Validating organ-on-chip assays for regulatory acceptance in preclinical safety testing
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Organ-on-Chip Technology Background and Objectives
Organ-on-chip (OoC) technology has evolved significantly over the past decade, emerging from the convergence of tissue engineering, microfluidics, and cell biology. This innovative platform aims to recapitulate the structural and functional complexity of human organs within microfluidic devices. The evolution began with simple 2D cell cultures, progressed through 3D organoid models, and has now reached sophisticated multi-compartmental systems that can simulate organ-level functions and interactions.
The technology's development trajectory has been characterized by increasing complexity and physiological relevance. Early systems focused on single tissue types, while contemporary platforms integrate multiple cell types, incorporate mechanical forces, and feature dynamic fluid flow to better mimic in vivo conditions. Recent advances include the integration of sensors for real-time monitoring and the development of interconnected multi-organ systems that can simulate systemic responses.
Current technological trends point toward enhanced biomimicry through incorporation of patient-derived cells, improved materials that better replicate native extracellular matrices, and advanced manufacturing techniques that allow for more precise control over microenvironments. The integration of artificial intelligence and machine learning for data analysis represents another significant trend, enabling more sophisticated interpretation of complex biological responses.
The primary objective of OoC technology in preclinical safety testing is to provide more predictive models of human response to pharmaceuticals and chemicals than traditional animal testing. Specific goals include developing standardized platforms that can reliably predict drug toxicity, establishing validated protocols that generate reproducible results across laboratories, and creating systems capable of long-term culture to assess chronic exposure effects.
A critical aim is to achieve regulatory acceptance of OoC assays as alternatives or complements to animal testing. This requires demonstrating that these systems can accurately recapitulate human physiology and predict adverse effects with equal or greater accuracy than current methods. The technology seeks to address the limitations of both animal models, which often fail to predict human responses due to species differences, and simple cell cultures, which lack the complexity of intact organs.
The ultimate technological objective is to create a "human-on-a-chip" – an integrated system of multiple organ models that can simulate whole-body responses to drugs and chemicals. This would revolutionize preclinical testing by providing a more comprehensive understanding of compound effects, including absorption, distribution, metabolism, excretion, and toxicity (ADMET) properties, while reducing reliance on animal testing in accordance with the 3Rs principles (replacement, reduction, refinement).
The technology's development trajectory has been characterized by increasing complexity and physiological relevance. Early systems focused on single tissue types, while contemporary platforms integrate multiple cell types, incorporate mechanical forces, and feature dynamic fluid flow to better mimic in vivo conditions. Recent advances include the integration of sensors for real-time monitoring and the development of interconnected multi-organ systems that can simulate systemic responses.
Current technological trends point toward enhanced biomimicry through incorporation of patient-derived cells, improved materials that better replicate native extracellular matrices, and advanced manufacturing techniques that allow for more precise control over microenvironments. The integration of artificial intelligence and machine learning for data analysis represents another significant trend, enabling more sophisticated interpretation of complex biological responses.
The primary objective of OoC technology in preclinical safety testing is to provide more predictive models of human response to pharmaceuticals and chemicals than traditional animal testing. Specific goals include developing standardized platforms that can reliably predict drug toxicity, establishing validated protocols that generate reproducible results across laboratories, and creating systems capable of long-term culture to assess chronic exposure effects.
A critical aim is to achieve regulatory acceptance of OoC assays as alternatives or complements to animal testing. This requires demonstrating that these systems can accurately recapitulate human physiology and predict adverse effects with equal or greater accuracy than current methods. The technology seeks to address the limitations of both animal models, which often fail to predict human responses due to species differences, and simple cell cultures, which lack the complexity of intact organs.
The ultimate technological objective is to create a "human-on-a-chip" – an integrated system of multiple organ models that can simulate whole-body responses to drugs and chemicals. This would revolutionize preclinical testing by providing a more comprehensive understanding of compound effects, including absorption, distribution, metabolism, excretion, and toxicity (ADMET) properties, while reducing reliance on animal testing in accordance with the 3Rs principles (replacement, reduction, refinement).
Market Analysis for Preclinical Safety Testing Alternatives
The global preclinical safety testing market is experiencing significant transformation, driven by increasing concerns about animal testing ethics, regulatory pressures, and the need for more human-relevant test systems. Currently valued at approximately $4.9 billion, this market is projected to grow at a CAGR of 8.2% through 2027, with alternative testing methods representing the fastest-growing segment.
Organ-on-chip technology represents a particularly promising alternative, with its market segment expected to reach $220 million by 2025. This growth is fueled by pharmaceutical companies seeking to reduce the 90% failure rate of drug candidates in clinical trials, many of which pass animal tests but fail in humans due to species-specific differences in drug metabolism and toxicity responses.
Regulatory bodies worldwide are increasingly receptive to alternative testing methods. The FDA's Predictive Toxicology Roadmap and the European Medicines Agency's guidelines on the use of non-animal alternatives demonstrate growing institutional support. Additionally, the FDA Modernization Act 2.0, signed into law in December 2022, removes the mandatory requirement for animal testing in drug development, creating a significant market opportunity for organ-on-chip technologies.
Cost considerations also drive market demand for alternatives. Traditional animal testing for a single compound can cost between $2-4 million and take 3-5 years. In contrast, organ-on-chip systems, while requiring initial investment, offer potential long-term cost savings through faster results, reduced compound requirements, and better predictive capabilities that can prevent costly late-stage clinical failures.
The pharmaceutical industry represents the largest market segment (65%) for preclinical safety testing alternatives, followed by academic research institutions (20%) and contract research organizations (15%). Geographically, North America leads with 45% market share, followed by Europe (30%) and Asia-Pacific (20%), with the latter showing the fastest growth rate due to increasing R&D investments and regulatory changes in countries like China and Japan.
Key market drivers include stringent regulatory requirements for drug safety, increasing R&D investments in pharmaceutical and biotechnology sectors, and growing public and corporate commitment to the 3Rs principle (Replacement, Reduction, Refinement) of animal testing. However, market barriers persist, including regulatory uncertainty around validation requirements, technical challenges in creating physiologically relevant models, and the need for standardization across different organ-on-chip platforms.
The COVID-19 pandemic has accelerated interest in alternative testing methods, as traditional animal testing facilities faced disruptions while the urgent need for therapeutic development intensified, highlighting the value of rapid, reliable in vitro testing platforms.
Organ-on-chip technology represents a particularly promising alternative, with its market segment expected to reach $220 million by 2025. This growth is fueled by pharmaceutical companies seeking to reduce the 90% failure rate of drug candidates in clinical trials, many of which pass animal tests but fail in humans due to species-specific differences in drug metabolism and toxicity responses.
Regulatory bodies worldwide are increasingly receptive to alternative testing methods. The FDA's Predictive Toxicology Roadmap and the European Medicines Agency's guidelines on the use of non-animal alternatives demonstrate growing institutional support. Additionally, the FDA Modernization Act 2.0, signed into law in December 2022, removes the mandatory requirement for animal testing in drug development, creating a significant market opportunity for organ-on-chip technologies.
Cost considerations also drive market demand for alternatives. Traditional animal testing for a single compound can cost between $2-4 million and take 3-5 years. In contrast, organ-on-chip systems, while requiring initial investment, offer potential long-term cost savings through faster results, reduced compound requirements, and better predictive capabilities that can prevent costly late-stage clinical failures.
The pharmaceutical industry represents the largest market segment (65%) for preclinical safety testing alternatives, followed by academic research institutions (20%) and contract research organizations (15%). Geographically, North America leads with 45% market share, followed by Europe (30%) and Asia-Pacific (20%), with the latter showing the fastest growth rate due to increasing R&D investments and regulatory changes in countries like China and Japan.
Key market drivers include stringent regulatory requirements for drug safety, increasing R&D investments in pharmaceutical and biotechnology sectors, and growing public and corporate commitment to the 3Rs principle (Replacement, Reduction, Refinement) of animal testing. However, market barriers persist, including regulatory uncertainty around validation requirements, technical challenges in creating physiologically relevant models, and the need for standardization across different organ-on-chip platforms.
The COVID-19 pandemic has accelerated interest in alternative testing methods, as traditional animal testing facilities faced disruptions while the urgent need for therapeutic development intensified, highlighting the value of rapid, reliable in vitro testing platforms.
Current Status and Challenges in Organ-on-Chip Validation
Organ-on-chip (OOC) technology has emerged as a promising alternative to traditional animal testing in preclinical safety assessment. However, despite significant advancements, the regulatory acceptance of OOC platforms faces numerous challenges. Currently, various OOC models representing different organ systems have been developed, including liver, kidney, heart, lung, and brain chips, with varying degrees of physiological relevance and validation status.
The global landscape of OOC development shows concentration in North America, Europe, and parts of Asia, with the United States leading in both academic research and commercial applications. European institutions have made substantial contributions to standardization efforts, while Asian countries, particularly China and Japan, are rapidly expanding their research capabilities in this domain.
A primary technical challenge in OOC validation is achieving reproducibility across different laboratories and manufacturing batches. The complex nature of these systems, involving multiple cell types, microfluidic components, and sensing elements, creates variability that complicates validation efforts. Furthermore, the lack of standardized protocols for cell sourcing, device fabrication, and experimental procedures hampers cross-laboratory comparisons.
Another significant hurdle is establishing relevant biological endpoints that correlate with in vivo responses. While OOC systems can replicate certain aspects of organ functionality, they often fail to capture the full complexity of systemic responses observed in whole organisms. This limitation raises questions about their predictive capacity for complex toxicological endpoints, particularly for chronic exposure scenarios and rare adverse events.
Regulatory frameworks present additional challenges, as current guidelines were primarily designed for traditional testing methods. The FDA, EMA, and other regulatory bodies have shown interest in OOC technology but require substantial evidence of reliability and relevance before formal acceptance. The qualification process for novel methodologies is rigorous and time-consuming, requiring extensive validation studies and comparison with established methods.
Data integration and interpretation pose further complications. OOC systems generate complex, multiparametric datasets that require sophisticated analytical approaches. The field currently lacks consensus on data analysis methodologies and reporting standards, making it difficult to compare results across studies and platforms.
Resource constraints also impede validation efforts, as comprehensive validation studies require significant investment in time, expertise, and funding. Many academic institutions and smaller companies struggle to conduct the extensive validation necessary for regulatory consideration, creating a gap between technological innovation and practical implementation.
The global landscape of OOC development shows concentration in North America, Europe, and parts of Asia, with the United States leading in both academic research and commercial applications. European institutions have made substantial contributions to standardization efforts, while Asian countries, particularly China and Japan, are rapidly expanding their research capabilities in this domain.
A primary technical challenge in OOC validation is achieving reproducibility across different laboratories and manufacturing batches. The complex nature of these systems, involving multiple cell types, microfluidic components, and sensing elements, creates variability that complicates validation efforts. Furthermore, the lack of standardized protocols for cell sourcing, device fabrication, and experimental procedures hampers cross-laboratory comparisons.
Another significant hurdle is establishing relevant biological endpoints that correlate with in vivo responses. While OOC systems can replicate certain aspects of organ functionality, they often fail to capture the full complexity of systemic responses observed in whole organisms. This limitation raises questions about their predictive capacity for complex toxicological endpoints, particularly for chronic exposure scenarios and rare adverse events.
Regulatory frameworks present additional challenges, as current guidelines were primarily designed for traditional testing methods. The FDA, EMA, and other regulatory bodies have shown interest in OOC technology but require substantial evidence of reliability and relevance before formal acceptance. The qualification process for novel methodologies is rigorous and time-consuming, requiring extensive validation studies and comparison with established methods.
Data integration and interpretation pose further complications. OOC systems generate complex, multiparametric datasets that require sophisticated analytical approaches. The field currently lacks consensus on data analysis methodologies and reporting standards, making it difficult to compare results across studies and platforms.
Resource constraints also impede validation efforts, as comprehensive validation studies require significant investment in time, expertise, and funding. Many academic institutions and smaller companies struggle to conduct the extensive validation necessary for regulatory consideration, creating a gap between technological innovation and practical implementation.
Current Validation Methodologies for Organ-on-Chip Platforms
01 Validation methods for organ-on-chip systems
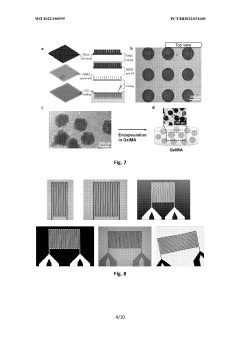
Various methods are employed to validate organ-on-chip systems to ensure their reliability and accuracy in mimicking human organ functions. These methods include comparing the chip's performance with established in vitro and in vivo models, using biomarkers to assess physiological responses, and implementing standardized protocols for validation. The validation process typically involves measuring key parameters such as cell viability, tissue functionality, and response to known stimuli to confirm that the chip accurately represents the target organ's behavior.- Microfluidic organ-on-chip validation methods: Microfluidic systems are used to validate organ-on-chip models by providing controlled environments that mimic physiological conditions. These validation methods include flow cytometry, microscopy imaging, and biochemical assays to assess cellular responses and functionality. The microfluidic platforms enable precise control of fluid dynamics, cellular interactions, and environmental parameters, ensuring reliable and reproducible validation of organ-on-chip models for drug screening and toxicity testing.
- Biomarker analysis for organ-on-chip validation: Biomarker analysis is essential for validating organ-on-chip models by measuring specific molecular indicators that reflect cellular functions and responses. This approach involves quantifying protein expression, gene transcription, metabolite production, and other biological markers to assess the physiological relevance of the chip model. Advanced analytical techniques such as mass spectrometry, immunoassays, and genomic profiling are employed to validate the organ-on-chip systems against established in vivo and clinical data.
- Integrated sensing technologies for real-time validation: Integrated sensing technologies enable real-time monitoring and validation of organ-on-chip systems through continuous measurement of key parameters. These technologies include electrochemical sensors, optical biosensors, impedance-based detection systems, and MEMS devices that can be directly incorporated into the chip architecture. Real-time validation allows for dynamic assessment of cellular responses, metabolic activities, and tissue functionality, providing immediate feedback on the physiological relevance and performance of the organ-on-chip model.
- Computational modeling for organ-on-chip validation: Computational modeling approaches are used to validate organ-on-chip systems by comparing experimental results with theoretical predictions. These models incorporate fluid dynamics, mass transport, cellular kinetics, and tissue mechanics to simulate physiological processes within the chip. Machine learning algorithms and statistical methods are applied to analyze large datasets generated from organ-on-chip experiments, enabling robust validation against established biological knowledge and clinical outcomes.
- Standardization protocols for organ-on-chip validation: Standardization protocols establish consistent methodologies for validating organ-on-chip systems across different laboratories and applications. These protocols define reference materials, quality control procedures, performance metrics, and reporting guidelines to ensure reproducibility and reliability of validation results. Standardized approaches include comparison with animal models, human tissue samples, and clinical data to verify the physiological relevance and predictive capacity of organ-on-chip platforms for drug development and disease modeling applications.
02 Microfluidic technology for organ-on-chip validation
Microfluidic technologies play a crucial role in organ-on-chip validation by enabling precise control of fluid flow, nutrient delivery, and waste removal that mimics physiological conditions. These technologies incorporate sensors and monitoring systems to continuously assess cellular responses and organ functionality in real-time. Advanced microfluidic platforms allow for the integration of multiple cell types and tissues, creating more complex and physiologically relevant models for validation studies. The controlled microenvironment helps ensure reproducibility and reliability of validation results.Expand Specific Solutions03 Integration of sensing technologies for real-time monitoring
Sensing technologies integrated into organ-on-chip platforms enable real-time monitoring of cellular responses and physiological parameters during validation studies. These include electrochemical sensors, optical sensors, and impedance-based detection systems that can measure metabolic activity, secretion of biomarkers, and changes in cellular morphology. The continuous monitoring capabilities allow researchers to validate the dynamic responses of the organ model to various stimuli and ensure that the chip accurately replicates the temporal aspects of organ function. This integration enhances the validation process by providing comprehensive data on chip performance.Expand Specific Solutions04 Standardization protocols for organ-on-chip validation
Standardization protocols are essential for ensuring consistent and reliable validation of organ-on-chip systems across different laboratories and applications. These protocols define specific parameters to be measured, reference materials to be used, and acceptance criteria for validation. They include guidelines for cell sourcing, culture conditions, device fabrication, and data analysis to minimize variability in validation results. The development of these standardized approaches facilitates regulatory acceptance of organ-on-chip technologies and enables meaningful comparison between different chip designs and validation studies.Expand Specific Solutions05 Multi-organ systems validation approaches
Validation of multi-organ-on-chip systems requires specialized approaches to assess the interactions between different organ models and ensure physiologically relevant responses. These approaches include evaluating metabolite exchange between connected organ compartments, assessing the propagation of drug effects across multiple tissues, and comparing the integrated response to established multi-organ physiological models. Validation methods for these complex systems often incorporate computational modeling to predict and verify the interconnected organ responses. The successful validation of multi-organ chips demonstrates their potential for studying systemic effects and organ-organ interactions in drug development and disease modeling.Expand Specific Solutions
Key Industry Players and Research Institutions
The organ-on-chip technology market is currently in a growth phase, transitioning from early adoption to broader implementation in preclinical safety testing. The global market is projected to reach approximately $220 million by 2025, with a CAGR of 30%. Regarding technical maturity, leading academic institutions (Harvard, MIT, Vanderbilt University) have established foundational research, while commercial entities are advancing practical applications. Companies like Beijing Daxiang Biotech and Stem Cell & Device Laboratory are developing specialized platforms, while established corporations such as Siemens AG are integrating these technologies into broader healthcare solutions. The primary challenge remains regulatory validation, with collaborative efforts between academic institutions (National University of Singapore, Southeast University) and regulatory bodies working to establish standardized validation protocols for wider acceptance in pharmaceutical development workflows.
Vanderbilt University
Technical Solution: Vanderbilt University has developed a comprehensive validation approach for their organ-on-chip platforms with specific focus on regulatory acceptance pathways. Their strategy centers on the PREDICT (Platform for Regulatory Evaluation and Development of Innovative Chip Technologies) framework, which establishes a systematic process for qualifying organ-chip models for specific regulatory contexts. Vanderbilt's validation methodology emphasizes three key dimensions: (1) biological fidelity - confirming tissue-specific structure and function through comprehensive biomarker profiling; (2) pharmacological relevance - demonstrating appropriate responses to reference compounds with known mechanisms; and (3) predictive capability - establishing statistical correlation between chip responses and human clinical outcomes. Their multi-organ platform has undergone extensive validation for ADME-Tox applications, with particular strength in modeling gut-liver interactions for first-pass metabolism. Vanderbilt researchers have collaborated with regulatory agencies to develop standardized protocols for qualification of organ-chip data, including establishing performance criteria and acceptance thresholds for different testing applications.
Strengths: Systematic qualification framework aligned with regulatory requirements; strong focus on multi-organ interactions; extensive validation datasets with reference compounds. Weaknesses: Complex systems require specialized expertise; challenges in standardizing primary cell sources; need for further validation with larger compound libraries.
President & Fellows of Harvard College
Technical Solution: Harvard's Wyss Institute has pioneered organ-on-chip technology with their human Organ Chips that recapitulate the microarchitecture and functions of living human organs. Their validation approach focuses on establishing physiological relevance through comprehensive characterization of tissue-specific markers, barrier function, and metabolic activities. They've developed multi-parametric validation protocols that combine molecular, cellular, and functional readouts to ensure chips accurately mimic in vivo responses. Harvard has collaborated with FDA through a Cooperative Research and Development Agreement to qualify their Organ Chips as "Context of Use" tools for drug development, establishing biomarkers and endpoints that correlate with clinical outcomes. Their validation framework includes reproducibility assessment across multiple laboratories and comparison with existing preclinical models and human clinical data to demonstrate predictive capabilities.
Strengths: Strong regulatory partnerships with FDA; comprehensive validation protocols; extensive peer-reviewed publications supporting technology. Weaknesses: Complex systems require specialized expertise; higher cost compared to traditional methods; challenges in achieving full systemic integration between different organ models.
Critical Technical Innovations in Organ-on-Chip Assays
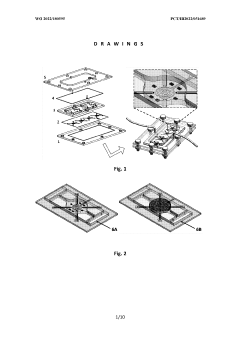
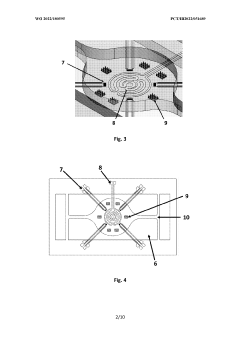
Multiorgan-on-a-chip device with integrated microbiosensors, methods and uses thereof
PatentWO2022180595A1
Innovation
- An advanced microfluidic system with in-situ implemented multiplexed micro(bio)sensors, including pH and temperature sensors, directly integrated with organ models, and a heating system for mimicking hyperthermia conditions, allowing real-time monitoring and automatic fluid replacement to maintain long-term cell culture homeostasis.
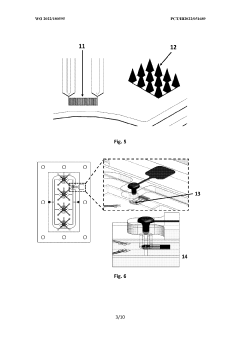
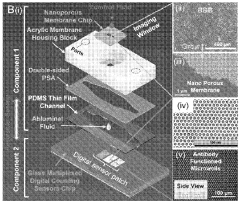
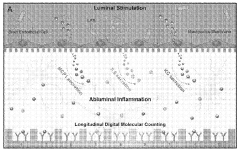
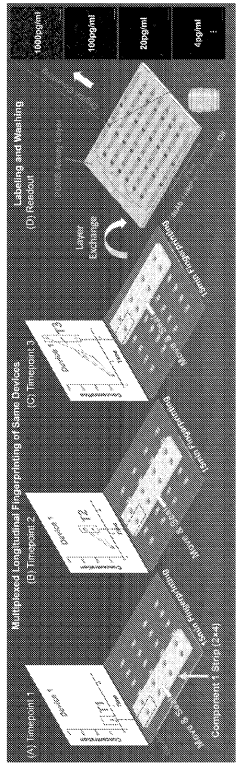
Digital molecular fingerprinting on organ on a chip systems
PatentWO2024137312A1
Innovation
- A Digital Tissue-BArrier-CytoKine-counting-on-a-chip (DigiTACK) platform integrating beadless digital immunosensors with nanoporous silicon-nitride membranes and microwells, allowing for multiplexed, ultrasensitive detection of cytokines with a low limit of detection (LOD) and enabling temporal profiling of cytokine secretion, overcoming the limitations of existing sensors by using a reversible sealing mechanism and optical detection of labeled analytes.
Regulatory Framework and Acceptance Criteria
The regulatory landscape for organ-on-chip (OOC) technologies in preclinical safety testing remains complex and evolving. Currently, no specific regulatory framework exists exclusively for OOC validation, requiring developers to navigate a patchwork of guidelines from multiple authorities. The FDA, EMA, and ICH have established general principles for alternative testing methods, but these must be adapted for the unique characteristics of OOC platforms.
Key regulatory considerations include demonstration of reproducibility, robustness, and relevance to human physiology. Regulatory bodies typically require extensive validation data comparing OOC results with both traditional animal models and human clinical outcomes where available. This triangulation approach helps establish confidence in the predictive capacity of these novel platforms.
The qualification process generally follows a stepwise approach beginning with analytical validation (demonstrating technical performance), followed by qualification (establishing biological relevance), and culminating in validation (confirming predictive capacity for specific contexts of use). Each regulatory authority maintains slightly different requirements, though efforts toward harmonization are ongoing through international initiatives like the OECD's Adverse Outcome Pathway framework.
Critical acceptance criteria include standardization of protocols, characterization of cellular components, demonstration of physiological functionality, and establishment of appropriate biomarkers. Regulatory bodies increasingly emphasize the need for transparent reporting of both positive and negative results, along with clear documentation of model limitations and boundary conditions.
The FDA's Predictive Toxicology Roadmap and the EMA's Innovation Task Force have created pathways for early engagement with developers of novel methodologies. These programs allow for scientific advice meetings where regulatory expectations can be clarified before significant resources are committed to validation studies. Similarly, the ICH has developed guidelines for qualification of novel methodologies that provide a framework for international acceptance.
Industry consortia and public-private partnerships play an essential role in developing consensus standards and validation frameworks. Organizations such as the IQ Consortium, EU-ToxRisk, and the NCATS Tissue Chip program are working to establish performance standards and reference compounds that can serve as benchmarks for regulatory acceptance. These collaborative efforts are critical for addressing the significant investment required for comprehensive validation studies.
Key regulatory considerations include demonstration of reproducibility, robustness, and relevance to human physiology. Regulatory bodies typically require extensive validation data comparing OOC results with both traditional animal models and human clinical outcomes where available. This triangulation approach helps establish confidence in the predictive capacity of these novel platforms.
The qualification process generally follows a stepwise approach beginning with analytical validation (demonstrating technical performance), followed by qualification (establishing biological relevance), and culminating in validation (confirming predictive capacity for specific contexts of use). Each regulatory authority maintains slightly different requirements, though efforts toward harmonization are ongoing through international initiatives like the OECD's Adverse Outcome Pathway framework.
Critical acceptance criteria include standardization of protocols, characterization of cellular components, demonstration of physiological functionality, and establishment of appropriate biomarkers. Regulatory bodies increasingly emphasize the need for transparent reporting of both positive and negative results, along with clear documentation of model limitations and boundary conditions.
The FDA's Predictive Toxicology Roadmap and the EMA's Innovation Task Force have created pathways for early engagement with developers of novel methodologies. These programs allow for scientific advice meetings where regulatory expectations can be clarified before significant resources are committed to validation studies. Similarly, the ICH has developed guidelines for qualification of novel methodologies that provide a framework for international acceptance.
Industry consortia and public-private partnerships play an essential role in developing consensus standards and validation frameworks. Organizations such as the IQ Consortium, EU-ToxRisk, and the NCATS Tissue Chip program are working to establish performance standards and reference compounds that can serve as benchmarks for regulatory acceptance. These collaborative efforts are critical for addressing the significant investment required for comprehensive validation studies.
Ethical and Animal Welfare Implications
The adoption of organ-on-chip technology for preclinical safety testing represents a significant paradigm shift with profound ethical and animal welfare implications. Traditional animal testing has long been criticized for ethical concerns related to animal suffering, raising questions about the moral justification of using sentient beings for human benefit. Organ-on-chip technology offers a promising alternative that could substantially reduce the number of animals used in preclinical studies, directly addressing the 3Rs principle (Replacement, Reduction, and Refinement) that guides ethical animal research globally.
The potential reduction in animal testing through organ-on-chip implementation is substantial. Current estimates suggest that a single drug development process may require 3,000-10,000 animals across various testing phases. Widespread adoption of validated organ-on-chip platforms could potentially reduce this number by 30-70%, depending on the specific application and regulatory acceptance levels. This represents not only a significant ethical advancement but also aligns with evolving societal expectations regarding animal welfare.
Beyond the quantitative reduction in animal use, organ-on-chip technology addresses qualitative ethical concerns by potentially eliminating particularly invasive or distressing procedures. Certain toxicity tests that cause significant suffering could be replaced entirely by in vitro alternatives that more accurately model human responses. This aspect of organ-on-chip technology resonates strongly with both ethical frameworks focused on reducing suffering and public sentiment regarding animal welfare.
The ethical implications extend to scientific validity as well. The species-specific differences that limit the translational value of animal models represent an ethical concern in themselves - subjecting animals to procedures that may yield data of questionable relevance to human outcomes. Organ-on-chip models derived from human cells potentially offer more predictive results, strengthening the ethical justification for any remaining animal testing by ensuring it occurs only when scientifically necessary and valid.
Regulatory bodies worldwide are increasingly acknowledging these ethical dimensions. The FDA Modernization Act 2.0 in the United States and similar initiatives in Europe explicitly recognize the ethical imperative to develop alternatives to animal testing. These regulatory shifts create a favorable environment for organ-on-chip validation, where ethical considerations serve not as barriers but as drivers for adoption.
However, the transition period presents its own ethical challenges. During validation, organ-on-chip technologies may temporarily increase animal use as comparative studies are required. This paradoxical situation necessitates careful ethical navigation to ensure that short-term increases in animal use are justified by long-term reductions. Transparent reporting of these validation studies and their animal welfare implications will be essential for maintaining public trust during this transition.
The potential reduction in animal testing through organ-on-chip implementation is substantial. Current estimates suggest that a single drug development process may require 3,000-10,000 animals across various testing phases. Widespread adoption of validated organ-on-chip platforms could potentially reduce this number by 30-70%, depending on the specific application and regulatory acceptance levels. This represents not only a significant ethical advancement but also aligns with evolving societal expectations regarding animal welfare.
Beyond the quantitative reduction in animal use, organ-on-chip technology addresses qualitative ethical concerns by potentially eliminating particularly invasive or distressing procedures. Certain toxicity tests that cause significant suffering could be replaced entirely by in vitro alternatives that more accurately model human responses. This aspect of organ-on-chip technology resonates strongly with both ethical frameworks focused on reducing suffering and public sentiment regarding animal welfare.
The ethical implications extend to scientific validity as well. The species-specific differences that limit the translational value of animal models represent an ethical concern in themselves - subjecting animals to procedures that may yield data of questionable relevance to human outcomes. Organ-on-chip models derived from human cells potentially offer more predictive results, strengthening the ethical justification for any remaining animal testing by ensuring it occurs only when scientifically necessary and valid.
Regulatory bodies worldwide are increasingly acknowledging these ethical dimensions. The FDA Modernization Act 2.0 in the United States and similar initiatives in Europe explicitly recognize the ethical imperative to develop alternatives to animal testing. These regulatory shifts create a favorable environment for organ-on-chip validation, where ethical considerations serve not as barriers but as drivers for adoption.
However, the transition period presents its own ethical challenges. During validation, organ-on-chip technologies may temporarily increase animal use as comparative studies are required. This paradoxical situation necessitates careful ethical navigation to ensure that short-term increases in animal use are justified by long-term reductions. Transparent reporting of these validation studies and their animal welfare implications will be essential for maintaining public trust during this transition.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!