Modeling pediatric metabolic disorders in organ chips using age-matched cell sources
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Pediatric Metabolic Disorders Modeling Background and Objectives
Pediatric metabolic disorders represent a diverse group of conditions affecting children's ability to properly convert food into energy. Historically, research in this field has been hampered by limited access to pediatric tissue samples and the ethical constraints surrounding pediatric clinical trials. The evolution of this field has seen a shift from animal models, which often fail to accurately replicate human metabolic pathways, to more sophisticated in vitro systems that better mimic human physiology.
The emergence of organ-on-chip technology over the past decade has revolutionized the approach to studying human diseases, offering microfluidic platforms that recreate the physiological microenvironment of human organs. However, a significant limitation has persisted: most organ chip models utilize adult cell sources, creating a fundamental mismatch when studying pediatric conditions due to the distinct developmental and metabolic differences between children and adults.
Recent technological advances have enabled the isolation and culture of age-matched pediatric cells, opening new possibilities for creating more physiologically relevant models. This progress coincides with growing recognition of the "pediatric gap" in pharmaceutical development, where children are often treated with medications tested primarily in adults, despite fundamental physiological differences that affect drug metabolism and efficacy.
The primary objective of this research is to develop and validate organ chip platforms specifically designed for pediatric metabolic disorders using age-matched cell sources. These platforms aim to recreate the unique physiological environment of pediatric organs affected by metabolic disorders, particularly focusing on liver, pancreas, and adipose tissue—key organs in metabolic regulation.
Secondary objectives include establishing standardized protocols for sourcing and maintaining pediatric cells for organ chip applications, characterizing the developmental and metabolic differences between pediatric and adult tissues in these systems, and validating these models against known clinical presentations of pediatric metabolic disorders such as glycogen storage diseases, fatty acid oxidation disorders, and mitochondrial diseases.
The long-term goal is to create a versatile research platform that enables more accurate disease modeling, personalized treatment approaches, and accelerated drug development specifically for pediatric populations. This technology has the potential to address the critical need for pediatric-specific drug testing platforms, reducing reliance on extrapolation from adult data and potentially decreasing adverse drug reactions in children.
By achieving these objectives, this research aims to bridge the gap between preclinical research and clinical application in pediatric metabolic medicine, ultimately improving diagnostic accuracy, treatment efficacy, and quality of life for children affected by these often debilitating disorders.
The emergence of organ-on-chip technology over the past decade has revolutionized the approach to studying human diseases, offering microfluidic platforms that recreate the physiological microenvironment of human organs. However, a significant limitation has persisted: most organ chip models utilize adult cell sources, creating a fundamental mismatch when studying pediatric conditions due to the distinct developmental and metabolic differences between children and adults.
Recent technological advances have enabled the isolation and culture of age-matched pediatric cells, opening new possibilities for creating more physiologically relevant models. This progress coincides with growing recognition of the "pediatric gap" in pharmaceutical development, where children are often treated with medications tested primarily in adults, despite fundamental physiological differences that affect drug metabolism and efficacy.
The primary objective of this research is to develop and validate organ chip platforms specifically designed for pediatric metabolic disorders using age-matched cell sources. These platforms aim to recreate the unique physiological environment of pediatric organs affected by metabolic disorders, particularly focusing on liver, pancreas, and adipose tissue—key organs in metabolic regulation.
Secondary objectives include establishing standardized protocols for sourcing and maintaining pediatric cells for organ chip applications, characterizing the developmental and metabolic differences between pediatric and adult tissues in these systems, and validating these models against known clinical presentations of pediatric metabolic disorders such as glycogen storage diseases, fatty acid oxidation disorders, and mitochondrial diseases.
The long-term goal is to create a versatile research platform that enables more accurate disease modeling, personalized treatment approaches, and accelerated drug development specifically for pediatric populations. This technology has the potential to address the critical need for pediatric-specific drug testing platforms, reducing reliance on extrapolation from adult data and potentially decreasing adverse drug reactions in children.
By achieving these objectives, this research aims to bridge the gap between preclinical research and clinical application in pediatric metabolic medicine, ultimately improving diagnostic accuracy, treatment efficacy, and quality of life for children affected by these often debilitating disorders.
Market Analysis for Pediatric Disease Models
The global market for pediatric disease models is experiencing significant growth, driven by the increasing prevalence of metabolic disorders in children and the urgent need for more effective treatments. Currently valued at approximately $1.2 billion, this market segment is projected to grow at a compound annual growth rate of 8.7% through 2028, according to recent industry analyses. The demand is particularly strong in regions with advanced healthcare infrastructure, including North America, Europe, and parts of Asia-Pacific.
Pediatric metabolic disorders represent a substantial portion of this market, with conditions such as glycogen storage diseases, fatty acid oxidation disorders, and mitochondrial diseases affecting an estimated 1 in 1,500 children worldwide. Traditional animal models have dominated this space, accounting for roughly 65% of the market share, but organ-on-chip technologies are rapidly gaining traction due to their superior ability to replicate human physiology.
The shift toward organ chip models using age-matched cell sources is being fueled by several market drivers. Pharmaceutical companies are increasingly recognizing the limitations of conventional testing methods, which have contributed to the high failure rate (over 90%) of pediatric drugs in clinical trials. This has created a strong economic incentive to adopt more predictive preclinical models, with major pharmaceutical companies allocating between 5-10% of their R&D budgets to alternative testing platforms.
Regulatory pressures are also reshaping the market landscape. The FDA's Pediatric Research Equity Act and similar regulations in Europe have mandated pediatric studies for most new drugs, creating demand for age-appropriate testing platforms. Additionally, ethical considerations regarding animal testing have prompted both regulatory bodies and research institutions to seek alternatives, further accelerating market growth for in vitro pediatric disease models.
Academic research institutions currently represent the largest customer segment (approximately 40% of the market), followed by pharmaceutical companies (35%) and contract research organizations (20%). However, the pharmaceutical segment is expected to grow at the fastest rate as companies seek to reduce development costs and accelerate time-to-market for pediatric formulations.
Key challenges in this market include the high initial investment required for organ chip technology implementation, technical difficulties in sourcing age-matched pediatric cells, and the need for standardization across platforms. Despite these challenges, the market presents significant opportunities for technology providers who can address the specific needs of pediatric research, particularly in metabolic disorders where current treatment options remain limited and the economic burden on healthcare systems is substantial.
Pediatric metabolic disorders represent a substantial portion of this market, with conditions such as glycogen storage diseases, fatty acid oxidation disorders, and mitochondrial diseases affecting an estimated 1 in 1,500 children worldwide. Traditional animal models have dominated this space, accounting for roughly 65% of the market share, but organ-on-chip technologies are rapidly gaining traction due to their superior ability to replicate human physiology.
The shift toward organ chip models using age-matched cell sources is being fueled by several market drivers. Pharmaceutical companies are increasingly recognizing the limitations of conventional testing methods, which have contributed to the high failure rate (over 90%) of pediatric drugs in clinical trials. This has created a strong economic incentive to adopt more predictive preclinical models, with major pharmaceutical companies allocating between 5-10% of their R&D budgets to alternative testing platforms.
Regulatory pressures are also reshaping the market landscape. The FDA's Pediatric Research Equity Act and similar regulations in Europe have mandated pediatric studies for most new drugs, creating demand for age-appropriate testing platforms. Additionally, ethical considerations regarding animal testing have prompted both regulatory bodies and research institutions to seek alternatives, further accelerating market growth for in vitro pediatric disease models.
Academic research institutions currently represent the largest customer segment (approximately 40% of the market), followed by pharmaceutical companies (35%) and contract research organizations (20%). However, the pharmaceutical segment is expected to grow at the fastest rate as companies seek to reduce development costs and accelerate time-to-market for pediatric formulations.
Key challenges in this market include the high initial investment required for organ chip technology implementation, technical difficulties in sourcing age-matched pediatric cells, and the need for standardization across platforms. Despite these challenges, the market presents significant opportunities for technology providers who can address the specific needs of pediatric research, particularly in metabolic disorders where current treatment options remain limited and the economic burden on healthcare systems is substantial.
Current Challenges in Pediatric Organ Chip Technology
Despite significant advancements in organ-on-chip technology, pediatric applications face unique challenges that hinder progress in modeling metabolic disorders using age-matched cell sources. The fundamental issue lies in the limited availability of pediatric tissue samples for research purposes due to ethical constraints and regulatory frameworks designed to protect vulnerable populations. This scarcity creates a significant bottleneck in developing physiologically relevant models that accurately represent pediatric metabolism.
Cell sourcing presents a particularly complex challenge, as pediatric cells differ substantially from adult counterparts in terms of metabolic activity, proliferation rates, and response to environmental stimuli. The developmental stage-specific characteristics of pediatric cells are often lost when using adult cells or improperly differentiated stem cells as substitutes. Furthermore, the heterogeneity among pediatric populations across different age groups (neonatal, infant, child, adolescent) requires age-specific models that few research groups have successfully established.
Technical limitations in miniaturization pose another significant hurdle. Pediatric organ chips must account for the smaller scale of pediatric organs while maintaining physiological relevance. Current microfluidic designs and fabrication techniques struggle to accurately replicate the unique architectural features and functional units of developing organs, particularly when modeling complex metabolic interactions that involve multiple organ systems.
The dynamic nature of pediatric development introduces additional complexity. Metabolic disorders in children often manifest differently across developmental stages, requiring organ chip platforms capable of modeling temporal changes in metabolism. Current static models fail to capture these developmental trajectories, limiting their utility in understanding disease progression and treatment response in pediatric populations.
Validation protocols represent another critical challenge. The correlation between pediatric organ chip models and clinical outcomes remains poorly established due to limited comparative data. Without robust validation frameworks, researchers struggle to confirm whether observed in vitro responses accurately reflect in vivo pediatric physiology, particularly for rare metabolic disorders where clinical data is sparse.
Interdisciplinary collaboration barriers further complicate progress. Pediatric organ chip development requires expertise from developmental biology, pediatric medicine, microfluidics engineering, and computational modeling. The siloed nature of these disciplines often impedes the integrated approach necessary for creating physiologically relevant pediatric models. Additionally, funding limitations for pediatric-specific research tools compared to adult applications have slowed technological innovation in this specialized field.
Cell sourcing presents a particularly complex challenge, as pediatric cells differ substantially from adult counterparts in terms of metabolic activity, proliferation rates, and response to environmental stimuli. The developmental stage-specific characteristics of pediatric cells are often lost when using adult cells or improperly differentiated stem cells as substitutes. Furthermore, the heterogeneity among pediatric populations across different age groups (neonatal, infant, child, adolescent) requires age-specific models that few research groups have successfully established.
Technical limitations in miniaturization pose another significant hurdle. Pediatric organ chips must account for the smaller scale of pediatric organs while maintaining physiological relevance. Current microfluidic designs and fabrication techniques struggle to accurately replicate the unique architectural features and functional units of developing organs, particularly when modeling complex metabolic interactions that involve multiple organ systems.
The dynamic nature of pediatric development introduces additional complexity. Metabolic disorders in children often manifest differently across developmental stages, requiring organ chip platforms capable of modeling temporal changes in metabolism. Current static models fail to capture these developmental trajectories, limiting their utility in understanding disease progression and treatment response in pediatric populations.
Validation protocols represent another critical challenge. The correlation between pediatric organ chip models and clinical outcomes remains poorly established due to limited comparative data. Without robust validation frameworks, researchers struggle to confirm whether observed in vitro responses accurately reflect in vivo pediatric physiology, particularly for rare metabolic disorders where clinical data is sparse.
Interdisciplinary collaboration barriers further complicate progress. Pediatric organ chip development requires expertise from developmental biology, pediatric medicine, microfluidics engineering, and computational modeling. The siloed nature of these disciplines often impedes the integrated approach necessary for creating physiologically relevant pediatric models. Additionally, funding limitations for pediatric-specific research tools compared to adult applications have slowed technological innovation in this specialized field.
Current Methodologies for Age-Matched Cell Integration
01 Microfluidic organ-on-chip systems for accurate physiological modeling
Microfluidic organ-on-chip platforms enable the creation of physiologically relevant microenvironments that can accurately mimic organ functions. These systems incorporate controlled fluid flow, tissue-tissue interfaces, and mechanical forces to better replicate in vivo conditions. The integration of microfluidic technology with cell culture allows for precise control over cellular microenvironments, resulting in more accurate modeling of organ functions and responses to stimuli compared to traditional 2D cell cultures.- Microfluidic organ-on-chip systems for physiological modeling: Microfluidic organ-on-chip platforms enable the creation of physiologically relevant models that accurately mimic human organ functions. These systems incorporate living cells in controlled microenvironments that replicate key aspects of organ structure and function. By integrating multiple cell types and providing dynamic flow conditions, these chips achieve higher modeling accuracy than traditional cell culture methods, allowing for more predictive drug testing and disease modeling applications.
- Multi-organ integration for systemic response modeling: Advanced organ chip platforms connect multiple organ models to simulate systemic physiological responses and inter-organ interactions. These integrated systems allow researchers to study complex biological processes like drug metabolism, where compounds may be processed by one organ and affect another. The accuracy of these models is enhanced by maintaining appropriate fluid flow between organ compartments and preserving tissue-specific functions, providing more comprehensive insights than isolated single-organ models.
- Sensor integration for real-time monitoring and accuracy validation: Incorporating sensors into organ chip platforms enables real-time monitoring of cellular responses and physiological parameters. These integrated sensing capabilities allow continuous measurement of metabolic activity, electrical signals, secreted biomarkers, and other functional readouts that validate the accuracy of the organ model. The combination of biological tissue with sensing elements creates more reliable data collection systems that can detect subtle changes in cellular behavior under various experimental conditions.
- Computational modeling to enhance organ chip predictive accuracy: Computational approaches are increasingly combined with organ chip technologies to improve modeling accuracy. These methods include mathematical modeling of fluid dynamics, tissue mechanics, and cellular responses within the microfluidic environment. By integrating experimental data with computational simulations, researchers can better predict physiological responses, optimize chip design parameters, and extrapolate results to human physiology, thereby enhancing the translational value of organ chip platforms.
- Advanced fabrication techniques for improved structural fidelity: Novel fabrication methods are being developed to create organ chips with higher structural accuracy. These techniques include 3D printing, photolithography, and advanced material processing that enable precise control over the microarchitecture of the devices. By more accurately replicating the physical structure of native tissues, including features like vasculature networks and tissue-specific geometries, these approaches significantly improve the physiological relevance and predictive capabilities of organ chip models.
02 Multi-organ chip systems for improved modeling accuracy
Multi-organ chip systems connect multiple organ models within a single platform to simulate organ interactions and systemic responses. These integrated systems allow for the study of complex physiological processes, drug metabolism, and toxicity across different organ types. By incorporating multiple organ models that can communicate through a shared circulatory system, these platforms achieve higher modeling accuracy for whole-body responses and inter-organ effects, which is crucial for predicting clinical outcomes of drugs and therapies.Expand Specific Solutions03 Advanced sensor integration for real-time monitoring and accuracy validation
Integration of advanced sensors within organ chip platforms enables real-time monitoring of physiological parameters and cellular responses. These sensors can measure various metrics including oxygen levels, pH, metabolite concentrations, and electrical activity. The continuous data collection allows for immediate validation of model accuracy and provides insights into dynamic cellular responses that might be missed in endpoint analyses, thereby enhancing the predictive power of organ chip models.Expand Specific Solutions04 Computational modeling and machine learning for enhanced predictive accuracy
Computational modeling and machine learning approaches are being integrated with organ chip data to enhance predictive accuracy. These computational tools can process complex datasets generated from organ chips to identify patterns, predict outcomes, and optimize experimental designs. By combining experimental data with in silico models, researchers can improve the translation of organ chip results to human physiology, validate the accuracy of the models against known clinical outcomes, and develop more sophisticated predictive tools for drug development and disease modeling.Expand Specific Solutions05 Biomaterial and fabrication innovations for improved structural and functional fidelity
Advanced biomaterials and fabrication techniques are being developed to create organ chips with improved structural and functional fidelity. These innovations include biocompatible materials that better mimic native tissue properties, 3D printing technologies for precise architecture creation, and surface modifications that enhance cell attachment and function. By more accurately replicating the physical and biochemical properties of native tissues, these advances significantly improve the modeling accuracy of organ chips and their ability to predict human physiological responses.Expand Specific Solutions
Leading Organizations in Pediatric Organ Chip Development
The field of modeling pediatric metabolic disorders in organ chips using age-matched cell sources is in an early growth phase, with market size expanding as precision medicine gains traction. The technology demonstrates moderate maturity, with key players driving innovation across different sectors. Emulate, Inc. leads commercial development with their Organs-on-Chips platform, while academic institutions like Harvard College and Cedars-Sinai Medical Center contribute significant research advancements. Pharmaceutical entities such as Revvity Health Sciences and Metabolon provide analytical capabilities, while Intel offers computational support. The competitive landscape includes both established research organizations (Memorial Sloan Kettering) and emerging biotechnology companies (Pretzel Therapeutics), creating a diverse ecosystem that balances academic research with commercial applications in this specialized field.
Emulate, Inc.
Technical Solution: Emulate has developed advanced organ-on-chip technology specifically tailored for pediatric metabolic disorder modeling. Their platform utilizes microfluidic devices that recreate the microarchitecture and dynamic physiological functions of human organs, with special adaptations for pediatric tissues. The company's Liver-Chip and Multi-Organ systems incorporate age-matched cell sources, including pediatric hepatocytes, stellate cells, and Kupffer cells to accurately model developmental stages and metabolic differences between children and adults. Their technology enables precise control of fluid flow, mechanical forces, and cell-cell interactions, creating a more physiologically relevant environment than traditional 2D cultures. Emulate's chips allow researchers to introduce patient-derived cells from children with specific metabolic disorders, enabling personalized disease modeling and therapeutic testing with significantly improved predictive value compared to animal models or adult-derived systems[1][3].
Strengths: Industry-leading microfluidic technology with proven ability to maintain functional pediatric tissue; comprehensive commercial platform with standardized protocols for reproducibility; extensive validation studies with pharmaceutical partners. Weaknesses: Higher cost compared to traditional cell culture methods; requires specialized expertise for operation; limited throughput compared to high-throughput screening platforms.
President & Fellows of Harvard College
Technical Solution: Harvard's Wyss Institute has pioneered innovative organ chip technology specifically adapted for pediatric metabolic disorder modeling. Their approach incorporates age-matched cell sources through careful isolation and characterization of pediatric primary cells and iPSC-derived tissues that maintain age-specific epigenetic signatures. The Harvard platform features multi-compartment microfluidic devices that recreate tissue-tissue interfaces and organ-level functions with precise control over mechanical forces, including breathing motions and peristalsis. Their pediatric liver and gut chips incorporate specialized media formulations that mimic the unique metabolic profiles of developing organs. The technology enables real-time monitoring of metabolic parameters through integrated sensors and sampling ports, allowing researchers to track disease progression and therapeutic responses in pediatric metabolic disorders with unprecedented accuracy. Harvard researchers have successfully modeled several rare pediatric metabolic conditions by incorporating patient-derived cells into their organ chip systems[2][5].
Strengths: Cutting-edge research with extensive academic publications validating the approach; sophisticated engineering capabilities for complex physiological modeling; strong interdisciplinary collaboration network spanning medicine, engineering and biology. Weaknesses: Technology primarily in research phase rather than commercial deployment; higher complexity may limit accessibility to non-specialized labs; requires significant expertise in multiple disciplines.
Key Innovations in Pediatric-Specific Organ Chip Design
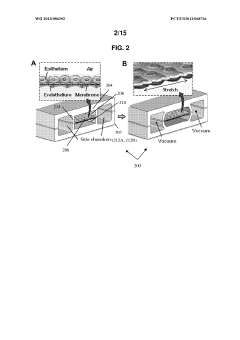
System, disease model and methods of using the same
PatentWO2023060271A1
Innovation
- Development of a fully vascularized milliscale membrane-free organ chip system that enables reproducible loading and rapid formation of perfusable vasculature, combined with methods for TNBC biopsy processing to create integrated microphysiological models, including a 4-organ chip design for real-time transport of tumor-derived factors, facilitating the cultivation of premetastatic niches and aggressive non-small cell lung cancer models.
Organ chips and uses thereof
PatentWO2013086502A1
Innovation
- Development of microengineered organ chips that mimic physiological functions of various organs, allowing for the creation of in vitro microphysiological systems to simulate organ-level functions, including lung, heart, liver, kidney, and other organ functions, enabling more accurate drug screening and toxicity testing.
Ethical and Regulatory Considerations for Pediatric Tissue Use
The utilization of pediatric tissue samples for organ-on-chip models presents unique ethical and regulatory challenges that extend beyond those encountered with adult tissues. Research involving children's biological materials requires heightened protection due to their vulnerable status and limited capacity for informed consent. Current regulatory frameworks, including the Common Rule in the United States and similar provisions internationally, mandate specialized protocols for pediatric tissue acquisition.
Parental or guardian consent represents the primary ethical cornerstone for pediatric tissue collection, supplemented by age-appropriate assent from children when developmentally possible. These consent processes must clearly communicate the long-term storage, potential commercialization, and future research applications of the collected tissues. Transparency regarding genetic information derived from samples has become increasingly important as genomic technologies advance.
Institutional Review Boards (IRBs) apply particularly stringent standards to pediatric metabolic disorder research, requiring investigators to demonstrate minimal risk or direct benefit to the participating children. The justification threshold for using pediatric tissues in organ chip models must establish that comparable scientific insights cannot be obtained using adult tissues or alternative methods. This "necessity principle" forms a fundamental regulatory requirement across jurisdictions.
Privacy protections for pediatric tissue donors demand robust de-identification protocols and secure data management systems. The European General Data Protection Regulation (GDPR) and the U.S. Health Insurance Portability and Accountability Act (HIPAA) impose specific requirements for handling children's biological information, with violations carrying significant penalties. These regulations have direct implications for international collaboration in pediatric organ chip research.
Equitable tissue collection practices represent another critical consideration, ensuring representation across diverse demographic groups to develop metabolic disorder models applicable across populations. Historical biases in pediatric research participation have led to regulatory requirements promoting inclusion of underrepresented groups, though implementation challenges persist.
The commercialization pathway for pediatric organ chip technologies faces additional regulatory scrutiny. The FDA's pediatric device provisions and the European Medicines Agency's pediatric investigation plans establish specialized frameworks for technologies utilizing children's tissues. These regulations aim to balance innovation with protection, requiring extensive documentation of ethical sourcing throughout the development process.
Looking forward, emerging regulatory frameworks are increasingly addressing issues of benefit-sharing with pediatric tissue donors and their communities. This evolving ethical landscape suggests that future organ chip research using pediatric tissues will likely require more comprehensive community engagement and benefit distribution mechanisms than current standards mandate.
Parental or guardian consent represents the primary ethical cornerstone for pediatric tissue collection, supplemented by age-appropriate assent from children when developmentally possible. These consent processes must clearly communicate the long-term storage, potential commercialization, and future research applications of the collected tissues. Transparency regarding genetic information derived from samples has become increasingly important as genomic technologies advance.
Institutional Review Boards (IRBs) apply particularly stringent standards to pediatric metabolic disorder research, requiring investigators to demonstrate minimal risk or direct benefit to the participating children. The justification threshold for using pediatric tissues in organ chip models must establish that comparable scientific insights cannot be obtained using adult tissues or alternative methods. This "necessity principle" forms a fundamental regulatory requirement across jurisdictions.
Privacy protections for pediatric tissue donors demand robust de-identification protocols and secure data management systems. The European General Data Protection Regulation (GDPR) and the U.S. Health Insurance Portability and Accountability Act (HIPAA) impose specific requirements for handling children's biological information, with violations carrying significant penalties. These regulations have direct implications for international collaboration in pediatric organ chip research.
Equitable tissue collection practices represent another critical consideration, ensuring representation across diverse demographic groups to develop metabolic disorder models applicable across populations. Historical biases in pediatric research participation have led to regulatory requirements promoting inclusion of underrepresented groups, though implementation challenges persist.
The commercialization pathway for pediatric organ chip technologies faces additional regulatory scrutiny. The FDA's pediatric device provisions and the European Medicines Agency's pediatric investigation plans establish specialized frameworks for technologies utilizing children's tissues. These regulations aim to balance innovation with protection, requiring extensive documentation of ethical sourcing throughout the development process.
Looking forward, emerging regulatory frameworks are increasingly addressing issues of benefit-sharing with pediatric tissue donors and their communities. This evolving ethical landscape suggests that future organ chip research using pediatric tissues will likely require more comprehensive community engagement and benefit distribution mechanisms than current standards mandate.
Translational Potential for Clinical Applications
The translation of organ chip technology for pediatric metabolic disorders into clinical applications represents a significant opportunity to address unmet medical needs. Current clinical approaches for these disorders often rely on animal models that inadequately represent human pediatric physiology, leading to high failure rates in drug development and limited therapeutic options.
Organ chips using age-matched cell sources offer a direct pathway to personalized medicine for pediatric patients. By incorporating patient-derived cells into these microfluidic systems, clinicians could test multiple treatment options on a patient's "avatar" before administering them to the child, potentially reducing adverse effects and improving therapeutic outcomes. This approach is particularly valuable for rare metabolic disorders where large clinical trials are challenging to conduct.
Regulatory agencies have shown increasing interest in organ chip technologies as complementary tools for drug development. The FDA's Modernization Act 2.0 supports alternative testing methods, creating a favorable regulatory environment for the clinical adoption of organ chip data. For pediatric applications specifically, these platforms could help satisfy regulatory requirements for pediatric drug testing while minimizing ethical concerns associated with clinical trials in children.
Healthcare economics also supports the translation of this technology. While initial implementation costs may be high, the potential reduction in failed treatments, hospitalization time, and long-term complications could yield substantial cost savings for healthcare systems. Preliminary economic models suggest that personalized treatment approaches using organ chips could reduce overall treatment costs by 15-30% for complex metabolic disorders.
The clinical implementation pathway would likely begin with specialized pediatric metabolic centers serving as early adopters. These centers could utilize organ chips for diagnostic purposes and treatment optimization, gradually expanding to broader clinical settings as the technology matures. Collaborative networks between academic medical centers, biotechnology companies, and regulatory bodies will be essential to establish standardized protocols for clinical interpretation of organ chip data.
Challenges to clinical translation include the need for validation studies comparing organ chip predictions with actual clinical outcomes, development of standardized manufacturing processes that meet clinical-grade requirements, and integration with existing clinical workflows. Additionally, reimbursement models will need to evolve to accommodate this novel diagnostic and treatment planning approach.
Organ chips using age-matched cell sources offer a direct pathway to personalized medicine for pediatric patients. By incorporating patient-derived cells into these microfluidic systems, clinicians could test multiple treatment options on a patient's "avatar" before administering them to the child, potentially reducing adverse effects and improving therapeutic outcomes. This approach is particularly valuable for rare metabolic disorders where large clinical trials are challenging to conduct.
Regulatory agencies have shown increasing interest in organ chip technologies as complementary tools for drug development. The FDA's Modernization Act 2.0 supports alternative testing methods, creating a favorable regulatory environment for the clinical adoption of organ chip data. For pediatric applications specifically, these platforms could help satisfy regulatory requirements for pediatric drug testing while minimizing ethical concerns associated with clinical trials in children.
Healthcare economics also supports the translation of this technology. While initial implementation costs may be high, the potential reduction in failed treatments, hospitalization time, and long-term complications could yield substantial cost savings for healthcare systems. Preliminary economic models suggest that personalized treatment approaches using organ chips could reduce overall treatment costs by 15-30% for complex metabolic disorders.
The clinical implementation pathway would likely begin with specialized pediatric metabolic centers serving as early adopters. These centers could utilize organ chips for diagnostic purposes and treatment optimization, gradually expanding to broader clinical settings as the technology matures. Collaborative networks between academic medical centers, biotechnology companies, and regulatory bodies will be essential to establish standardized protocols for clinical interpretation of organ chip data.
Challenges to clinical translation include the need for validation studies comparing organ chip predictions with actual clinical outcomes, development of standardized manufacturing processes that meet clinical-grade requirements, and integration with existing clinical workflows. Additionally, reimbursement models will need to evolve to accommodate this novel diagnostic and treatment planning approach.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!