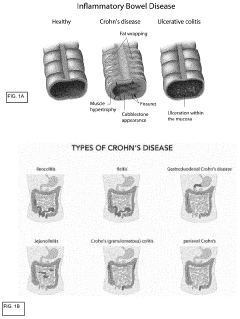
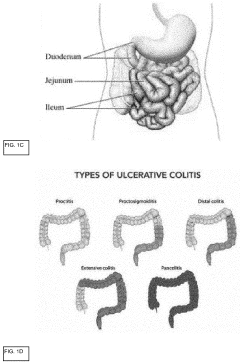
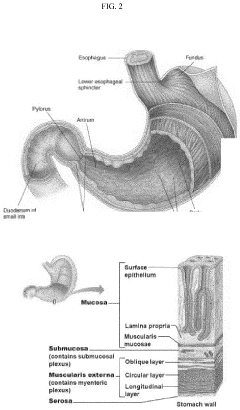
Development of label-free impedance sensors for continuous barrier integrity monitoring in gut-on-chip
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Gut-on-Chip Impedance Sensing Background and Objectives
Gut-on-chip (GoC) technology has emerged as a revolutionary platform in biomedical research over the past decade, offering unprecedented capabilities for modeling the human intestinal environment in vitro. This microfluidic system replicates the dynamic mechanical and biochemical microenvironment of the human gut, enabling more physiologically relevant studies compared to traditional cell culture methods. The integration of this technology with real-time monitoring systems represents a critical advancement in the field of organ-on-chip research.
The evolution of gut barrier integrity monitoring has progressed from end-point destructive assays to non-invasive continuous monitoring techniques. Early approaches relied heavily on fluorescent markers and permeability assays that provided only snapshots of barrier function at specific timepoints. The technological trajectory has steadily moved toward label-free methods that allow for continuous assessment without disrupting the cellular microenvironment or introducing potentially confounding factors.
Impedance sensing technology has emerged as a particularly promising approach for monitoring epithelial barrier integrity. This technique measures electrical resistance across cellular monolayers, providing real-time data on tight junction functionality and overall barrier health. The principles of transepithelial/transendothelial electrical resistance (TEER) measurement have been established for decades in traditional cell culture systems but adapting these principles to the microscale dimensions and dynamic conditions of gut-on-chip platforms presents unique technical challenges.
The primary objective of developing label-free impedance sensors for gut-on-chip platforms is to establish a non-invasive, continuous monitoring system that can detect subtle changes in intestinal barrier function in real-time. This capability would significantly enhance the utility of gut-on-chip models for studying intestinal pathophysiology, drug absorption, and toxicity screening. Furthermore, such technology aims to provide quantitative metrics for assessing the impact of various stimuli on gut barrier integrity, including exposure to pathogens, inflammatory mediators, and therapeutic compounds.
Additional technical goals include miniaturization of sensing components, integration with existing microfluidic architectures, reduction of measurement artifacts, and development of automated data acquisition and analysis systems. The ultimate aim is to create a standardized, reliable platform that can be widely adopted by researchers and pharmaceutical companies for advanced intestinal modeling and drug development applications, potentially reducing reliance on animal models and accelerating the translation of research findings to clinical applications.
The evolution of gut barrier integrity monitoring has progressed from end-point destructive assays to non-invasive continuous monitoring techniques. Early approaches relied heavily on fluorescent markers and permeability assays that provided only snapshots of barrier function at specific timepoints. The technological trajectory has steadily moved toward label-free methods that allow for continuous assessment without disrupting the cellular microenvironment or introducing potentially confounding factors.
Impedance sensing technology has emerged as a particularly promising approach for monitoring epithelial barrier integrity. This technique measures electrical resistance across cellular monolayers, providing real-time data on tight junction functionality and overall barrier health. The principles of transepithelial/transendothelial electrical resistance (TEER) measurement have been established for decades in traditional cell culture systems but adapting these principles to the microscale dimensions and dynamic conditions of gut-on-chip platforms presents unique technical challenges.
The primary objective of developing label-free impedance sensors for gut-on-chip platforms is to establish a non-invasive, continuous monitoring system that can detect subtle changes in intestinal barrier function in real-time. This capability would significantly enhance the utility of gut-on-chip models for studying intestinal pathophysiology, drug absorption, and toxicity screening. Furthermore, such technology aims to provide quantitative metrics for assessing the impact of various stimuli on gut barrier integrity, including exposure to pathogens, inflammatory mediators, and therapeutic compounds.
Additional technical goals include miniaturization of sensing components, integration with existing microfluidic architectures, reduction of measurement artifacts, and development of automated data acquisition and analysis systems. The ultimate aim is to create a standardized, reliable platform that can be widely adopted by researchers and pharmaceutical companies for advanced intestinal modeling and drug development applications, potentially reducing reliance on animal models and accelerating the translation of research findings to clinical applications.
Market Analysis for Label-Free Barrier Integrity Monitoring
The global market for label-free impedance sensors in gut-on-chip applications is experiencing significant growth, driven by increasing demand for more physiologically relevant in vitro models in drug development and toxicology testing. The market size for organ-on-chip technologies was valued at approximately $103 million in 2022 and is projected to reach $594 million by 2030, with gut-on-chip devices representing a substantial segment of this market.
Label-free barrier integrity monitoring addresses critical needs in pharmaceutical research, where traditional cell culture models fail to accurately predict drug absorption and toxicity in the human intestine. This technology enables real-time, continuous assessment of intestinal barrier function without disrupting the cellular environment, providing more reliable data for drug permeability studies and reducing the need for animal testing.
The primary market segments for this technology include pharmaceutical companies conducting preclinical drug development, academic research institutions studying intestinal physiology and disease, and contract research organizations offering specialized testing services. Pharmaceutical companies represent the largest market share, as they seek more predictive models to reduce late-stage drug failures related to absorption, distribution, metabolism, and excretion (ADME) issues.
Regionally, North America dominates the market due to substantial R&D investments and the presence of major pharmaceutical companies and research institutions. Europe follows closely, with significant growth observed in countries with strong biomedical research sectors such as Germany, the UK, and Switzerland. The Asia-Pacific region, particularly China, Japan, and South Korea, is emerging as the fastest-growing market due to increasing investment in life sciences research and pharmaceutical development.
Key market drivers include the push to reduce animal testing through the development of alternative methods, regulatory support for organ-on-chip technologies, and the need for more predictive preclinical models to address the high failure rate of drugs in clinical trials. The FDA's Predictive Toxicology Roadmap and similar initiatives in Europe have created a favorable regulatory environment for the adoption of these technologies.
Market challenges include the relatively high cost of gut-on-chip systems compared to traditional cell culture methods, technical complexity requiring specialized expertise, and the need for standardization and validation to ensure reproducibility across different laboratories. Additionally, integration with existing laboratory workflows and data management systems presents practical barriers to widespread adoption.
Despite these challenges, the market outlook remains positive, with a compound annual growth rate (CAGR) of approximately 17% projected through 2030. This growth is supported by ongoing technological advancements, increasing validation studies demonstrating the predictive power of gut-on-chip models, and growing acceptance within the pharmaceutical industry as a valuable tool for drug development and safety assessment.
Label-free barrier integrity monitoring addresses critical needs in pharmaceutical research, where traditional cell culture models fail to accurately predict drug absorption and toxicity in the human intestine. This technology enables real-time, continuous assessment of intestinal barrier function without disrupting the cellular environment, providing more reliable data for drug permeability studies and reducing the need for animal testing.
The primary market segments for this technology include pharmaceutical companies conducting preclinical drug development, academic research institutions studying intestinal physiology and disease, and contract research organizations offering specialized testing services. Pharmaceutical companies represent the largest market share, as they seek more predictive models to reduce late-stage drug failures related to absorption, distribution, metabolism, and excretion (ADME) issues.
Regionally, North America dominates the market due to substantial R&D investments and the presence of major pharmaceutical companies and research institutions. Europe follows closely, with significant growth observed in countries with strong biomedical research sectors such as Germany, the UK, and Switzerland. The Asia-Pacific region, particularly China, Japan, and South Korea, is emerging as the fastest-growing market due to increasing investment in life sciences research and pharmaceutical development.
Key market drivers include the push to reduce animal testing through the development of alternative methods, regulatory support for organ-on-chip technologies, and the need for more predictive preclinical models to address the high failure rate of drugs in clinical trials. The FDA's Predictive Toxicology Roadmap and similar initiatives in Europe have created a favorable regulatory environment for the adoption of these technologies.
Market challenges include the relatively high cost of gut-on-chip systems compared to traditional cell culture methods, technical complexity requiring specialized expertise, and the need for standardization and validation to ensure reproducibility across different laboratories. Additionally, integration with existing laboratory workflows and data management systems presents practical barriers to widespread adoption.
Despite these challenges, the market outlook remains positive, with a compound annual growth rate (CAGR) of approximately 17% projected through 2030. This growth is supported by ongoing technological advancements, increasing validation studies demonstrating the predictive power of gut-on-chip models, and growing acceptance within the pharmaceutical industry as a valuable tool for drug development and safety assessment.
Current Challenges in Gut-on-Chip Impedance Sensing
Despite significant advancements in gut-on-chip technology, impedance sensing for continuous barrier integrity monitoring faces several critical challenges that impede widespread adoption and reliable implementation. The integration of electrodes into microfluidic systems presents complex fabrication issues, as traditional photolithography techniques often struggle with the three-dimensional structures of organ-on-chip platforms. Electrode materials must balance conductivity, biocompatibility, and stability in culture conditions, with noble metals like gold and platinum being preferred but adding significant cost to manufacturing.
Signal quality remains a persistent challenge, with measurements frequently compromised by background noise from cellular activities, media composition fluctuations, and environmental factors. The small signal changes associated with barrier integrity alterations can be easily obscured by these noise sources, necessitating sophisticated signal processing algorithms that are not yet standardized across the field.
Electrode fouling represents another significant obstacle, as prolonged exposure to cell culture media and biological materials leads to protein adsorption and cellular debris accumulation on electrode surfaces. This biofouling progressively degrades sensor performance and introduces measurement drift, complicating long-term monitoring applications that are essential for chronic disease modeling.
Spatial resolution limitations constrain current impedance sensing approaches, as most systems can only provide averaged measurements across the entire barrier rather than detecting localized defects. This limitation is particularly problematic when studying heterogeneous barrier disruption patterns that occur in many intestinal pathologies, where focal lesions may develop before widespread barrier breakdown.
The correlation between electrical impedance measurements and actual biological barrier function remains incompletely understood. While transepithelial/transendothelial electrical resistance (TEER) is widely used as a surrogate marker for barrier integrity, its relationship with specific molecular transport mechanisms and paracellular permeability requires further validation across different experimental conditions and cell types.
Standardization issues persist throughout the field, with various electrode configurations, measurement frequencies, and data analysis methods being employed across different research groups. This lack of standardization complicates cross-study comparisons and hinders the establishment of reference values for normal and compromised barrier function in gut-on-chip models.
Integration with other sensing modalities represents an emerging challenge, as comprehensive barrier function assessment ideally requires simultaneous monitoring of multiple parameters beyond electrical impedance, including molecular permeability, secretory function, and immune cell interactions. Developing multiplexed sensing platforms that maintain the advantages of label-free impedance measurements while incorporating complementary data streams remains technically demanding.
Signal quality remains a persistent challenge, with measurements frequently compromised by background noise from cellular activities, media composition fluctuations, and environmental factors. The small signal changes associated with barrier integrity alterations can be easily obscured by these noise sources, necessitating sophisticated signal processing algorithms that are not yet standardized across the field.
Electrode fouling represents another significant obstacle, as prolonged exposure to cell culture media and biological materials leads to protein adsorption and cellular debris accumulation on electrode surfaces. This biofouling progressively degrades sensor performance and introduces measurement drift, complicating long-term monitoring applications that are essential for chronic disease modeling.
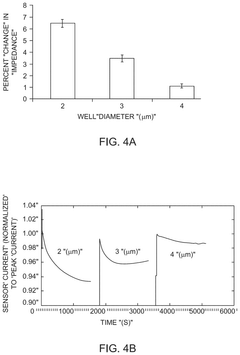
Spatial resolution limitations constrain current impedance sensing approaches, as most systems can only provide averaged measurements across the entire barrier rather than detecting localized defects. This limitation is particularly problematic when studying heterogeneous barrier disruption patterns that occur in many intestinal pathologies, where focal lesions may develop before widespread barrier breakdown.
The correlation between electrical impedance measurements and actual biological barrier function remains incompletely understood. While transepithelial/transendothelial electrical resistance (TEER) is widely used as a surrogate marker for barrier integrity, its relationship with specific molecular transport mechanisms and paracellular permeability requires further validation across different experimental conditions and cell types.
Standardization issues persist throughout the field, with various electrode configurations, measurement frequencies, and data analysis methods being employed across different research groups. This lack of standardization complicates cross-study comparisons and hinders the establishment of reference values for normal and compromised barrier function in gut-on-chip models.
Integration with other sensing modalities represents an emerging challenge, as comprehensive barrier function assessment ideally requires simultaneous monitoring of multiple parameters beyond electrical impedance, including molecular permeability, secretory function, and immune cell interactions. Developing multiplexed sensing platforms that maintain the advantages of label-free impedance measurements while incorporating complementary data streams remains technically demanding.
Existing Label-Free Impedance Sensing Approaches
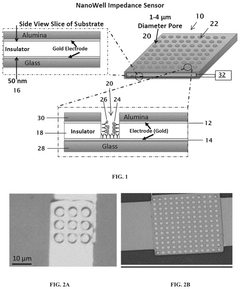
01 Label-free impedance sensing for barrier integrity monitoring
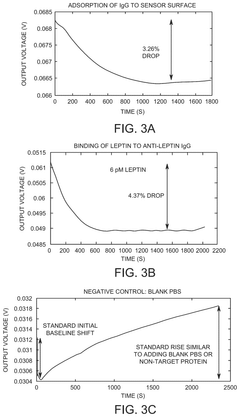
Label-free impedance sensors can be used to monitor barrier integrity in various systems by measuring electrical impedance changes across barriers. These sensors detect changes in resistance or capacitance when barrier integrity is compromised, without requiring fluorescent or radioactive labels. This technology enables real-time, non-invasive monitoring of barrier function in biological tissues, cell cultures, and other barrier systems.- Label-free impedance sensing for cellular barrier integrity: Label-free impedance sensors can be used to monitor the integrity of cellular barriers by measuring electrical impedance across cell monolayers. These systems detect changes in barrier function without the need for fluorescent or radioactive labels, allowing for real-time, non-invasive monitoring of barrier integrity in various cell types including epithelial and endothelial cells. The technology enables detection of barrier disruption caused by toxins, pathogens, or pharmaceutical compounds.
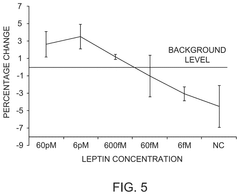
- Microelectrode arrays for impedance-based barrier monitoring: Specialized microelectrode array systems have been developed for impedance-based monitoring of barrier integrity. These systems utilize patterned electrode arrays on substrates where cells are cultured, allowing for precise measurement of transepithelial/transendothelial electrical resistance (TEER). The microelectrode configuration enables spatial resolution of barrier function across the cell layer and can detect localized changes in barrier integrity that might be missed by traditional methods.
- Automated systems for continuous barrier integrity monitoring: Automated impedance-based systems enable continuous, long-term monitoring of barrier integrity in laboratory and industrial settings. These systems incorporate data acquisition hardware, analysis software, and sometimes environmental control components to maintain optimal conditions for cell cultures. The automation allows for high-throughput screening of compounds that might affect barrier function and can generate alerts when barrier integrity is compromised beyond set thresholds.
- Wireless and portable impedance sensors for barrier monitoring: Wireless and portable impedance sensing technologies have been developed for monitoring barrier integrity in various settings, including point-of-care applications. These systems utilize miniaturized electronics and wireless data transmission to enable remote monitoring of barrier function. The portable nature of these devices allows for barrier integrity assessment in field conditions or clinical settings where traditional laboratory equipment is not available.
- Integration of impedance sensing with other analytical techniques: Advanced barrier monitoring systems integrate impedance sensing with complementary analytical techniques to provide comprehensive assessment of barrier function. These integrated platforms may combine impedance measurements with optical imaging, molecular analysis, or microfluidic systems. The multi-modal approach enables correlation between electrical barrier properties and other cellular parameters, providing deeper insights into barrier physiology and pathology.
02 Cell-based barrier integrity assessment systems
Impedance-based systems for monitoring cellular barrier integrity use electrodes to measure transepithelial/transendothelial electrical resistance (TEER) across cell monolayers. These systems can detect subtle changes in tight junction formation, cell-cell adhesion, and barrier function in response to various stimuli. Applications include drug permeability studies, toxicity testing, and disease modeling using cell culture models of biological barriers such as intestinal, pulmonary, or blood-brain barriers.Expand Specific Solutions03 Microfluidic and chip-based impedance sensing platforms
Microfluidic devices and chip-based platforms incorporate impedance sensors for barrier integrity monitoring in miniaturized systems. These platforms integrate electrodes within microchannels or chambers to measure impedance across cellular barriers under flow conditions. The technology enables high-throughput screening, reduced sample volumes, and more physiologically relevant barrier models compared to traditional static systems.Expand Specific Solutions04 Environmental and structural barrier monitoring applications
Impedance sensors are applied to monitor integrity of non-biological barriers such as building structures, environmental containment systems, and protective barriers. These sensors can detect breaches, moisture intrusion, or structural changes that compromise barrier function. The technology enables continuous monitoring of critical infrastructure and early detection of potential failures in protective systems.Expand Specific Solutions05 Advanced data processing for impedance-based barrier monitoring
Sophisticated algorithms and data processing techniques enhance the capabilities of impedance-based barrier monitoring systems. These approaches include machine learning for pattern recognition, signal processing to filter noise, and statistical methods to identify significant changes in barrier integrity. Advanced data processing improves sensitivity, specificity, and predictive capabilities of impedance measurements for barrier integrity assessment.Expand Specific Solutions
Leading Organizations in Gut-on-Chip Sensor Development
The development of label-free impedance sensors for gut-on-chip barrier integrity monitoring is in an emerging growth phase, with market size expanding as organ-on-chip technologies gain traction in drug development and toxicology testing. The competitive landscape features established players like Emulate, Inc., which specializes in organ-on-chip platforms, alongside major instrumentation companies including Agilent Technologies and Molecular Devices that bring expertise in analytical measurement systems. Academic institutions such as Harvard, University of Pennsylvania, and Nanyang Technological University are driving fundamental research innovations. Technology maturity remains moderate, with companies like Corning contributing materials expertise and Taiwan Semiconductor Manufacturing providing microelectronics capabilities essential for sensor miniaturization and integration. The field represents a convergence of microfluidics, biosensing, and semiconductor technologies advancing toward commercial applications.
President & Fellows of Harvard College
Technical Solution: Harvard has developed advanced label-free impedance sensing technology for gut-on-chip platforms that utilizes transepithelial electrical resistance (TEER) measurements to continuously monitor intestinal barrier integrity. Their approach integrates microelectrodes directly into the microfluidic device architecture, allowing for real-time, non-invasive monitoring of epithelial barrier function. The system employs four-point electrode configurations to minimize electrode polarization effects and improve measurement accuracy. Harvard's technology incorporates automated impedance spectroscopy across multiple frequency ranges (100Hz-1MHz) to distinguish between paracellular and transcellular pathways of ion transport, providing deeper insights into barrier function dynamics. Their platform has been validated with intestinal epithelial models exposed to various inflammatory stimuli and pharmaceutical compounds, demonstrating sensitivity to detect subtle changes in barrier integrity before morphological changes become apparent[1][3].
Strengths: Superior sensitivity for detecting early barrier disruption; non-invasive continuous monitoring capability; validated with pharmaceutical compounds for toxicity screening. Weaknesses: Requires specialized microfabrication techniques; potential for electrode fouling during long-term culture; higher implementation complexity compared to end-point assays.
Corning, Inc.
Technical Solution: Corning has developed a label-free impedance sensing technology for gut-on-chip applications that builds upon their established Electric Cell-substrate Impedance Sensing (ECIS) expertise. Their approach integrates transparent indium tin oxide (ITO) electrodes into microfluidic gut-on-chip devices, enabling simultaneous impedance measurements and optical microscopy. The system features a proprietary electrode coating that enhances cell adhesion while maintaining electrode sensitivity. Corning's technology employs multi-frequency impedance analysis (250Hz-64kHz) to distinguish between changes in barrier function and cell coverage/adhesion. Their platform includes automated calibration protocols that account for temperature fluctuations and medium evaporation, enhancing measurement stability during long-term experiments. The system has been validated with intestinal epithelial models under flow conditions, demonstrating correlation between impedance measurements and permeability of fluorescent tracers. Corning's technology has been applied to study the effects of probiotic bacteria and dietary components on intestinal barrier function in gut-on-chip models[6][8].
Strengths: Integration with established Corning cell culture products; transparent electrode design allowing simultaneous imaging; robust commercial support infrastructure. Weaknesses: Less specialized for gut-specific applications compared to dedicated gut-on-chip systems; limited customization options; higher cost compared to academic solutions.
Critical Patents and Research in Impedance Sensing Technology
Apparatus and Methods For Monitoring Of Biomarkers In Blood
PatentPendingUS20250041866A1
Innovation
- A label-free impedance sensor with a pair of conducting electrodes separated by a gap and an insulator, featuring plural wells to expose the other electrode, which modulates impedance in response to the presence of a target analyte, allowing for concentration measurement.
In vitro gastrointestinal model comprising lamina propria-derived cells
PatentInactiveUS20220081663A1
Innovation
- An in vitro microfluidic gut-on-chip system is developed, featuring a multicellular, layered culture of intestinal epithelial cells, lamina propria-derived cells, and endothelial cells, allowing for interactions and simulations of healthy, pre-disease, and diseased states, enabling drug testing and inflammation reduction.
Biocompatibility and Material Science Considerations
The development of label-free impedance sensors for gut-on-chip devices necessitates careful consideration of biocompatibility and material science aspects. Materials used in these sensors must maintain long-term compatibility with biological tissues while preserving their electrical and mechanical properties. Polydimethylsiloxane (PDMS) remains the predominant substrate material due to its optical transparency, gas permeability, and mechanical flexibility, which closely mimics the natural gut environment. However, PDMS presents challenges including hydrophobicity and potential absorption of small hydrophobic molecules, which may interfere with sensor readings over extended monitoring periods.
Electrode materials represent another critical consideration, with gold and platinum being preferred choices due to their excellent biocompatibility and electrochemical stability. Recent advancements have introduced carbon-based electrodes, including graphene and carbon nanotubes, which offer enhanced sensitivity and reduced biofouling compared to traditional metal electrodes. These materials demonstrate superior long-term stability in biological environments while maintaining consistent impedance measurements.
Surface functionalization techniques have evolved to address the interface between sensor materials and biological components. Approaches such as plasma treatment, chemical modification with hydrophilic polymers, and protein coating (e.g., fibronectin, collagen) significantly improve cell adhesion and proliferation on sensor surfaces. These modifications must be carefully optimized to enhance biocompatibility without compromising the electrical properties essential for impedance sensing.
The mechanical properties of materials used in gut-on-chip impedance sensors must accommodate the dynamic nature of the gut microenvironment. Materials should withstand peristaltic-like motions and fluid flow while maintaining structural integrity. Recent research has explored composite materials that combine flexibility with durability, including hydrogel-based substrates that better mimic the extracellular matrix of intestinal tissue.
Sterilization compatibility represents another significant challenge, as sensor materials must withstand common sterilization methods without degradation. Ethylene oxide, gamma irradiation, and autoclave sterilization can potentially alter material properties, affecting sensor performance. Novel approaches utilizing UV-C sterilization and antimicrobial coatings have shown promise in maintaining material integrity while ensuring sterility.
Long-term stability of materials in culture conditions presents ongoing challenges. Continuous exposure to cell culture media, enzymes, and metabolic byproducts can lead to material degradation or surface fouling. Research efforts have focused on developing bioinert coatings and surface treatments that resist protein adsorption and bacterial adhesion, thereby extending sensor lifespan and measurement reliability in continuous monitoring applications.
Electrode materials represent another critical consideration, with gold and platinum being preferred choices due to their excellent biocompatibility and electrochemical stability. Recent advancements have introduced carbon-based electrodes, including graphene and carbon nanotubes, which offer enhanced sensitivity and reduced biofouling compared to traditional metal electrodes. These materials demonstrate superior long-term stability in biological environments while maintaining consistent impedance measurements.
Surface functionalization techniques have evolved to address the interface between sensor materials and biological components. Approaches such as plasma treatment, chemical modification with hydrophilic polymers, and protein coating (e.g., fibronectin, collagen) significantly improve cell adhesion and proliferation on sensor surfaces. These modifications must be carefully optimized to enhance biocompatibility without compromising the electrical properties essential for impedance sensing.
The mechanical properties of materials used in gut-on-chip impedance sensors must accommodate the dynamic nature of the gut microenvironment. Materials should withstand peristaltic-like motions and fluid flow while maintaining structural integrity. Recent research has explored composite materials that combine flexibility with durability, including hydrogel-based substrates that better mimic the extracellular matrix of intestinal tissue.
Sterilization compatibility represents another significant challenge, as sensor materials must withstand common sterilization methods without degradation. Ethylene oxide, gamma irradiation, and autoclave sterilization can potentially alter material properties, affecting sensor performance. Novel approaches utilizing UV-C sterilization and antimicrobial coatings have shown promise in maintaining material integrity while ensuring sterility.
Long-term stability of materials in culture conditions presents ongoing challenges. Continuous exposure to cell culture media, enzymes, and metabolic byproducts can lead to material degradation or surface fouling. Research efforts have focused on developing bioinert coatings and surface treatments that resist protein adsorption and bacterial adhesion, thereby extending sensor lifespan and measurement reliability in continuous monitoring applications.
Regulatory Pathway for Organ-on-Chip Diagnostic Tools
The regulatory landscape for organ-on-chip (OOC) diagnostic tools, particularly those incorporating label-free impedance sensors for gut barrier monitoring, presents a complex pathway requiring strategic navigation. These innovative technologies occupy an intersection between medical devices and in vitro diagnostic tools, necessitating careful consideration of multiple regulatory frameworks.
In the United States, the FDA's regulatory approach to OOC technologies remains evolving, with most systems currently classified under the medical device pathway. Label-free impedance sensors for gut-on-chip would likely fall under Class II medical devices, requiring premarket notification (510(k)) or potentially De Novo classification if deemed sufficiently novel. The FDA's Center for Devices and Radiological Health (CDRH) has established the Emerging Technology Program specifically to facilitate regulatory processes for innovative technologies like impedance-based barrier monitoring systems.
European regulation follows the In Vitro Diagnostic Regulation (IVDR 2017/746) and Medical Device Regulation (MDR 2017/745), which implemented stricter requirements in 2022. Impedance-based gut barrier monitoring systems would require conformity assessment procedures and CE marking, with classification likely as Class C under IVDR due to their monitoring capabilities of potentially critical physiological parameters.
Regulatory validation for these systems presents unique challenges, as traditional clinical validation protocols may not directly apply. Establishing performance standards specifically for impedance-based barrier integrity measurements requires demonstration of correlation between electrical measurements and actual biological barrier function. Regulatory bodies increasingly recognize the need for specialized validation frameworks for OOC technologies, with the FDA-NCATS collaboration developing specific guidance documents.
Quality Management Systems (QMS) compliance represents another critical regulatory component, with manufacturers needing to implement ISO 13485 standards throughout development. For continuous monitoring applications, additional considerations regarding data integrity, security, and management must be addressed to meet regulatory requirements for both the hardware sensors and associated software systems.
International harmonization efforts are gradually emerging through initiatives like the International Medical Device Regulators Forum (IMDRF), which aims to standardize approaches to novel technologies including OOC platforms. However, significant regional variations persist, requiring manufacturers to develop market-specific regulatory strategies, particularly for technologies combining multiple innovative elements like label-free impedance sensing and microfluidic organ models.
In the United States, the FDA's regulatory approach to OOC technologies remains evolving, with most systems currently classified under the medical device pathway. Label-free impedance sensors for gut-on-chip would likely fall under Class II medical devices, requiring premarket notification (510(k)) or potentially De Novo classification if deemed sufficiently novel. The FDA's Center for Devices and Radiological Health (CDRH) has established the Emerging Technology Program specifically to facilitate regulatory processes for innovative technologies like impedance-based barrier monitoring systems.
European regulation follows the In Vitro Diagnostic Regulation (IVDR 2017/746) and Medical Device Regulation (MDR 2017/745), which implemented stricter requirements in 2022. Impedance-based gut barrier monitoring systems would require conformity assessment procedures and CE marking, with classification likely as Class C under IVDR due to their monitoring capabilities of potentially critical physiological parameters.
Regulatory validation for these systems presents unique challenges, as traditional clinical validation protocols may not directly apply. Establishing performance standards specifically for impedance-based barrier integrity measurements requires demonstration of correlation between electrical measurements and actual biological barrier function. Regulatory bodies increasingly recognize the need for specialized validation frameworks for OOC technologies, with the FDA-NCATS collaboration developing specific guidance documents.
Quality Management Systems (QMS) compliance represents another critical regulatory component, with manufacturers needing to implement ISO 13485 standards throughout development. For continuous monitoring applications, additional considerations regarding data integrity, security, and management must be addressed to meet regulatory requirements for both the hardware sensors and associated software systems.
International harmonization efforts are gradually emerging through initiatives like the International Medical Device Regulators Forum (IMDRF), which aims to standardize approaches to novel technologies including OOC platforms. However, significant regional variations persist, requiring manufacturers to develop market-specific regulatory strategies, particularly for technologies combining multiple innovative elements like label-free impedance sensing and microfluidic organ models.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!