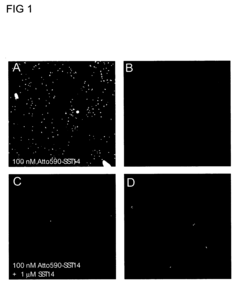
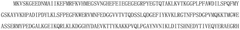High-content imaging pipelines for phenotypic drug screening on cardiac and hepatic chips
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
High-Content Imaging Background and Objectives
High-content imaging (HCI) has emerged as a transformative technology in drug discovery and development, evolving from simple microscopy techniques to sophisticated automated systems capable of capturing and analyzing complex cellular phenotypes. This technology integrates advanced microscopy, automated image acquisition, and computational analysis to extract quantitative data from biological samples at unprecedented scale and resolution. The evolution of HCI has been driven by advances in optical technologies, fluorescent probes, automation systems, and computational algorithms over the past two decades.
The integration of HCI with organ-on-chip technologies represents a significant advancement in preclinical drug screening methodologies. Cardiac and hepatic chips, which mimic the physiological functions of heart and liver tissues respectively, provide more physiologically relevant models compared to traditional 2D cell cultures. When combined with HCI, these platforms enable real-time monitoring of cellular responses to drug compounds in a microenvironment that better recapitulates in vivo conditions.
The primary objective of developing HCI pipelines for phenotypic drug screening on cardiac and hepatic chips is to enhance the predictive power of preclinical drug testing, thereby reducing the high attrition rates in pharmaceutical development. Specifically, these pipelines aim to identify cardiotoxicity and hepatotoxicity—two major causes of drug failure in clinical trials—at earlier stages of drug development.
Technical goals include optimizing image acquisition parameters for 3D tissue structures, developing robust image analysis algorithms capable of extracting meaningful phenotypic data from complex microenvironments, and establishing standardized workflows that ensure reproducibility across different laboratories and experimental conditions.
Another critical objective is to enable longitudinal studies that track cellular responses over extended periods, providing insights into both acute and chronic effects of drug compounds. This requires developing non-invasive imaging techniques that minimize phototoxicity while maintaining high spatial and temporal resolution.
Furthermore, these pipelines aim to facilitate multiparametric analysis, allowing simultaneous assessment of multiple cellular functions and structures. This comprehensive approach provides a more holistic understanding of drug effects compared to traditional single-endpoint assays.
The ultimate goal is to establish HCI pipelines that bridge the gap between in vitro and in vivo drug testing, providing pharmaceutical researchers with tools that can more accurately predict human responses to drug candidates before entering costly clinical trials, thereby accelerating drug development while enhancing patient safety.
The integration of HCI with organ-on-chip technologies represents a significant advancement in preclinical drug screening methodologies. Cardiac and hepatic chips, which mimic the physiological functions of heart and liver tissues respectively, provide more physiologically relevant models compared to traditional 2D cell cultures. When combined with HCI, these platforms enable real-time monitoring of cellular responses to drug compounds in a microenvironment that better recapitulates in vivo conditions.
The primary objective of developing HCI pipelines for phenotypic drug screening on cardiac and hepatic chips is to enhance the predictive power of preclinical drug testing, thereby reducing the high attrition rates in pharmaceutical development. Specifically, these pipelines aim to identify cardiotoxicity and hepatotoxicity—two major causes of drug failure in clinical trials—at earlier stages of drug development.
Technical goals include optimizing image acquisition parameters for 3D tissue structures, developing robust image analysis algorithms capable of extracting meaningful phenotypic data from complex microenvironments, and establishing standardized workflows that ensure reproducibility across different laboratories and experimental conditions.
Another critical objective is to enable longitudinal studies that track cellular responses over extended periods, providing insights into both acute and chronic effects of drug compounds. This requires developing non-invasive imaging techniques that minimize phototoxicity while maintaining high spatial and temporal resolution.
Furthermore, these pipelines aim to facilitate multiparametric analysis, allowing simultaneous assessment of multiple cellular functions and structures. This comprehensive approach provides a more holistic understanding of drug effects compared to traditional single-endpoint assays.
The ultimate goal is to establish HCI pipelines that bridge the gap between in vitro and in vivo drug testing, providing pharmaceutical researchers with tools that can more accurately predict human responses to drug candidates before entering costly clinical trials, thereby accelerating drug development while enhancing patient safety.
Market Analysis for Phenotypic Drug Screening
The phenotypic drug screening market has experienced substantial growth in recent years, driven by the increasing recognition of its advantages over traditional target-based approaches. The global market for phenotypic screening was valued at approximately $1.5 billion in 2022 and is projected to reach $3.2 billion by 2028, representing a compound annual growth rate (CAGR) of 13.5%. This growth trajectory is particularly pronounced in the cardiac and hepatic drug development segments, where organ-on-chip technologies are gaining significant traction.
The demand for high-content imaging pipelines in phenotypic drug screening is primarily fueled by the pharmaceutical industry's need to reduce late-stage drug failures. With the average cost of bringing a new drug to market exceeding $2.6 billion and failure rates in clinical trials remaining high (approximately 90%), there is an urgent need for more predictive preclinical models. Cardiac and hepatic toxicities account for nearly 40% of drug withdrawals from the market, highlighting the critical importance of improved screening methods for these specific organ systems.
Regional market analysis reveals that North America currently dominates the phenotypic screening market with a 42% share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 15.7% during the forecast period, driven by increasing R&D investments and the establishment of new research centers in China, Japan, and South Korea.
Key customer segments include large pharmaceutical companies (45% market share), biotechnology firms (30%), academic research institutions (15%), and contract research organizations (10%). Large pharmaceutical companies are increasingly integrating phenotypic screening into their drug discovery workflows, with 65% of major pharma companies reporting increased investment in these technologies over the past five years.
The economic value proposition of high-content imaging for cardiac and hepatic chips is compelling. Studies indicate that implementing these advanced screening methods can reduce preclinical development costs by up to 25% through earlier identification of toxic compounds. Furthermore, the return on investment is enhanced by the potential to repurpose existing drugs for new indications, a strategy that can reduce development timelines by 3-5 years and costs by up to 60% compared to developing new molecular entities.
Market barriers include high initial investment costs for equipment and expertise, challenges in data management and analysis, and regulatory uncertainties regarding the validation of organ-on-chip models for preclinical testing. Despite these challenges, the market outlook remains highly positive, with increasing adoption rates across the pharmaceutical industry and growing recognition from regulatory bodies regarding the value of these advanced screening approaches.
The demand for high-content imaging pipelines in phenotypic drug screening is primarily fueled by the pharmaceutical industry's need to reduce late-stage drug failures. With the average cost of bringing a new drug to market exceeding $2.6 billion and failure rates in clinical trials remaining high (approximately 90%), there is an urgent need for more predictive preclinical models. Cardiac and hepatic toxicities account for nearly 40% of drug withdrawals from the market, highlighting the critical importance of improved screening methods for these specific organ systems.
Regional market analysis reveals that North America currently dominates the phenotypic screening market with a 42% share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 15.7% during the forecast period, driven by increasing R&D investments and the establishment of new research centers in China, Japan, and South Korea.
Key customer segments include large pharmaceutical companies (45% market share), biotechnology firms (30%), academic research institutions (15%), and contract research organizations (10%). Large pharmaceutical companies are increasingly integrating phenotypic screening into their drug discovery workflows, with 65% of major pharma companies reporting increased investment in these technologies over the past five years.
The economic value proposition of high-content imaging for cardiac and hepatic chips is compelling. Studies indicate that implementing these advanced screening methods can reduce preclinical development costs by up to 25% through earlier identification of toxic compounds. Furthermore, the return on investment is enhanced by the potential to repurpose existing drugs for new indications, a strategy that can reduce development timelines by 3-5 years and costs by up to 60% compared to developing new molecular entities.
Market barriers include high initial investment costs for equipment and expertise, challenges in data management and analysis, and regulatory uncertainties regarding the validation of organ-on-chip models for preclinical testing. Despite these challenges, the market outlook remains highly positive, with increasing adoption rates across the pharmaceutical industry and growing recognition from regulatory bodies regarding the value of these advanced screening approaches.
Current Challenges in Organ-on-Chip Imaging
Despite significant advancements in organ-on-chip (OoC) technology, high-content imaging for phenotypic drug screening on cardiac and hepatic chips faces several critical challenges. The integration of complex biological systems with imaging technologies creates unique obstacles that researchers and industry professionals must overcome to fully realize the potential of these platforms.
Resolution limitations present a significant barrier when attempting to capture cellular and subcellular responses in three-dimensional tissue structures. While conventional microscopy techniques work well for monolayer cultures, they struggle to provide adequate depth penetration and clarity when imaging the multilayered, three-dimensional structures typical of cardiac and hepatic chips. This limitation restricts the ability to observe deep tissue responses to drug compounds.
Temporal constraints also pose considerable challenges. Cardiac tissues exhibit rapid contractile movements occurring within milliseconds, requiring extremely high frame rates to capture accurately. Conversely, hepatic responses to drugs may develop over hours or days, necessitating long-term, stable imaging conditions. These divergent temporal requirements demand sophisticated imaging systems capable of adapting to different time scales.
Phototoxicity emerges as another significant concern, particularly for longitudinal studies. Prolonged or intense light exposure during imaging can damage sensitive cardiac and hepatic tissues, potentially altering their physiological responses to test compounds and compromising experimental validity. This creates a difficult balance between image quality and tissue viability.
Data management presents overwhelming challenges as high-content imaging generates massive datasets. A single experiment may produce terabytes of multidimensional data, creating bottlenecks in storage, processing, and analysis pipelines. The complexity increases when attempting to extract meaningful phenotypic information from these vast datasets.
Standardization issues further complicate the field. The lack of universally accepted imaging protocols and analysis methods for cardiac and hepatic chips makes cross-laboratory comparisons difficult. This absence of standardization impedes validation efforts and slows the broader adoption of these technologies in drug discovery pipelines.
Automation integration remains incomplete in many systems. While automated imaging platforms exist, their seamless integration with microfluidic organ-on-chip systems presents engineering challenges, particularly regarding maintaining sterility, temperature control, and precise positioning during extended experiments.
Label compatibility issues also persist. Many fluorescent labels and contrast agents developed for traditional cell cultures may behave differently in the complex microenvironments of organ chips, potentially affecting cellular function or yielding inconsistent results. Developing chip-specific, non-invasive imaging methods remains an active area of research.
Resolution limitations present a significant barrier when attempting to capture cellular and subcellular responses in three-dimensional tissue structures. While conventional microscopy techniques work well for monolayer cultures, they struggle to provide adequate depth penetration and clarity when imaging the multilayered, three-dimensional structures typical of cardiac and hepatic chips. This limitation restricts the ability to observe deep tissue responses to drug compounds.
Temporal constraints also pose considerable challenges. Cardiac tissues exhibit rapid contractile movements occurring within milliseconds, requiring extremely high frame rates to capture accurately. Conversely, hepatic responses to drugs may develop over hours or days, necessitating long-term, stable imaging conditions. These divergent temporal requirements demand sophisticated imaging systems capable of adapting to different time scales.
Phototoxicity emerges as another significant concern, particularly for longitudinal studies. Prolonged or intense light exposure during imaging can damage sensitive cardiac and hepatic tissues, potentially altering their physiological responses to test compounds and compromising experimental validity. This creates a difficult balance between image quality and tissue viability.
Data management presents overwhelming challenges as high-content imaging generates massive datasets. A single experiment may produce terabytes of multidimensional data, creating bottlenecks in storage, processing, and analysis pipelines. The complexity increases when attempting to extract meaningful phenotypic information from these vast datasets.
Standardization issues further complicate the field. The lack of universally accepted imaging protocols and analysis methods for cardiac and hepatic chips makes cross-laboratory comparisons difficult. This absence of standardization impedes validation efforts and slows the broader adoption of these technologies in drug discovery pipelines.
Automation integration remains incomplete in many systems. While automated imaging platforms exist, their seamless integration with microfluidic organ-on-chip systems presents engineering challenges, particularly regarding maintaining sterility, temperature control, and precise positioning during extended experiments.
Label compatibility issues also persist. Many fluorescent labels and contrast agents developed for traditional cell cultures may behave differently in the complex microenvironments of organ chips, potentially affecting cellular function or yielding inconsistent results. Developing chip-specific, non-invasive imaging methods remains an active area of research.
Current High-Content Imaging Methodologies
01 Automated high-content imaging systems for drug screening
Automated high-content imaging systems enable efficient phenotypic drug screening by capturing and analyzing cellular responses to compounds. These systems integrate hardware components like microscopes, robotic sample handling, and incubation chambers with software for image acquisition and analysis. The automation allows for high-throughput screening of large compound libraries while maintaining the quality and reproducibility of results, significantly accelerating the drug discovery process.- Automated high-content imaging systems for drug screening: Automated high-content imaging systems enable efficient phenotypic drug screening by capturing and analyzing cellular responses to compounds. These systems integrate hardware components like microscopes, robotic sample handlers, and incubators with software for image acquisition and analysis. The automation allows for high-throughput screening of large compound libraries while maintaining data quality and reproducibility, significantly accelerating the drug discovery process.
- Image analysis algorithms and machine learning for phenotypic screening: Advanced image analysis algorithms and machine learning techniques are essential for extracting meaningful data from high-content images in phenotypic drug screening. These computational methods can identify subtle cellular changes, classify phenotypes, and quantify biological responses to test compounds. Machine learning models improve the accuracy of phenotype identification and can detect complex patterns that might be missed by conventional analysis, enabling more predictive drug discovery.
- Cell-based assay development for phenotypic screening: Specialized cell-based assays are developed to model disease states and detect compound effects in phenotypic screening. These assays utilize various cell types, including primary cells, stem cells, and engineered cell lines that express disease-relevant targets or reporter systems. The assays are designed to measure multiple parameters simultaneously, such as cell morphology, protein expression, and subcellular localization, providing a comprehensive view of compound effects on cellular physiology.
- 3D cell culture and organoid models for high-content screening: Three-dimensional cell cultures and organoid models provide more physiologically relevant systems for phenotypic drug screening compared to traditional 2D cultures. These advanced models better recapitulate tissue architecture, cell-cell interactions, and microenvironmental factors that influence drug responses. High-content imaging of these 3D structures requires specialized techniques for image acquisition and analysis, but yields more translatable data for predicting in vivo drug efficacy and toxicity.
- Integration of multi-omics data with high-content imaging: Combining high-content imaging with multi-omics approaches (genomics, transcriptomics, proteomics) creates powerful platforms for comprehensive phenotypic drug screening. This integration allows researchers to correlate visual phenotypes with molecular mechanisms, providing deeper insights into drug action. Computational methods that integrate these diverse data types help identify biomarkers, predict mechanisms of action, and select promising drug candidates with greater confidence, ultimately improving the success rate of drug development programs.
02 Machine learning and AI in image analysis for phenotypic screening
Machine learning and artificial intelligence algorithms are increasingly applied to analyze complex cellular phenotypes in high-content screening. These computational approaches can identify subtle phenotypic changes, classify cellular responses, and predict compound efficacy or toxicity. Deep learning models in particular have enhanced the ability to extract meaningful features from microscopy images without manual intervention, improving both the speed and accuracy of phenotypic drug screening campaigns.Expand Specific Solutions03 Multiparametric phenotypic assays for compound profiling
Multiparametric phenotypic assays measure multiple cellular parameters simultaneously to create comprehensive profiles of compound effects. These assays capture diverse cellular responses including morphological changes, protein expression, organelle integrity, and cell cycle progression. By analyzing multiple parameters from a single experiment, researchers can better understand compound mechanisms of action, identify off-target effects, and group compounds with similar phenotypic signatures, enabling more informed decisions in drug development.Expand Specific Solutions04 3D cell culture models for advanced phenotypic screening
Three-dimensional cell culture models provide more physiologically relevant environments for phenotypic drug screening compared to traditional 2D cultures. These models include spheroids, organoids, and tissue-engineered constructs that better recapitulate in vivo cellular organization, differentiation, and drug responses. High-content imaging of 3D models requires specialized techniques for image acquisition and analysis, but offers improved predictive value for compound efficacy and toxicity in human tissues.Expand Specific Solutions05 Integration of genomic data with phenotypic screening results
Integrating genomic data with phenotypic screening results creates powerful approaches for target identification and personalized medicine. This strategy combines the unbiased nature of phenotypic screening with molecular insights from genomics, enabling researchers to correlate phenotypic responses with genetic backgrounds. Such integration helps identify genetic biomarkers of drug sensitivity, elucidate mechanisms of action, and develop targeted therapies for specific patient populations based on their genetic profiles.Expand Specific Solutions
Leading Companies in Organ-on-Chip Imaging
High-content imaging pipelines for phenotypic drug screening on cardiac and hepatic chips represent an emerging technology at the intersection of microfluidics, tissue engineering, and advanced imaging. The market is in its early growth phase, with an estimated global market size of $1.5-2 billion and projected annual growth of 15-20%. Leading pharmaceutical companies like Janssen Pharmaceutica, F. Hoffmann-La Roche, and Bristol Myers Squibb are investing heavily in this technology to reduce drug development costs and improve predictive accuracy. Technology innovators such as Recursion Pharmaceuticals have developed proprietary platforms combining high-throughput imaging with AI analytics, while Canon and Illumina provide specialized imaging equipment. Academic-industry partnerships, particularly involving institutions like Tsinghua University and University of Washington, are accelerating technology maturation through collaborative research initiatives focused on improving throughput, reproducibility, and physiological relevance.
Recursion Pharmaceuticals, Inc.
Technical Solution: Recursion has developed a high-content imaging platform that combines automated microscopy with machine learning for phenotypic drug screening. Their approach utilizes a proprietary Cell Painting assay to capture morphological features across multiple cellular compartments in cardiac and hepatic organ-on-chip models. The platform employs a high-throughput automated microscopy system capable of capturing thousands of cellular images per hour at subcellular resolution[1]. Their RxRx1 dataset contains over 300TB of biological images across various cell types including cardiac and hepatic cells. Recursion's AI-driven image analysis pipeline extracts over 1,000 morphological features from each cell, creating a multidimensional phenotypic fingerprint that can detect subtle drug-induced changes in cell morphology and function[2]. For cardiac chips specifically, their system can detect changes in cardiomyocyte contractility, calcium handling, and structural organization, while their hepatic chip analysis focuses on metabolic function, bile canaliculi formation, and toxicity markers[3].
Strengths: Industry-leading scale of image data collection and processing capabilities; proprietary deep learning algorithms specifically optimized for biological image analysis; integrated cloud computing infrastructure allowing rapid processing of massive datasets. Weaknesses: Highly dependent on computational infrastructure requiring significant investment; potential challenges in translating in vitro phenotypic findings to in vivo efficacy; requires specialized expertise spanning biology, imaging, and machine learning.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has pioneered an advanced high-content imaging platform for phenotypic screening on organ-chip models, particularly focusing on cardiac and hepatic tissues. Their system integrates automated confocal microscopy with sophisticated image analysis algorithms specifically designed for 3D tissue structures. The platform employs a multi-parametric approach to simultaneously assess structural integrity, functional parameters, and molecular markers in living microtissues[1]. For cardiac applications, their technology can measure contractile function, calcium transients, and electrophysiological parameters in real-time, while continuously monitoring tissue architecture. Their hepatic chip imaging system focuses on bile canaliculi formation, metabolic enzyme activity, and transport protein function[2]. Roche's platform incorporates machine learning algorithms that analyze temporal changes in tissue morphology and function, enabling the detection of both acute and chronic drug effects. The system is designed to work with their proprietary organ-chip models that recapitulate key aspects of cardiac and hepatic physiology, including cell-cell interactions and tissue-specific microenvironments[3].
Strengths: Exceptional integration of imaging with physiologically relevant 3D tissue models; robust validation against known cardiotoxic and hepatotoxic compounds; seamless integration with Roche's broader drug discovery pipeline. Weaknesses: Proprietary nature of the platform may limit broader adoption in the scientific community; complex system requiring specialized expertise to operate and maintain; higher cost compared to traditional 2D cell culture screening approaches.
Key Innovations in Cardiac and Hepatic Imaging
Method for screening drug for tumor disease, program product, high-content imaging analysis system, and method for analyzing phase separation conditions
PatentWO2024160270A1
Innovation
- Development of a high-throughput imaging system that enables continuous monitoring of protein aggregate dynamics in cells for drug screening in tumor diseases.
- Use of labeled fusion proteins containing phase separation (PS) domains and DNA-binding domains (DBD) to form identifiable aggregates associated with tumor diseases.
- Time-series imaging approach across multiple fields of view to comprehensively track the dynamic effects of small molecule compounds on intracellular aggregates.
Ligand-receptor tracking assays
PatentInactiveUS20060105393A1
Innovation
- A multiplex method using image-based analysis with fluorescently labeled ligands and receptors, allowing for the simultaneous measurement of multiple cellular parameters in individual cells or cell populations, including receptor-ligand internalization, Ca2+ flux, and gene expression, using a high content screening reader like the Cellomics ArrayScan 4.0.
Regulatory Considerations for Drug Discovery Platforms
The regulatory landscape for high-content imaging pipelines in phenotypic drug screening using cardiac and hepatic chips presents significant complexities that must be navigated carefully. The FDA and EMA have established frameworks for evaluating novel drug discovery platforms, with specific guidance documents addressing the use of in vitro models and imaging-based assays. These regulatory bodies increasingly recognize the value of organ-on-chip technologies as potential alternatives to traditional animal testing, aligning with the 3Rs principle (Replacement, Reduction, Refinement).
For high-content imaging systems integrated with cardiac and hepatic chips, developers must address validation requirements that demonstrate reproducibility, accuracy, and clinical relevance. The FDA's qualification process for drug development tools provides a pathway for formal recognition of these platforms, though the burden of proof remains substantial. Regulatory acceptance typically requires extensive correlation studies comparing chip-based results with established in vivo and clinical outcomes.
Data integrity and standardization represent critical regulatory considerations. Imaging pipelines generate massive datasets requiring robust quality control measures and standardized analysis protocols. Regulatory bodies increasingly scrutinize the algorithms and computational methods used to extract phenotypic endpoints, demanding transparency in how machine learning models are trained and validated.
Privacy and ethical considerations also factor into regulatory compliance, particularly when human-derived cells are utilized in cardiac and hepatic chips. Proper informed consent documentation and data anonymization protocols must be implemented in accordance with regulations like GDPR in Europe and HIPAA in the United States.
International harmonization efforts, such as those led by the International Council for Harmonisation (ICH), are gradually establishing consensus guidelines for advanced in vitro models. However, significant regional variations persist in regulatory approaches to novel drug discovery platforms, creating challenges for global implementation.
Looking forward, regulatory frameworks are evolving to accommodate innovative technologies like high-content imaging on organ chips. The FDA's Emerging Technology Program and similar initiatives in other regions provide opportunities for early engagement with regulators. Companies developing these platforms should proactively pursue regulatory discussions during development phases rather than waiting until pre-clinical or clinical stages.
For high-content imaging systems integrated with cardiac and hepatic chips, developers must address validation requirements that demonstrate reproducibility, accuracy, and clinical relevance. The FDA's qualification process for drug development tools provides a pathway for formal recognition of these platforms, though the burden of proof remains substantial. Regulatory acceptance typically requires extensive correlation studies comparing chip-based results with established in vivo and clinical outcomes.
Data integrity and standardization represent critical regulatory considerations. Imaging pipelines generate massive datasets requiring robust quality control measures and standardized analysis protocols. Regulatory bodies increasingly scrutinize the algorithms and computational methods used to extract phenotypic endpoints, demanding transparency in how machine learning models are trained and validated.
Privacy and ethical considerations also factor into regulatory compliance, particularly when human-derived cells are utilized in cardiac and hepatic chips. Proper informed consent documentation and data anonymization protocols must be implemented in accordance with regulations like GDPR in Europe and HIPAA in the United States.
International harmonization efforts, such as those led by the International Council for Harmonisation (ICH), are gradually establishing consensus guidelines for advanced in vitro models. However, significant regional variations persist in regulatory approaches to novel drug discovery platforms, creating challenges for global implementation.
Looking forward, regulatory frameworks are evolving to accommodate innovative technologies like high-content imaging on organ chips. The FDA's Emerging Technology Program and similar initiatives in other regions provide opportunities for early engagement with regulators. Companies developing these platforms should proactively pursue regulatory discussions during development phases rather than waiting until pre-clinical or clinical stages.
Data Management and AI Integration
The integration of advanced data management systems and artificial intelligence (AI) has become a critical component in high-content imaging pipelines for phenotypic drug screening on cardiac and hepatic chips. These organ-on-chip platforms generate massive volumes of complex imaging data that require sophisticated handling and analysis approaches to extract meaningful biological insights.
Current data management systems for organ-on-chip imaging typically employ hierarchical database architectures that organize information based on experimental parameters, temporal sequences, and spatial dimensions. Cloud-based solutions have emerged as the preferred infrastructure, offering scalable storage capacity and computational resources necessary for processing high-resolution microscopy images. Leading platforms such as Amazon Web Services' HealthLake and Google Cloud's Healthcare API have developed specialized modules for biomedical imaging data that maintain regulatory compliance while enabling collaborative access.
AI integration in these pipelines has evolved significantly, with deep learning algorithms demonstrating particular efficacy in automated image analysis. Convolutional neural networks (CNNs) have become the standard for feature extraction and cellular phenotype classification, achieving accuracy rates exceeding 95% in identifying drug-induced morphological changes in cardiac and hepatic tissues. Recent advances in transformer-based architectures have further improved temporal analysis capabilities, enabling the detection of subtle functional changes over extended experimental periods.
Real-time analysis frameworks represent the cutting edge of this technological integration, where streaming analytics platforms process imaging data as it is generated. This approach has reduced the time from data acquisition to actionable insights from days to minutes, dramatically accelerating the drug screening workflow. Companies like Genentech and AstraZeneca have reported 60-70% reductions in screening cycle times through implementation of these systems.
Federated learning models are gaining traction as a solution to data privacy concerns, allowing multiple research institutions to collaboratively train AI models without sharing sensitive experimental data. This approach has facilitated unprecedented cross-institutional validation of phenotypic drug responses, enhancing the reliability of screening results across diverse tissue sources and experimental conditions.
Challenges remain in standardizing data formats and analysis protocols across different imaging modalities and organ-chip platforms. The International Alliance for Biological Standardization has proposed a unified data schema for organ-chip imaging that aims to address interoperability issues, though adoption remains fragmented. Additionally, the computational demands of processing three-dimensional time-lapse imaging data continue to pose resource constraints, particularly for smaller research organizations without access to high-performance computing infrastructure.
Current data management systems for organ-on-chip imaging typically employ hierarchical database architectures that organize information based on experimental parameters, temporal sequences, and spatial dimensions. Cloud-based solutions have emerged as the preferred infrastructure, offering scalable storage capacity and computational resources necessary for processing high-resolution microscopy images. Leading platforms such as Amazon Web Services' HealthLake and Google Cloud's Healthcare API have developed specialized modules for biomedical imaging data that maintain regulatory compliance while enabling collaborative access.
AI integration in these pipelines has evolved significantly, with deep learning algorithms demonstrating particular efficacy in automated image analysis. Convolutional neural networks (CNNs) have become the standard for feature extraction and cellular phenotype classification, achieving accuracy rates exceeding 95% in identifying drug-induced morphological changes in cardiac and hepatic tissues. Recent advances in transformer-based architectures have further improved temporal analysis capabilities, enabling the detection of subtle functional changes over extended experimental periods.
Real-time analysis frameworks represent the cutting edge of this technological integration, where streaming analytics platforms process imaging data as it is generated. This approach has reduced the time from data acquisition to actionable insights from days to minutes, dramatically accelerating the drug screening workflow. Companies like Genentech and AstraZeneca have reported 60-70% reductions in screening cycle times through implementation of these systems.
Federated learning models are gaining traction as a solution to data privacy concerns, allowing multiple research institutions to collaboratively train AI models without sharing sensitive experimental data. This approach has facilitated unprecedented cross-institutional validation of phenotypic drug responses, enhancing the reliability of screening results across diverse tissue sources and experimental conditions.
Challenges remain in standardizing data formats and analysis protocols across different imaging modalities and organ-chip platforms. The International Alliance for Biological Standardization has proposed a unified data schema for organ-chip imaging that aims to address interoperability issues, though adoption remains fragmented. Additionally, the computational demands of processing three-dimensional time-lapse imaging data continue to pose resource constraints, particularly for smaller research organizations without access to high-performance computing infrastructure.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!