platform. This technology integrates advanced microfluidic engineering with computational modeling to create physiologically relevant organ-on-chip systems specifically designed for pharmacokinetic applications. MIT's platform features modular organ units representing key metabolic and excretory tissues (liver, kidney, intestine) connected through a recirculating endothelialized vascular network that mimics the human circulatory system. Their system incorporates real-time monitoring capabilities through integrated microsensors that track drug concentrations, metabolites, and physiological parameters across different tissue compartments. MIT's computational framework combines physiologically-based pharmacokinetic (PBPK) modeling with machine learning approaches to translate chip-derived data to human predictions. The platform includes sophisticated mathematical models that account for scaling differences between chip systems and human physiology, with algorithms that adjust for organ-specific blood flow rates, tissue volumes, and metabolic capacities. MIT researchers have validated their system against clinical data for various drug classes, demonstrating strong correlations between chip-predicted and human PK parameters.
Strengths: Exceptional engineering precision with highly controlled microfluidic environment; strong computational modeling capabilities; modular design allows customization for specific research questions. Weaknesses: Higher technical complexity requires specialized expertise; academic system may have challenges in scaling for high-throughput applications; limited commercial availability restricts widespread adoption.
President & Fellows of Harvard College
Technical Solution: Harvard's approach to incorporating dynamic PK/PD modeling into multi-organ chips represents one of the most sophisticated academic efforts in this field. Their technology, developed through the Wyss Institute, features a "human-on-a-chip" platform that integrates up to 10 different organ systems connected by a physiologically relevant circulatory system. The platform incorporates microengineered organ chips that recapitulate key functional aspects of human tissues, including tissue-tissue interfaces and mechanical forces. Harvard's system employs advanced microfluidic engineering to control inter-organ communication and drug distribution, with integrated biosensors providing real-time monitoring of physiological parameters and drug concentrations. Their computational framework combines compartmental PK modeling with systems pharmacology approaches to translate chip-derived data to human predictions. The platform incorporates mathematical models that account for scaling differences between chip systems and human physiology, including adjustments for relative organ sizes, flow rates, and metabolic capacities. Harvard researchers have demonstrated the platform's ability to recapitulate complex drug-drug interactions and predict unexpected toxicities not captured by conventional methods.
Strengths: Exceptional biological fidelity with multiple integrated organ systems; strong scientific foundation with extensive peer-reviewed publications; comprehensive modeling framework. Weaknesses: Complex academic system may face challenges in commercial translation; higher cost and lower throughput than some commercial alternatives; requires significant expertise to operate effectively.
Critical Technologies for Dynamic PK/PD-MOC Integration
Performing pharmacodynamics evaluations using microfluidic devices
PatentPendingUS20220074924A1
Innovation
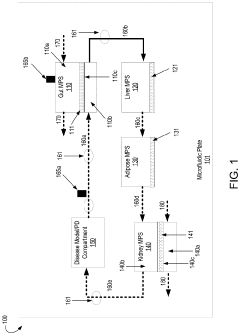
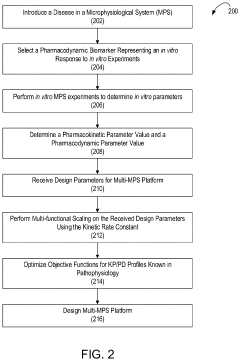
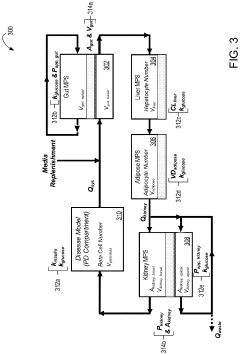
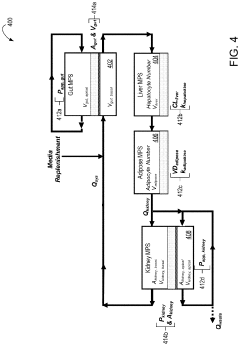
- A multi-MPS platform is designed with interconnected organ constructs that simulate human organ functions and interactions, using fluidic channels for continuous drug flow and applying fluid shear stress to mimic physiological conditions, allowing for the estimation of PK/PD parameters and optimization of drug profiles.
Regulatory Pathway for Novel Drug Testing Platforms
The regulatory landscape for novel drug testing platforms, particularly those incorporating dynamic PK/PD modeling with multi-organ chips, presents both challenges and opportunities for pharmaceutical development. Current regulatory frameworks were largely established before the emergence of these advanced in vitro technologies, creating a need for updated guidance and approval pathways.
The FDA has begun addressing these innovations through its Advancing Regulatory Science initiative, which recognizes the potential of organ-on-chip technologies to provide more predictive human exposure data than traditional animal models. In 2022, the FDA issued draft guidance on microphysiological systems, acknowledging their potential role in drug development while outlining expectations for validation and qualification.
Similarly, the European Medicines Agency (EMA) has established the Innovation Task Force specifically to engage with developers of novel methodologies. Their qualification procedure provides a pathway for formal recognition of innovative drug development tools, including multi-organ chip platforms with integrated PK/PD modeling capabilities.
International harmonization efforts are underway through the International Council for Harmonisation (ICH), which is developing guidelines for incorporating alternative testing methods into regulatory submissions. The ICH M10 guideline on bioanalytical method validation provides some relevant frameworks that could be adapted for multi-organ chip validation.
Regulatory acceptance requires substantial validation data demonstrating that these platforms can reliably predict human systemic exposure. Key requirements include reproducibility across laboratories, correlation with known human PK/PD data for reference compounds, and clear documentation of the platform's limitations and applicability domain.
A phased approach to regulatory integration is emerging as the most viable strategy. Initially, multi-organ chips with PK/PD modeling are being positioned as complementary tools alongside traditional methods, providing supplementary data in regulatory submissions. As confidence in these platforms grows, they may eventually replace certain animal studies, particularly for specialized applications like rare diseases or personalized medicine approaches.
Engagement with regulatory agencies through formal consultation programs is essential for developers. The FDA's Critical Path Innovation Meetings and EMA's Innovation Task Force consultations provide opportunities to discuss development plans and receive feedback on validation strategies before formal submission, potentially accelerating the path to regulatory acceptance.
The FDA has begun addressing these innovations through its Advancing Regulatory Science initiative, which recognizes the potential of organ-on-chip technologies to provide more predictive human exposure data than traditional animal models. In 2022, the FDA issued draft guidance on microphysiological systems, acknowledging their potential role in drug development while outlining expectations for validation and qualification.
Similarly, the European Medicines Agency (EMA) has established the Innovation Task Force specifically to engage with developers of novel methodologies. Their qualification procedure provides a pathway for formal recognition of innovative drug development tools, including multi-organ chip platforms with integrated PK/PD modeling capabilities.
International harmonization efforts are underway through the International Council for Harmonisation (ICH), which is developing guidelines for incorporating alternative testing methods into regulatory submissions. The ICH M10 guideline on bioanalytical method validation provides some relevant frameworks that could be adapted for multi-organ chip validation.
Regulatory acceptance requires substantial validation data demonstrating that these platforms can reliably predict human systemic exposure. Key requirements include reproducibility across laboratories, correlation with known human PK/PD data for reference compounds, and clear documentation of the platform's limitations and applicability domain.
A phased approach to regulatory integration is emerging as the most viable strategy. Initially, multi-organ chips with PK/PD modeling are being positioned as complementary tools alongside traditional methods, providing supplementary data in regulatory submissions. As confidence in these platforms grows, they may eventually replace certain animal studies, particularly for specialized applications like rare diseases or personalized medicine approaches.
Engagement with regulatory agencies through formal consultation programs is essential for developers. The FDA's Critical Path Innovation Meetings and EMA's Innovation Task Force consultations provide opportunities to discuss development plans and receive feedback on validation strategies before formal submission, potentially accelerating the path to regulatory acceptance.
Translational Challenges from In Vitro to Clinical Applications
The translation of multi-organ chip technologies from laboratory settings to clinical applications faces significant challenges that must be addressed to realize their full potential. One primary obstacle is the validation gap between in vitro models and human physiological responses. Despite sophisticated engineering, multi-organ chips with integrated PK/PD modeling still struggle to fully replicate the complex interactions between organs and systemic circulation observed in humans.
Regulatory frameworks present another substantial hurdle, as current approval pathways are not optimized for these novel hybrid technologies. Regulatory agencies require extensive validation data comparing chip-based predictions with traditional animal models and clinical outcomes before accepting them as alternatives for drug development decision-making.
Scaling issues further complicate translation, as laboratory prototypes must be adapted for high-throughput screening environments while maintaining physiological relevance. The balance between complexity and practicality remains difficult to achieve, particularly when incorporating dynamic modeling components that require real-time data integration.
Technical standardization represents a critical challenge, with variability in chip design, cell sourcing, and analytical endpoints making cross-platform comparisons problematic. The field lacks consensus on reference standards for calibrating PK/PD models across different multi-organ chip platforms, hampering reproducibility and clinical translation.
Data integration challenges emerge when attempting to correlate in vitro observations with clinical outcomes. The mathematical frameworks for translating chip-derived PK/PD parameters to human-relevant dosing regimens remain underdeveloped, creating uncertainty in predictive accuracy for diverse patient populations.
Cost considerations significantly impact clinical adoption, as current multi-organ chip technologies with integrated modeling capabilities require substantial investment in specialized equipment and expertise. The economic value proposition must be clearly demonstrated against established methods before widespread implementation can occur.
Biological complexity presents perhaps the most fundamental challenge, as incorporating patient-specific factors such as genetic polymorphisms, disease states, and aging into PK/PD models remains technically difficult. Capturing inter-individual variability within chip-based systems requires advances in both biological understanding and computational approaches to personalized medicine.
Regulatory frameworks present another substantial hurdle, as current approval pathways are not optimized for these novel hybrid technologies. Regulatory agencies require extensive validation data comparing chip-based predictions with traditional animal models and clinical outcomes before accepting them as alternatives for drug development decision-making.
Scaling issues further complicate translation, as laboratory prototypes must be adapted for high-throughput screening environments while maintaining physiological relevance. The balance between complexity and practicality remains difficult to achieve, particularly when incorporating dynamic modeling components that require real-time data integration.
Technical standardization represents a critical challenge, with variability in chip design, cell sourcing, and analytical endpoints making cross-platform comparisons problematic. The field lacks consensus on reference standards for calibrating PK/PD models across different multi-organ chip platforms, hampering reproducibility and clinical translation.
Data integration challenges emerge when attempting to correlate in vitro observations with clinical outcomes. The mathematical frameworks for translating chip-derived PK/PD parameters to human-relevant dosing regimens remain underdeveloped, creating uncertainty in predictive accuracy for diverse patient populations.
Cost considerations significantly impact clinical adoption, as current multi-organ chip technologies with integrated modeling capabilities require substantial investment in specialized equipment and expertise. The economic value proposition must be clearly demonstrated against established methods before widespread implementation can occur.
Biological complexity presents perhaps the most fundamental challenge, as incorporating patient-specific factors such as genetic polymorphisms, disease states, and aging into PK/PD models remains technically difficult. Capturing inter-individual variability within chip-based systems requires advances in both biological understanding and computational approaches to personalized medicine.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!