Multi-organ chip assessment of nanoparticle biodistribution, clearance, and off-target toxicity
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nanoparticle MOC Technology Background and Objectives
Nanoparticle technology has evolved significantly over the past three decades, transitioning from theoretical concepts to practical applications across multiple industries. The integration of nanoparticles in medical diagnostics and therapeutics represents one of the most promising developments in modern medicine, with applications ranging from targeted drug delivery to enhanced imaging capabilities. However, the biological interactions of these engineered nanomaterials remain incompletely understood, particularly regarding their distribution across multiple organ systems, clearance mechanisms, and potential toxicity profiles.
Traditional in vitro and animal models have provided valuable but limited insights into nanoparticle behavior in biological systems. Single-organ models fail to capture the complex inter-organ interactions that characterize human physiology, while animal models often poorly predict human responses due to species-specific differences in metabolism, immune function, and organ structure. This translational gap has hindered the clinical advancement of numerous promising nanomedicine candidates.
Multi-organ chip (MOC) technology has emerged as a revolutionary platform to address these limitations. These microfluidic devices integrate multiple engineered human tissue constructs within a physiologically relevant environment, enabling the study of complex biological processes in a controlled setting. The technology represents a convergence of advances in microfluidics, tissue engineering, sensors, and computational modeling, creating systems that can mimic aspects of human physiology with unprecedented fidelity.
The primary objective of this technology assessment is to evaluate the current capabilities and limitations of multi-organ chip platforms for studying nanoparticle biodistribution, clearance mechanisms, and off-target toxicity. We aim to determine whether these systems can provide more predictive and mechanistically informative data than conventional testing methods, potentially accelerating the translation of nanomedicines to clinical applications while reducing reliance on animal testing.
Secondary objectives include identifying technological gaps in current MOC systems that limit their utility for nanoparticle assessment, evaluating standardization needs for broader adoption, and exploring how computational approaches might complement experimental data to create more comprehensive predictive models. Additionally, we seek to map the trajectory of MOC technology development and anticipate future innovations that could further enhance their relevance to nanomedicine research and development.
The ultimate goal is to determine whether investment in MOC technology for nanoparticle assessment represents a strategically sound direction for pharmaceutical and biotech companies seeking more efficient, cost-effective, and predictive preclinical testing platforms. This assessment will consider both the technical feasibility and the potential return on investment from implementing these advanced in vitro systems in nanomedicine development pipelines.
Traditional in vitro and animal models have provided valuable but limited insights into nanoparticle behavior in biological systems. Single-organ models fail to capture the complex inter-organ interactions that characterize human physiology, while animal models often poorly predict human responses due to species-specific differences in metabolism, immune function, and organ structure. This translational gap has hindered the clinical advancement of numerous promising nanomedicine candidates.
Multi-organ chip (MOC) technology has emerged as a revolutionary platform to address these limitations. These microfluidic devices integrate multiple engineered human tissue constructs within a physiologically relevant environment, enabling the study of complex biological processes in a controlled setting. The technology represents a convergence of advances in microfluidics, tissue engineering, sensors, and computational modeling, creating systems that can mimic aspects of human physiology with unprecedented fidelity.
The primary objective of this technology assessment is to evaluate the current capabilities and limitations of multi-organ chip platforms for studying nanoparticle biodistribution, clearance mechanisms, and off-target toxicity. We aim to determine whether these systems can provide more predictive and mechanistically informative data than conventional testing methods, potentially accelerating the translation of nanomedicines to clinical applications while reducing reliance on animal testing.
Secondary objectives include identifying technological gaps in current MOC systems that limit their utility for nanoparticle assessment, evaluating standardization needs for broader adoption, and exploring how computational approaches might complement experimental data to create more comprehensive predictive models. Additionally, we seek to map the trajectory of MOC technology development and anticipate future innovations that could further enhance their relevance to nanomedicine research and development.
The ultimate goal is to determine whether investment in MOC technology for nanoparticle assessment represents a strategically sound direction for pharmaceutical and biotech companies seeking more efficient, cost-effective, and predictive preclinical testing platforms. This assessment will consider both the technical feasibility and the potential return on investment from implementing these advanced in vitro systems in nanomedicine development pipelines.
Market Analysis for Multi-organ Chip Platforms
The multi-organ chip market is experiencing rapid growth, driven by increasing demand for more physiologically relevant in vitro models for drug development and toxicity testing. Currently valued at approximately $38 million in 2023, the market is projected to reach $120 million by 2028, representing a compound annual growth rate (CAGR) of 25.8%. This growth trajectory reflects the significant potential of multi-organ chip platforms to revolutionize preclinical testing methodologies.
Pharmaceutical and biotechnology companies constitute the largest market segment, accounting for nearly 65% of the total market share. These organizations are increasingly adopting multi-organ chip technologies to reduce drug development costs and timelines while improving predictive accuracy. Academic research institutions represent the second-largest market segment at 25%, followed by contract research organizations at 10%.
Geographically, North America dominates the market with approximately 45% share, attributed to substantial research funding, presence of major pharmaceutical companies, and favorable regulatory environment. Europe follows closely at 30%, with significant contributions from countries like Germany, Switzerland, and the United Kingdom. The Asia-Pacific region, particularly China, Japan, and South Korea, is emerging as the fastest-growing market with a CAGR of 30%, driven by increasing R&D investments and government initiatives supporting biotechnology advancement.
The market for multi-organ chips specifically designed for nanoparticle testing represents a specialized but rapidly expanding segment. With nanomedicine market projected to reach $350 billion by 2027, the demand for platforms capable of accurately assessing nanoparticle biodistribution, clearance mechanisms, and potential toxicity is intensifying. Currently, this specialized segment accounts for approximately 18% of the total multi-organ chip market but is expected to grow at an accelerated rate of 32% annually.
Key market drivers include increasing regulatory scrutiny of nanoparticle-based therapeutics, growing concerns about nanomaterial safety, and the need for more predictive preclinical models. The FDA and EMA have both issued guidance documents emphasizing the importance of comprehensive safety assessment for nanomedicines, creating regulatory pressure that favors adoption of advanced testing platforms like multi-organ chips.
Customer pain points in this market include high initial investment costs, technical complexity in operation, and challenges in data interpretation. Additionally, end-users express concerns regarding standardization, validation, and regulatory acceptance of data generated using these platforms. These challenges represent significant market entry barriers but also create opportunities for companies offering comprehensive solutions that address these concerns.
Pharmaceutical and biotechnology companies constitute the largest market segment, accounting for nearly 65% of the total market share. These organizations are increasingly adopting multi-organ chip technologies to reduce drug development costs and timelines while improving predictive accuracy. Academic research institutions represent the second-largest market segment at 25%, followed by contract research organizations at 10%.
Geographically, North America dominates the market with approximately 45% share, attributed to substantial research funding, presence of major pharmaceutical companies, and favorable regulatory environment. Europe follows closely at 30%, with significant contributions from countries like Germany, Switzerland, and the United Kingdom. The Asia-Pacific region, particularly China, Japan, and South Korea, is emerging as the fastest-growing market with a CAGR of 30%, driven by increasing R&D investments and government initiatives supporting biotechnology advancement.
The market for multi-organ chips specifically designed for nanoparticle testing represents a specialized but rapidly expanding segment. With nanomedicine market projected to reach $350 billion by 2027, the demand for platforms capable of accurately assessing nanoparticle biodistribution, clearance mechanisms, and potential toxicity is intensifying. Currently, this specialized segment accounts for approximately 18% of the total multi-organ chip market but is expected to grow at an accelerated rate of 32% annually.
Key market drivers include increasing regulatory scrutiny of nanoparticle-based therapeutics, growing concerns about nanomaterial safety, and the need for more predictive preclinical models. The FDA and EMA have both issued guidance documents emphasizing the importance of comprehensive safety assessment for nanomedicines, creating regulatory pressure that favors adoption of advanced testing platforms like multi-organ chips.
Customer pain points in this market include high initial investment costs, technical complexity in operation, and challenges in data interpretation. Additionally, end-users express concerns regarding standardization, validation, and regulatory acceptance of data generated using these platforms. These challenges represent significant market entry barriers but also create opportunities for companies offering comprehensive solutions that address these concerns.
Current Challenges in Nanoparticle Toxicity Assessment
The assessment of nanoparticle toxicity faces significant challenges due to the complex interactions between nanomaterials and biological systems. Traditional toxicity testing methods often fail to capture the unique properties of nanoparticles, including their size-dependent behavior, surface characteristics, and tendency to aggregate in biological environments. These limitations have led to inconsistent and sometimes contradictory results across studies, hampering the development of standardized safety protocols.
One major challenge is the lack of physiologically relevant in vitro models that can accurately predict in vivo responses. Conventional cell culture systems are typically two-dimensional and fail to replicate the complex three-dimensional architecture of human tissues. This discrepancy leads to poor correlation between laboratory findings and clinical outcomes, particularly when assessing long-term effects and chronic exposure scenarios.
The biodistribution of nanoparticles presents another significant hurdle. Nanoparticles can cross biological barriers that are impermeable to larger particles, including the blood-brain barrier and placental barrier. Current assessment methods struggle to track these distribution patterns accurately, especially when considering secondary organ accumulation and clearance pathways that may occur days or weeks after initial exposure.
Inter-laboratory variability further complicates toxicity assessment. Differences in nanoparticle synthesis, characterization techniques, dosing protocols, and biological models lead to poor reproducibility across research groups. The absence of standardized reference materials and validated testing procedures exacerbates this issue, making it difficult to compare results and establish definitive safety thresholds.
The translation of dose metrics from in vitro to in vivo settings remains problematic. Traditional mass-based dosing fails to account for the unique surface area and particle number considerations that are critical for nanomaterials. Additionally, the dynamic nature of nanoparticles in biological media—including protein corona formation, dissolution, and agglomeration—can dramatically alter their toxicological profile during experiments.
Regulatory frameworks have struggled to keep pace with nanotechnology advancements. Current guidelines often apply conventional toxicology approaches that may not adequately address nano-specific concerns. The diversity of nanomaterials, with variations in composition, size, shape, and surface modifications, creates a vast parameter space that cannot be comprehensively evaluated using existing regulatory paradigms.
Emerging evidence suggests that nanoparticles may induce subtle biological effects that are not captured by standard toxicity endpoints. These include alterations to the immune system, disruption of the microbiome, epigenetic changes, and potential developmental effects that may manifest only after prolonged exposure or in subsequent generations.
One major challenge is the lack of physiologically relevant in vitro models that can accurately predict in vivo responses. Conventional cell culture systems are typically two-dimensional and fail to replicate the complex three-dimensional architecture of human tissues. This discrepancy leads to poor correlation between laboratory findings and clinical outcomes, particularly when assessing long-term effects and chronic exposure scenarios.
The biodistribution of nanoparticles presents another significant hurdle. Nanoparticles can cross biological barriers that are impermeable to larger particles, including the blood-brain barrier and placental barrier. Current assessment methods struggle to track these distribution patterns accurately, especially when considering secondary organ accumulation and clearance pathways that may occur days or weeks after initial exposure.
Inter-laboratory variability further complicates toxicity assessment. Differences in nanoparticle synthesis, characterization techniques, dosing protocols, and biological models lead to poor reproducibility across research groups. The absence of standardized reference materials and validated testing procedures exacerbates this issue, making it difficult to compare results and establish definitive safety thresholds.
The translation of dose metrics from in vitro to in vivo settings remains problematic. Traditional mass-based dosing fails to account for the unique surface area and particle number considerations that are critical for nanomaterials. Additionally, the dynamic nature of nanoparticles in biological media—including protein corona formation, dissolution, and agglomeration—can dramatically alter their toxicological profile during experiments.
Regulatory frameworks have struggled to keep pace with nanotechnology advancements. Current guidelines often apply conventional toxicology approaches that may not adequately address nano-specific concerns. The diversity of nanomaterials, with variations in composition, size, shape, and surface modifications, creates a vast parameter space that cannot be comprehensively evaluated using existing regulatory paradigms.
Emerging evidence suggests that nanoparticles may induce subtle biological effects that are not captured by standard toxicity endpoints. These include alterations to the immune system, disruption of the microbiome, epigenetic changes, and potential developmental effects that may manifest only after prolonged exposure or in subsequent generations.
Existing Multi-organ Chip Assessment Methodologies
01 Multi-organ chip platforms for toxicity assessment
Multi-organ chip platforms integrate multiple tissue types to simulate organ interactions for toxicity testing. These systems allow for the assessment of compound toxicity across different organ systems simultaneously, providing more physiologically relevant data than single-organ models. The platforms can detect organ-specific toxicity, systemic effects, and potential adverse reactions that might not be apparent in conventional testing methods.- Multi-organ chip platforms for toxicity assessment: Multi-organ chip platforms enable integrated toxicity assessment by mimicking the interactions between different organ systems. These platforms allow for the evaluation of compound toxicity across multiple tissues simultaneously, providing more physiologically relevant data than single-organ models. The chips incorporate microfluidic systems that connect different organ compartments, allowing for the circulation of test compounds and observation of their effects on various tissues, which is crucial for comprehensive toxicity profiling.
- Biodistribution analysis using microfluidic systems: Microfluidic systems in multi-organ chips enable real-time monitoring of compound biodistribution across different tissue types. These systems utilize various detection methods including fluorescence imaging, mass spectrometry, and biosensors to track how compounds distribute, accumulate, and transfer between organ compartments. The ability to observe biodistribution patterns in an interconnected system provides insights into tissue-specific uptake and compound targeting that cannot be achieved with traditional in vitro or animal models.
- Clearance mechanisms and pharmacokinetic modeling: Multi-organ chips incorporate functional metabolic organs like liver and kidney models to study compound clearance mechanisms. These systems allow researchers to observe metabolic transformations, excretion pathways, and elimination rates in a controlled environment. By integrating sensors and analytical techniques, the chips enable continuous monitoring of compound concentrations and metabolites, facilitating the development of pharmacokinetic models that can predict clearance profiles in humans with greater accuracy than conventional methods.
- Integration of biosensors for real-time monitoring: Advanced biosensors integrated into multi-organ chips allow for real-time monitoring of cellular responses to test compounds. These sensors can detect changes in cellular metabolism, membrane integrity, electrical activity, and secreted biomarkers that indicate toxicity. The continuous data collection enables researchers to observe the onset and progression of toxic effects across different organ systems simultaneously, providing temporal information about compound safety profiles that is difficult to obtain through traditional endpoint assays.
- Validation and correlation with in vivo data: Methods for validating multi-organ chip results against established in vivo toxicity data ensure the reliability of these platforms for safety assessment. Comparative studies between chip-based assessments and animal or human data help establish predictive correlations and identify the strengths and limitations of the technology. Standardized protocols and reference compounds are used to calibrate multi-organ chip responses, enabling consistent interpretation of results across different laboratories and improving the translational value of the technology for drug development and chemical safety evaluation.
02 Biodistribution analysis using microfluidic systems
Microfluidic systems enable real-time monitoring of compound biodistribution across multiple organ compartments. These systems utilize sensors and imaging techniques to track how substances distribute between different tissues, providing insights into absorption, distribution patterns, and tissue-specific accumulation. The technology allows for continuous monitoring without disrupting the system, offering dynamic biodistribution data rather than static endpoints.Expand Specific Solutions03 Clearance mechanisms and pharmacokinetic modeling
Multi-organ chips incorporate clearance mechanisms to mimic metabolic elimination pathways found in the body. These systems often include liver and kidney components to replicate first-pass metabolism and excretion processes. The integrated approach allows for more accurate pharmacokinetic modeling by accounting for metabolite formation and elimination rates across connected organ systems, providing better predictions of compound half-life and clearance profiles.Expand Specific Solutions04 Sensor integration for real-time monitoring
Advanced multi-organ chips incorporate integrated sensor systems for continuous monitoring of various parameters. These sensors can detect changes in cellular responses, metabolite production, and biomarkers of toxicity in real-time. The technology enables immediate detection of adverse effects, allowing for temporal correlation between exposure and toxicity onset, which is crucial for understanding the progression of toxic responses across different organ systems.Expand Specific Solutions05 Validation and correlation with in vivo models
Multi-organ chip platforms require validation against established in vivo models to ensure predictive accuracy. Comparative studies between chip-based assessments and animal or clinical data help establish the reliability of these systems for toxicity prediction. Validation protocols typically involve testing well-characterized compounds with known toxicity profiles to demonstrate that the chip can accurately replicate in vivo responses, supporting their use as alternatives to traditional animal testing.Expand Specific Solutions
Leading Organizations in Nanoparticle MOC Research
The multi-organ chip technology for nanoparticle assessment is in an early growth phase, with market size expanding as pharmaceutical and biomedical industries seek better preclinical testing methods. The technology demonstrates moderate maturity, with academic institutions leading research efforts while commercial applications are emerging. Key players include university research centers (Harvard, Vanderbilt, Zhejiang University, Tsinghua Shenzhen International Graduate School) collaborating with specialized companies like Humanase and Suzhou Jiyan Biomedical Technology. Pharmaceutical giants such as Hoffmann-La Roche are investing in this field, while research institutions like the National Center for Nanoscience & Technology provide critical infrastructure. The competitive landscape shows a balance between academic innovation and commercial development, with increasing cross-sector partnerships accelerating technology advancement.
President & Fellows of Harvard College
Technical Solution: Harvard's Wyss Institute has pioneered human Organ-on-a-Chip technology for nanoparticle toxicity assessment. Their multi-organ chip platform integrates microfluidic devices with living human cells to create functional organ models including liver, kidney, lung, and intestine. The system enables real-time monitoring of nanoparticle distribution across multiple organ compartments connected by a circulatory system that mimics blood flow. Their proprietary microengineered technology incorporates mechanical forces to recreate physiological organ functions, allowing for more accurate prediction of nanoparticle behavior than traditional cell cultures or animal models. Harvard researchers have demonstrated the platform's ability to track fluorescently labeled nanoparticles across organ boundaries, measure clearance rates, and detect organ-specific toxicity markers through integrated biosensors and imaging capabilities[1][3]. The system supports extended culture periods (>2 weeks) enabling assessment of both acute and chronic nanoparticle exposure effects.
Strengths: Industry-leading microfluidic engineering expertise; comprehensive physiological modeling including mechanical forces; established commercialization pathway through Emulate, Inc. Weaknesses: Complex system requires specialized expertise to operate; higher cost compared to traditional testing methods; challenges in achieving full physiological representation of all organ interactions.
École Polytechnique Fédérale de Lausanne
Technical Solution: EPFL has developed a precision-engineered multi-organ chip platform for nanoparticle safety assessment featuring microstructured compartments that recreate tissue-specific architectures. Their system incorporates advanced microfluidic valving technology that enables programmable control of inter-organ communication and sampling from specific compartments. EPFL's platform utilizes impedance spectroscopy and integrated optical sensors for real-time, label-free monitoring of cellular responses to nanoparticle exposure. The technology incorporates specialized modules representing key clearance organs (liver, kidney) with physiologically relevant cell ratios and zonation. EPFL researchers have implemented machine learning algorithms that analyze multi-parameter data outputs to identify early markers of nanoparticle toxicity before conventional endpoints become apparent[6]. Their platform includes a simulated blood-brain barrier module with integrated electrical resistance measurement for assessing nanoparticle neurological safety. The system supports co-culture of immune cells with organ-specific cells to evaluate immunological responses to nanoparticles, including inflammation and complement activation. EPFL has validated their platform using a library of nanoparticles with varying physicochemical properties to establish structure-activity relationships for toxicity prediction.
Strengths: Advanced sensor integration provides comprehensive real-time monitoring; sophisticated microfluidic control enables precise inter-organ communication modeling; machine learning integration enhances predictive capabilities. Weaknesses: High technical complexity requires specialized expertise; limited validation with diverse nanoparticle types; challenges in achieving physiological scaling of different organ compartments.
Key Innovations in Nanoparticle Biodistribution Tracking
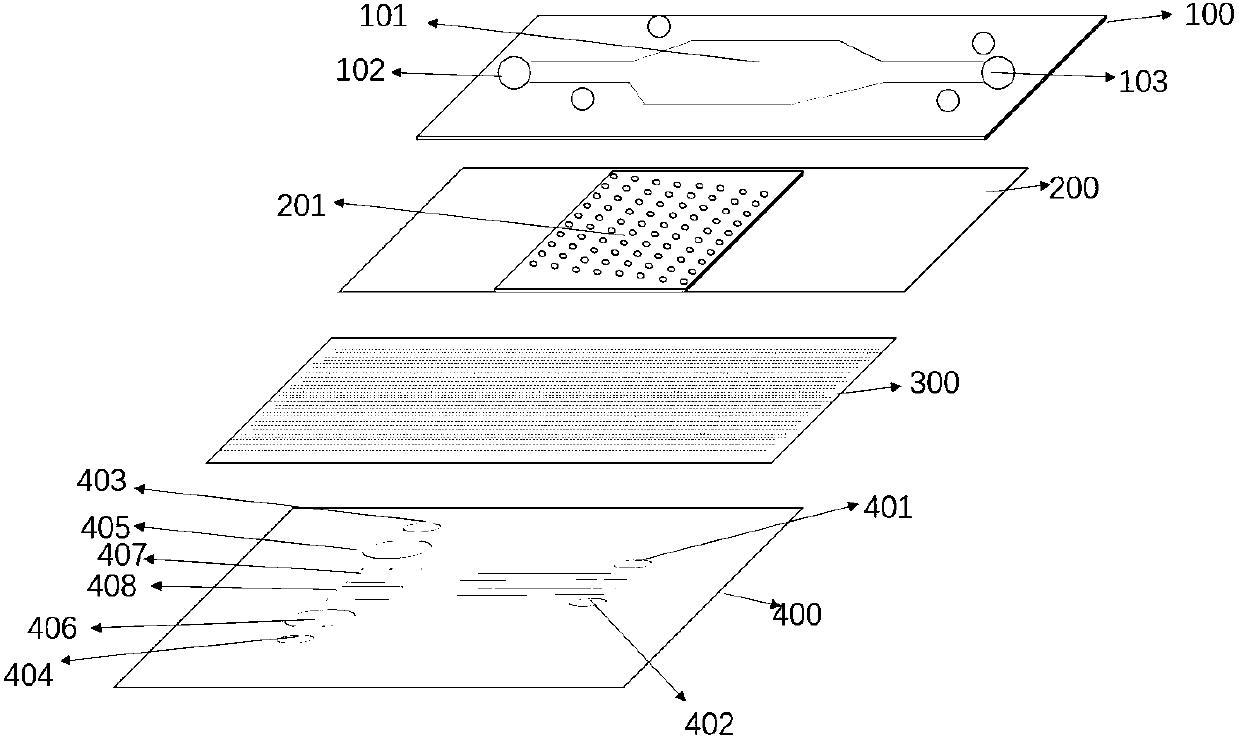
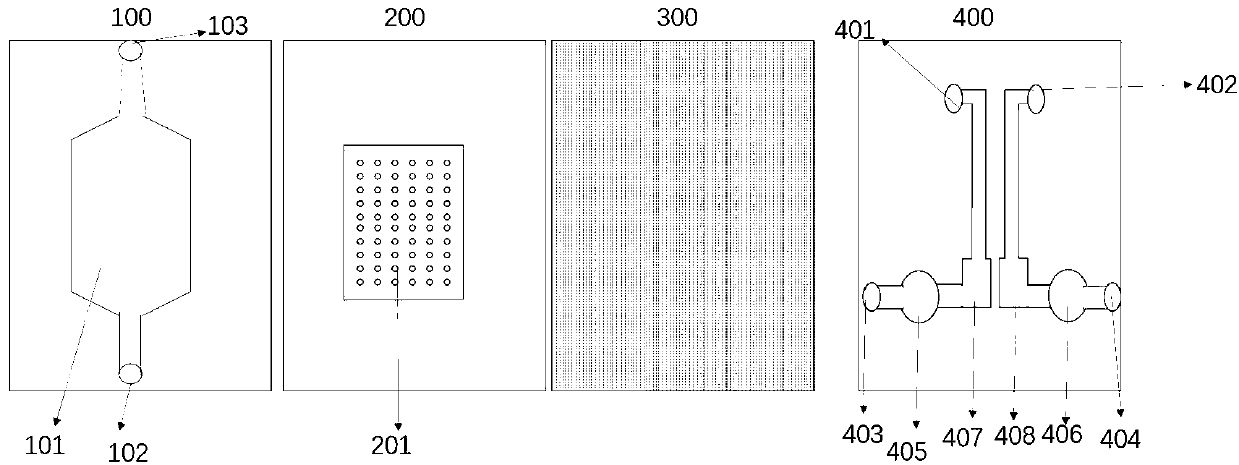
Bionic multi-organ chip and preparation method therefor and application of bionic multi-organ chip
PatentInactiveCN111218404A
Innovation
- A bionic multi-organ chip with a four-layer structure is designed, including a fluid channel layer, a 3D tissue cell culture layer, a porous membrane layer and a fluid channel layer containing a cell culture area. It is prepared through 3D printing and ion sealing technology to achieve a variety of functions. The three-dimensional culture of tissue cells and the exchange of materials between the upper and lower layers simulate the interaction of human organs.
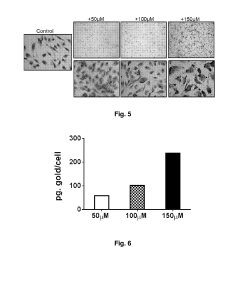
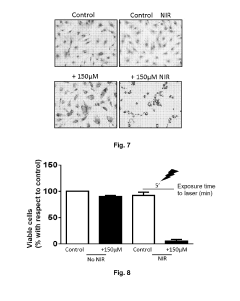
Cells loaded with gold nanoparticles for use in the diagnosis and/or treatment of melanoma
PatentActiveUS20190142980A1
Innovation
- Utilizing endothelial colony-forming cells (ECFC) loaded with gold nanoparticles, which selectively localize in tumor masses and can be marked for diagnostic purposes, allowing for targeted thermoablation while minimizing off-target toxicity and facilitating rapid excretion.
Regulatory Framework for Nanoparticle Safety Evaluation
The regulatory landscape for nanoparticle safety evaluation has evolved significantly in response to the rapid advancement of nanotechnology applications in medicine, particularly in multi-organ chip systems. Currently, regulatory frameworks across major jurisdictions exhibit considerable variation, creating challenges for researchers and manufacturers developing nanoparticle-based therapeutics and diagnostics.
In the United States, the FDA has established a tiered approach to nanoparticle regulation through its Nanotechnology Task Force, which evaluates nanomaterials based on their intended use rather than solely on their physical characteristics. For multi-organ chip assessments specifically, the FDA has published guidance documents addressing the evaluation of nanoparticle biodistribution and clearance pathways, though these remain non-binding recommendations rather than formal regulations.
The European Medicines Agency (EMA) has implemented more stringent requirements through the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation, which mandates comprehensive safety data for nanomaterials. The EMA specifically addresses multi-organ toxicity assessment through its reflection papers on nanotechnology-based medicinal products, emphasizing the need for specialized testing methodologies that can accurately predict in vivo behavior.
International harmonization efforts are being led by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), which is developing standardized protocols for nanoparticle safety evaluation across organ systems. These initiatives aim to establish consistent methodologies for assessing biodistribution patterns and potential off-target effects.
A significant regulatory gap exists in the validation of multi-organ chip platforms themselves as predictive tools for nanoparticle safety assessment. While these systems offer promising alternatives to traditional animal testing, regulatory acceptance requires extensive correlation studies between chip-based results and in vivo outcomes. The OECD Working Party on Manufactured Nanomaterials has been instrumental in developing standardized test guidelines, though specific protocols for multi-organ chip systems remain under development.
Regulatory challenges are particularly pronounced for novel nanoparticle formulations with unique physicochemical properties that may influence biodistribution and clearance kinetics. Current frameworks often struggle to address the complex interactions between nanoparticles and biological systems across multiple organ compartments, necessitating case-by-case evaluation approaches rather than standardized pathways.
Moving forward, regulatory evolution will likely focus on developing adaptive frameworks that can accommodate rapid technological advances while maintaining rigorous safety standards. This includes the potential implementation of adverse outcome pathway (AOP) approaches that link molecular initiating events to adverse outcomes across organ systems, providing a mechanistic basis for regulatory decision-making in nanoparticle safety assessment.
In the United States, the FDA has established a tiered approach to nanoparticle regulation through its Nanotechnology Task Force, which evaluates nanomaterials based on their intended use rather than solely on their physical characteristics. For multi-organ chip assessments specifically, the FDA has published guidance documents addressing the evaluation of nanoparticle biodistribution and clearance pathways, though these remain non-binding recommendations rather than formal regulations.
The European Medicines Agency (EMA) has implemented more stringent requirements through the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation, which mandates comprehensive safety data for nanomaterials. The EMA specifically addresses multi-organ toxicity assessment through its reflection papers on nanotechnology-based medicinal products, emphasizing the need for specialized testing methodologies that can accurately predict in vivo behavior.
International harmonization efforts are being led by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), which is developing standardized protocols for nanoparticle safety evaluation across organ systems. These initiatives aim to establish consistent methodologies for assessing biodistribution patterns and potential off-target effects.
A significant regulatory gap exists in the validation of multi-organ chip platforms themselves as predictive tools for nanoparticle safety assessment. While these systems offer promising alternatives to traditional animal testing, regulatory acceptance requires extensive correlation studies between chip-based results and in vivo outcomes. The OECD Working Party on Manufactured Nanomaterials has been instrumental in developing standardized test guidelines, though specific protocols for multi-organ chip systems remain under development.
Regulatory challenges are particularly pronounced for novel nanoparticle formulations with unique physicochemical properties that may influence biodistribution and clearance kinetics. Current frameworks often struggle to address the complex interactions between nanoparticles and biological systems across multiple organ compartments, necessitating case-by-case evaluation approaches rather than standardized pathways.
Moving forward, regulatory evolution will likely focus on developing adaptive frameworks that can accommodate rapid technological advances while maintaining rigorous safety standards. This includes the potential implementation of adverse outcome pathway (AOP) approaches that link molecular initiating events to adverse outcomes across organ systems, providing a mechanistic basis for regulatory decision-making in nanoparticle safety assessment.
Ethical Implications of MOC Technology in Drug Development
The integration of Multi-Organ Chip (MOC) technology into drug development processes raises significant ethical considerations that must be addressed by researchers, regulatory bodies, and pharmaceutical companies. These advanced in vitro systems, which simulate the complex interactions between multiple human organs, present both opportunities and challenges from an ethical perspective.
The reduction of animal testing represents one of the most compelling ethical advantages of MOC technology. Traditional drug development relies heavily on animal models, raising concerns about animal welfare and suffering. MOCs offer a potential alternative that could substantially decrease the number of animals used in preclinical studies, particularly for nanoparticle toxicity assessment, aligning with the 3Rs principle (Replacement, Reduction, Refinement) in scientific research.
However, the transition to MOC systems introduces new ethical questions regarding human tissue sourcing. The fabrication of these chips often requires human cells or tissues, necessitating clear consent procedures and privacy protections for donors. Ensuring equitable representation of diverse populations in cell sourcing is crucial to develop drugs that are effective across different demographic groups, addressing historical disparities in pharmaceutical research.
Data management and ownership present another ethical dimension. MOC systems generate vast amounts of biological data that may have implications beyond the immediate research context. Establishing transparent protocols for data handling, storage, and sharing becomes essential, particularly when commercial interests intersect with public health objectives. Questions about intellectual property rights and benefit-sharing must be resolved to promote both innovation and equitable access.
The validation and standardization of MOC technologies also raise ethical concerns. As these platforms increasingly influence drug development decisions, ensuring their reliability becomes an ethical imperative. Premature adoption of inadequately validated MOC systems could lead to misleading safety profiles for nanoparticle-based therapeutics, potentially endangering patients in subsequent clinical trials.
Furthermore, MOC technology may create new forms of responsibility in the drug development ecosystem. Researchers must carefully communicate the capabilities and limitations of these systems to prevent misinterpretation of results or overconfidence in their predictive power. Regulatory frameworks need adaptation to appropriately incorporate MOC-derived data in approval processes, balancing innovation with patient safety.
Ultimately, the ethical implementation of MOC technology in nanoparticle assessment requires ongoing dialogue between scientists, ethicists, regulators, and patient advocates. Developing governance structures that evolve alongside technological advancements will be crucial to maximize the benefits while minimizing potential harms of this promising approach to drug development.
The reduction of animal testing represents one of the most compelling ethical advantages of MOC technology. Traditional drug development relies heavily on animal models, raising concerns about animal welfare and suffering. MOCs offer a potential alternative that could substantially decrease the number of animals used in preclinical studies, particularly for nanoparticle toxicity assessment, aligning with the 3Rs principle (Replacement, Reduction, Refinement) in scientific research.
However, the transition to MOC systems introduces new ethical questions regarding human tissue sourcing. The fabrication of these chips often requires human cells or tissues, necessitating clear consent procedures and privacy protections for donors. Ensuring equitable representation of diverse populations in cell sourcing is crucial to develop drugs that are effective across different demographic groups, addressing historical disparities in pharmaceutical research.
Data management and ownership present another ethical dimension. MOC systems generate vast amounts of biological data that may have implications beyond the immediate research context. Establishing transparent protocols for data handling, storage, and sharing becomes essential, particularly when commercial interests intersect with public health objectives. Questions about intellectual property rights and benefit-sharing must be resolved to promote both innovation and equitable access.
The validation and standardization of MOC technologies also raise ethical concerns. As these platforms increasingly influence drug development decisions, ensuring their reliability becomes an ethical imperative. Premature adoption of inadequately validated MOC systems could lead to misleading safety profiles for nanoparticle-based therapeutics, potentially endangering patients in subsequent clinical trials.
Furthermore, MOC technology may create new forms of responsibility in the drug development ecosystem. Researchers must carefully communicate the capabilities and limitations of these systems to prevent misinterpretation of results or overconfidence in their predictive power. Regulatory frameworks need adaptation to appropriately incorporate MOC-derived data in approval processes, balancing innovation with patient safety.
Ultimately, the ethical implementation of MOC technology in nanoparticle assessment requires ongoing dialogue between scientists, ethicists, regulators, and patient advocates. Developing governance structures that evolve alongside technological advancements will be crucial to maximize the benefits while minimizing potential harms of this promising approach to drug development.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!