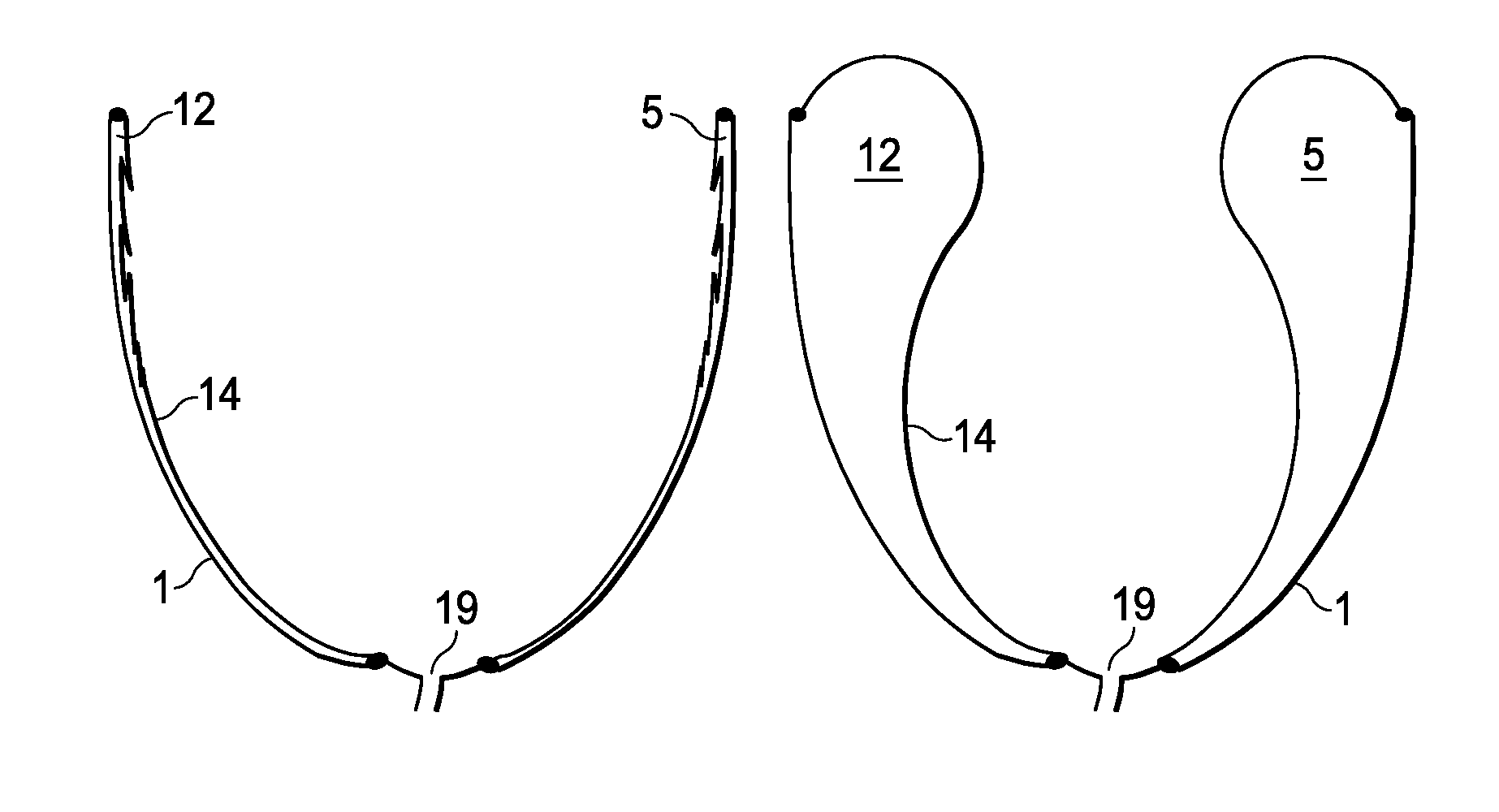
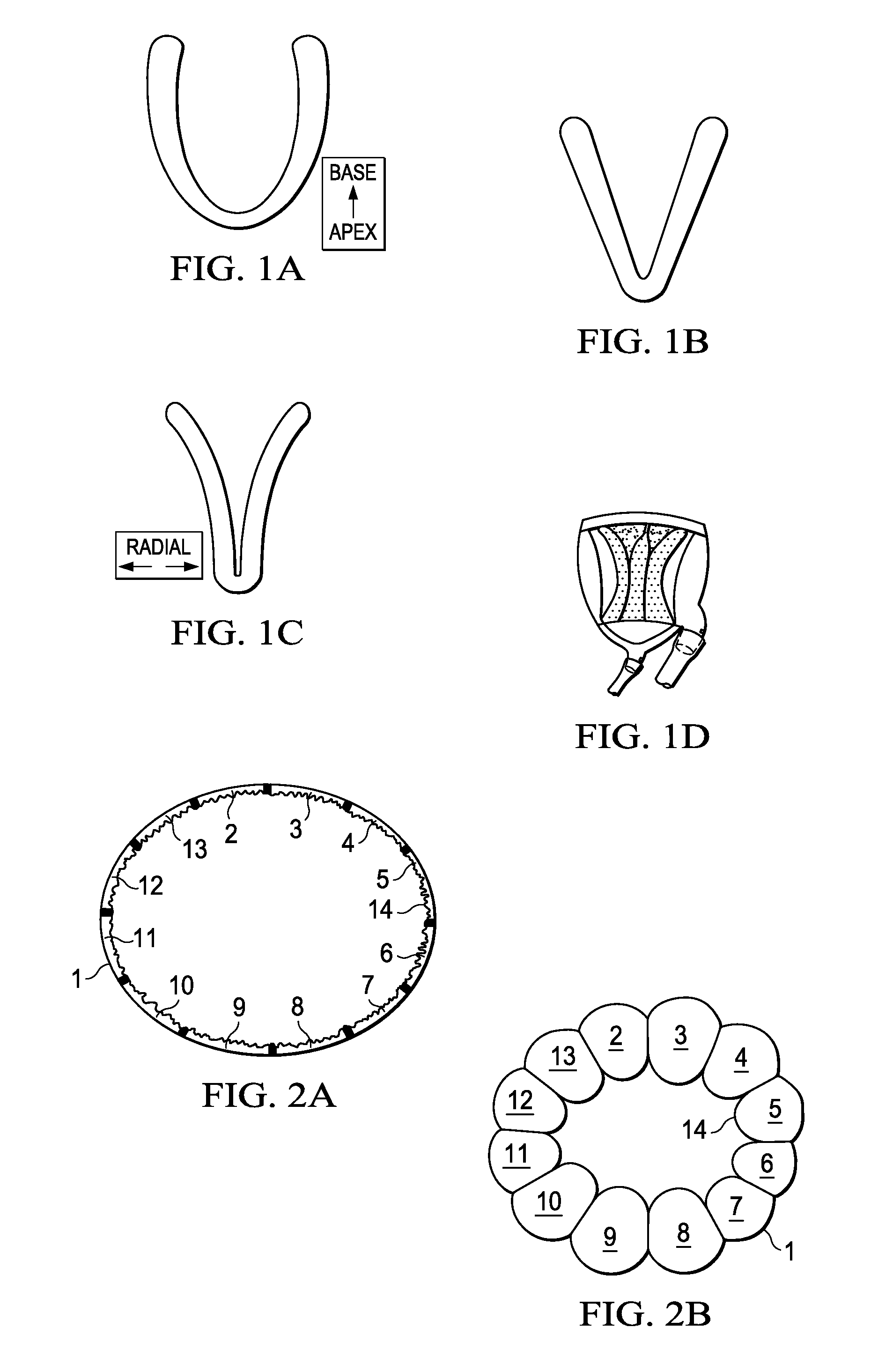
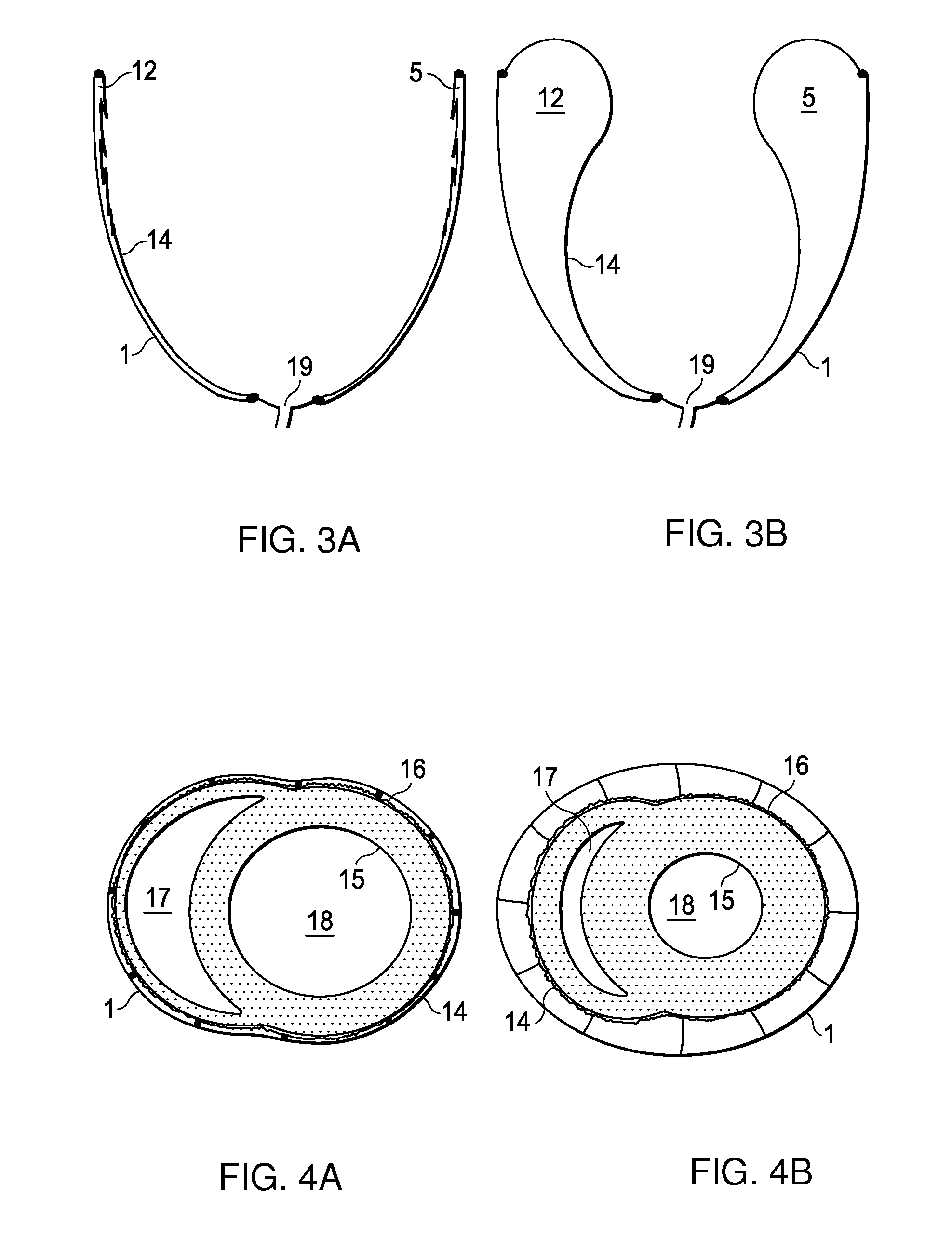
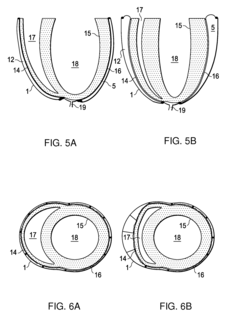
Design rules for pneumatic actuation to replicate cyclic mechanical strain in cardiac and lung chips
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Pneumatic Actuation Background and Objectives
Pneumatic actuation systems have emerged as a cornerstone technology in the development of organ-on-chip platforms, particularly for cardiac and lung models where cyclic mechanical strain is essential for physiological relevance. The evolution of this technology can be traced back to early microfluidic systems in the late 1990s, with significant advancements occurring over the past decade as biomimetic in vitro models gained prominence in drug development and disease modeling applications.
The fundamental principle behind pneumatic actuation involves the controlled application of positive or negative pressure to deform flexible membranes, thereby creating mechanical forces that simulate the dynamic mechanical environment experienced by cells in vivo. This approach has proven particularly valuable for replicating the rhythmic mechanical strains present in cardiac tissue during heartbeats and in lung alveoli during breathing cycles.
Current technological trends in pneumatic actuation for organ chips are moving toward more precise control systems, miniaturization, and integration with sensing technologies. The field is witnessing a shift from simple on/off pressure systems to sophisticated controllers capable of generating complex waveforms that better mimic physiological strain patterns. Additionally, there is growing interest in developing systems that can simultaneously actuate multiple chambers with independent control parameters.
The primary technical objectives for pneumatic actuation in cardiac and lung chips include achieving physiologically relevant strain magnitudes (typically 5-15% for cardiac tissue and 5-25% for lung tissue), appropriate strain rates, and cycle frequencies that match human physiology (approximately 1 Hz for cardiac and 0.2-0.5 Hz for breathing). Furthermore, these systems must maintain stable performance over extended periods (days to weeks) to support long-term cell culture and chronic studies.
Another critical objective is the standardization of design parameters to ensure reproducibility across different research platforms. This includes establishing clear relationships between applied pressure and resulting strain, optimizing membrane materials and geometries, and developing robust fabrication protocols that can be widely adopted by the research community.
The integration of pneumatic actuation with other organ chip functionalities, such as perfusion flow, electrical stimulation, and real-time monitoring, represents an additional technical goal. This integration is essential for creating comprehensive organ models that can recapitulate multiple aspects of tissue physiology simultaneously, thereby enhancing their predictive value for drug development and disease modeling applications.
The fundamental principle behind pneumatic actuation involves the controlled application of positive or negative pressure to deform flexible membranes, thereby creating mechanical forces that simulate the dynamic mechanical environment experienced by cells in vivo. This approach has proven particularly valuable for replicating the rhythmic mechanical strains present in cardiac tissue during heartbeats and in lung alveoli during breathing cycles.
Current technological trends in pneumatic actuation for organ chips are moving toward more precise control systems, miniaturization, and integration with sensing technologies. The field is witnessing a shift from simple on/off pressure systems to sophisticated controllers capable of generating complex waveforms that better mimic physiological strain patterns. Additionally, there is growing interest in developing systems that can simultaneously actuate multiple chambers with independent control parameters.
The primary technical objectives for pneumatic actuation in cardiac and lung chips include achieving physiologically relevant strain magnitudes (typically 5-15% for cardiac tissue and 5-25% for lung tissue), appropriate strain rates, and cycle frequencies that match human physiology (approximately 1 Hz for cardiac and 0.2-0.5 Hz for breathing). Furthermore, these systems must maintain stable performance over extended periods (days to weeks) to support long-term cell culture and chronic studies.
Another critical objective is the standardization of design parameters to ensure reproducibility across different research platforms. This includes establishing clear relationships between applied pressure and resulting strain, optimizing membrane materials and geometries, and developing robust fabrication protocols that can be widely adopted by the research community.
The integration of pneumatic actuation with other organ chip functionalities, such as perfusion flow, electrical stimulation, and real-time monitoring, represents an additional technical goal. This integration is essential for creating comprehensive organ models that can recapitulate multiple aspects of tissue physiology simultaneously, thereby enhancing their predictive value for drug development and disease modeling applications.
Market Analysis for Organ-on-Chip Technologies
The organ-on-chip (OOC) market is experiencing significant growth, driven by increasing demand for alternatives to animal testing and more physiologically relevant in vitro models. The global OOC market was valued at approximately $30 million in 2019 and is projected to reach $220 million by 2025, representing a compound annual growth rate (CAGR) of 39.9%. This remarkable growth trajectory underscores the transformative potential of this technology across multiple sectors.
Cardiac and lung chips represent two of the most commercially significant segments within the OOC market. The cardiac chip segment currently holds about 25% of the market share, while lung chips account for approximately 20%. These segments are particularly valuable due to the high failure rates of cardiac and pulmonary drugs in clinical trials, creating strong demand for better preclinical models that can accurately replicate the mechanical strains and physiological conditions of these organs.
Pharmaceutical companies constitute the largest end-user segment, accounting for over 60% of the OOC market. These companies are increasingly adopting organ chips to reduce drug development costs, which can exceed $2.6 billion per approved drug, and to address the 90% failure rate of drug candidates in clinical trials. Academic research institutions represent the second-largest market segment at 25%, followed by cosmetics companies at 10%.
Regionally, North America dominates the OOC market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region is expected to witness the fastest growth rate of 42% CAGR through 2025, primarily driven by increasing R&D investments in China, Japan, and South Korea.
Key market drivers include stringent regulatory requirements limiting animal testing, rising R&D expenditure in pharmaceutical industries, and growing awareness about the limitations of traditional cell culture and animal models. The pneumatic actuation systems specifically designed for cardiac and lung chips represent a specialized sub-segment estimated at $8 million in 2020, with projected growth to $45 million by 2025.
Market challenges include high development costs, technical complexities in designing reliable pneumatic actuation systems that can precisely replicate physiological mechanical strains, and the need for standardization across platforms. Additionally, the integration of these systems with existing laboratory workflows and analytical instruments presents adoption barriers that manufacturers must address to expand market penetration.
Cardiac and lung chips represent two of the most commercially significant segments within the OOC market. The cardiac chip segment currently holds about 25% of the market share, while lung chips account for approximately 20%. These segments are particularly valuable due to the high failure rates of cardiac and pulmonary drugs in clinical trials, creating strong demand for better preclinical models that can accurately replicate the mechanical strains and physiological conditions of these organs.
Pharmaceutical companies constitute the largest end-user segment, accounting for over 60% of the OOC market. These companies are increasingly adopting organ chips to reduce drug development costs, which can exceed $2.6 billion per approved drug, and to address the 90% failure rate of drug candidates in clinical trials. Academic research institutions represent the second-largest market segment at 25%, followed by cosmetics companies at 10%.
Regionally, North America dominates the OOC market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region is expected to witness the fastest growth rate of 42% CAGR through 2025, primarily driven by increasing R&D investments in China, Japan, and South Korea.
Key market drivers include stringent regulatory requirements limiting animal testing, rising R&D expenditure in pharmaceutical industries, and growing awareness about the limitations of traditional cell culture and animal models. The pneumatic actuation systems specifically designed for cardiac and lung chips represent a specialized sub-segment estimated at $8 million in 2020, with projected growth to $45 million by 2025.
Market challenges include high development costs, technical complexities in designing reliable pneumatic actuation systems that can precisely replicate physiological mechanical strains, and the need for standardization across platforms. Additionally, the integration of these systems with existing laboratory workflows and analytical instruments presents adoption barriers that manufacturers must address to expand market penetration.
Current Challenges in Biomimetic Mechanical Strain
Despite significant advancements in organ-on-chip technology, creating biomimetic mechanical strain that accurately replicates physiological conditions remains a formidable challenge. Current pneumatic actuation systems for cardiac and lung chips struggle to precisely mimic the complex, multi-directional strain patterns observed in native tissues. The primary limitation lies in achieving spatiotemporal control over strain parameters, including magnitude, frequency, and directionality, which vary significantly across different regions of organs during physiological function.
Material selection presents another substantial hurdle. The mechanical properties of PDMS, the most commonly used substrate, differ considerably from native tissue. While PDMS offers excellent optical transparency and fabrication versatility, its stiffness (typically 1-3 MPa) far exceeds that of cardiac tissue (~10-15 kPa) or lung tissue (~2-5 kPa), resulting in non-physiological mechanotransduction signaling in cultured cells.
Scaling and reproducibility issues further complicate biomimetic strain implementation. Current pneumatic actuation systems often exhibit inconsistent performance across different devices and laboratories, hampering standardization efforts. The miniaturized nature of these systems makes precise control of pressure distribution challenging, leading to variability in strain patterns even within a single device.
Integration with sensing technologies represents another significant challenge. Real-time monitoring of applied strain is essential for validating biomimetic conditions, yet current systems rarely incorporate feedback mechanisms to adjust actuation parameters based on actual tissue deformation. This open-loop approach limits the ability to maintain physiologically relevant conditions throughout extended experimental periods.
The biological complexity of native tissues adds another layer of difficulty. Both cardiac and lung tissues exhibit heterogeneous cellular compositions and extracellular matrix organizations that influence local mechanical properties. Current pneumatic actuation systems typically apply uniform strain across the entire culture chamber, failing to recapitulate this heterogeneity and potentially misrepresenting tissue-level responses.
Long-term stability remains problematic for extended studies. Pneumatic systems often suffer from pressure leakage, membrane fatigue, and altered mechanical properties over time. These issues become particularly pronounced during studies requiring continuous cyclic strain for days or weeks, such as those investigating chronic disease models or tissue maturation processes.
Finally, computational modeling tools for predicting strain distributions in these complex systems remain underdeveloped. The lack of robust simulation capabilities hampers rational design approaches, forcing researchers to rely heavily on empirical optimization that is both time-consuming and resource-intensive.
Material selection presents another substantial hurdle. The mechanical properties of PDMS, the most commonly used substrate, differ considerably from native tissue. While PDMS offers excellent optical transparency and fabrication versatility, its stiffness (typically 1-3 MPa) far exceeds that of cardiac tissue (~10-15 kPa) or lung tissue (~2-5 kPa), resulting in non-physiological mechanotransduction signaling in cultured cells.
Scaling and reproducibility issues further complicate biomimetic strain implementation. Current pneumatic actuation systems often exhibit inconsistent performance across different devices and laboratories, hampering standardization efforts. The miniaturized nature of these systems makes precise control of pressure distribution challenging, leading to variability in strain patterns even within a single device.
Integration with sensing technologies represents another significant challenge. Real-time monitoring of applied strain is essential for validating biomimetic conditions, yet current systems rarely incorporate feedback mechanisms to adjust actuation parameters based on actual tissue deformation. This open-loop approach limits the ability to maintain physiologically relevant conditions throughout extended experimental periods.
The biological complexity of native tissues adds another layer of difficulty. Both cardiac and lung tissues exhibit heterogeneous cellular compositions and extracellular matrix organizations that influence local mechanical properties. Current pneumatic actuation systems typically apply uniform strain across the entire culture chamber, failing to recapitulate this heterogeneity and potentially misrepresenting tissue-level responses.
Long-term stability remains problematic for extended studies. Pneumatic systems often suffer from pressure leakage, membrane fatigue, and altered mechanical properties over time. These issues become particularly pronounced during studies requiring continuous cyclic strain for days or weeks, such as those investigating chronic disease models or tissue maturation processes.
Finally, computational modeling tools for predicting strain distributions in these complex systems remain underdeveloped. The lack of robust simulation capabilities hampers rational design approaches, forcing researchers to rely heavily on empirical optimization that is both time-consuming and resource-intensive.
Existing Pneumatic Actuation Methodologies
01 Pneumatic actuation systems for mechanical strain cycling
Pneumatic systems designed to generate controlled cyclic mechanical strain for various applications. These systems use compressed air to create repetitive movement patterns, allowing for precise control of force, frequency, and amplitude of the mechanical strain. The pneumatic actuation enables reliable operation in environments requiring consistent cyclic loading patterns while maintaining system durability over extended operational periods.- Pneumatic actuation systems for vehicle applications: Pneumatic actuation systems are utilized in various vehicle applications to provide controlled mechanical strain and movement. These systems use compressed air to generate force for operations such as braking, suspension adjustment, and seat positioning. The cyclic mechanical strain produced by these systems allows for reliable and repeatable operations in automotive environments, offering advantages in weight reduction and responsive control compared to hydraulic alternatives.
- Cyclic strain mechanisms in industrial automation: Industrial automation systems employ pneumatic actuators to create controlled cyclic mechanical strain for manufacturing processes. These systems are designed to provide precise, repetitive movements necessary for assembly lines, material handling, and quality testing. The pneumatic components are engineered to withstand continuous operation while maintaining consistent force output and timing, which is essential for production efficiency and product quality.
- Pneumatic control systems with strain monitoring: Advanced pneumatic actuation systems incorporate strain monitoring capabilities to optimize performance and prevent mechanical failures. These systems use sensors to measure the cyclic mechanical strain experienced by components during operation, allowing for real-time adjustments to air pressure and flow. This monitoring enables predictive maintenance, extends system lifespan, and ensures consistent operation even under varying load conditions.
- Energy-efficient pneumatic strain generation: Energy-efficient designs for pneumatic actuation systems focus on optimizing the generation and application of cyclic mechanical strain while minimizing compressed air consumption. These innovations include improved valve designs, air recovery systems, and intelligent control algorithms that adjust pressure based on required force. By reducing energy waste, these systems lower operational costs and environmental impact while maintaining the necessary mechanical strain for effective operation.
- Biomechanical applications of pneumatic strain systems: Pneumatic actuation systems are increasingly used in biomechanical applications to create controlled cyclic mechanical strain for medical devices, rehabilitation equipment, and prosthetics. These systems mimic natural movement patterns by providing adjustable resistance and support through precisely controlled air pressure. The gentle yet consistent force application makes pneumatic systems particularly suitable for therapeutic applications where comfort and safety are paramount while still delivering the necessary mechanical strain.
02 Vehicle suspension and damping systems using pneumatic actuation
Pneumatic actuation systems implemented in vehicle suspension technologies to manage cyclic mechanical strain from road conditions. These systems utilize compressed air chambers and valves to absorb and distribute mechanical forces, providing adaptive damping capabilities. The pneumatic components allow for real-time adjustment of suspension characteristics based on driving conditions, improving both comfort and handling while extending component lifespan through better strain management.Expand Specific Solutions03 Industrial automation with pneumatic strain control
Industrial applications utilizing pneumatic systems to control cyclic mechanical strain in manufacturing processes. These systems incorporate pressure regulators, valves, and actuators to deliver precise mechanical movements for operations such as forming, testing, and assembly. The pneumatic control architecture enables programmable strain patterns that can be adjusted for different material properties and production requirements, ensuring consistent quality while minimizing equipment wear.Expand Specific Solutions04 Biomedical applications of pneumatic strain systems
Specialized pneumatic actuation systems designed for biomedical applications requiring controlled cyclic mechanical strain. These systems are used in tissue engineering, cell culture studies, and medical device testing where biological materials need to be subjected to physiologically relevant mechanical forces. The pneumatic mechanisms provide gentle, precisely controlled strain patterns that can simulate in vivo conditions while maintaining sterility and biocompatibility requirements.Expand Specific Solutions05 Energy-efficient pneumatic strain cycling technologies
Advanced pneumatic actuation systems designed with energy efficiency as a primary consideration for cyclic strain applications. These systems incorporate innovative valve designs, pressure recovery mechanisms, and smart control algorithms to minimize compressed air consumption while maintaining precise strain control. The technologies include air recycling components, proportional control valves, and adaptive pressure management to reduce operational costs and environmental impact in applications requiring continuous cyclic mechanical strain.Expand Specific Solutions
Leading Developers in Organ-on-Chip Platforms
The pneumatic actuation for cardiac and lung chips market is in an early growth phase, characterized by increasing adoption of organ-on-chip technologies for drug development and disease modeling. The global market size is expanding rapidly, driven by pharmaceutical research needs and reduced animal testing requirements. Technical maturity varies significantly among key players, with established medical device companies like Edwards Lifesciences and CorInnova leading commercial applications, while academic institutions (University of Bern, Texas A&M, Tongji University) focus on fundamental research advancements. Emulate has emerged as a specialized leader in organ-chip platforms with proprietary pneumatic actuation systems. The technology is transitioning from research-focused applications toward standardized commercial solutions, with increasing collaboration between academic institutions and industry partners to overcome challenges in replicating physiologically relevant mechanical strain patterns.
Edwards Lifesciences Corp.
Technical Solution: Edwards Lifesciences has developed sophisticated pneumatic actuation systems for cardiac tissue models that precisely replicate physiological mechanical strains. Their approach utilizes a dual-chamber design with a central tissue compartment flanked by pneumatically actuated chambers. The system employs precision-controlled air pressure modulators that can generate complex waveforms mimicking cardiac cycle mechanics, with frequencies adjustable from 0.5-3 Hz to simulate different heart rates. Their design rules specify membrane thickness optimization (typically 15-30 μm) based on the specific cardiac tissue being modeled, with thinner membranes for more sensitive applications requiring higher strain transmission efficiency. The pneumatic channels are designed with specific width-to-depth ratios (typically 5:1 to 10:1) to ensure uniform strain distribution across the culture area. Edwards' system incorporates real-time pressure monitoring with feedback control loops that maintain precise strain parameters despite changes in tissue stiffness during culture. Their technology allows for independent control of multiple pneumatic lines, enabling the creation of complex, anisotropic strain patterns that better replicate the directional nature of cardiac tissue mechanics in vivo.
Strengths: Exceptional precision in replicating complex cardiac strain patterns; robust engineering with minimal drift over extended operation periods; comprehensive validation with human cardiac tissues. Weaknesses: Relatively high system complexity requiring specialized technical expertise; higher cost compared to simpler actuation systems; limited flexibility for rapid protocol modifications.
CorInnova, Inc.
Technical Solution: CorInnova has developed a specialized pneumatic actuation system focused primarily on cardiac tissue applications that require precise cyclic mechanical strain. Their technology utilizes a biomimetic approach with pneumatically actuated chambers designed to replicate the three-dimensional strain patterns experienced by cardiac tissues in vivo. The system employs a multi-layer design with flexible elastomeric membranes (typically 25-50 μm thick) that separate cell culture chambers from pneumatic control channels. Their design rules emphasize the importance of membrane material selection, with specific elastomers chosen to match the mechanical properties of native cardiac extracellular matrix (elastic modulus range of 10-50 kPa). The pneumatic control system incorporates precision regulators capable of generating physiologically relevant pressure waveforms with frequencies adjustable between 0.5-2.5 Hz to simulate various cardiac conditions. CorInnova's approach includes careful optimization of chamber geometry, with aspect ratios specifically designed to create anisotropic strain patterns that mimic the directional nature of cardiac tissue contraction. Their system allows for independent control of multiple pneumatic lines, enabling the creation of complex mechanical microenvironments that better replicate regional variations in cardiac strain patterns.
Strengths: Highly specialized for cardiac applications with excellent biomimicry of physiological strain patterns; robust design suitable for extended culture periods; validated correlation with cardiac tissue responses. Weaknesses: Limited flexibility for adaptation to non-cardiac applications; relatively complex setup requiring specialized technical knowledge; higher cost compared to simpler actuation systems.
Key Technical Innovations in Cyclic Strain Generation
Diastolic recoil method and device for treatment of cardiac pathologies
PatentActiveUS20150165104A1
Innovation
- A biphasic and dynamic diastolic recoil device with intrinsic pneumatic attachment to the exterior surface of the heart, capable of modulating end-systolic and end-diastolic configurations without inverting curvature, providing adjustable passive support and active assist to treat both systolic and diastolic heart failure, and promoting a physiological mechanical environment for cardiac stem cell therapies.
Materials Science Considerations for Pneumatic Systems
The selection of appropriate materials for pneumatic actuation systems in organ-on-chip devices is critical for achieving reliable and physiologically relevant mechanical strain patterns. Materials used in these systems must balance several competing requirements including biocompatibility, mechanical properties, and manufacturing considerations.
Polydimethylsiloxane (PDMS) remains the gold standard material for pneumatic actuation components in cardiac and lung chips due to its excellent elasticity, optical transparency, and gas permeability. The elastic modulus of PDMS can be tuned between 0.1-3 MPa by adjusting the base-to-curing agent ratio, allowing customization for specific strain requirements. However, PDMS presents challenges including hydrophobicity, potential for protein adsorption, and absorption of small hydrophobic molecules that may affect cell behavior.
Alternative elastomeric materials gaining traction include thermoplastic polyurethanes (TPUs) and styrenic block copolymers, which offer improved manufacturing scalability and batch-to-batch consistency compared to PDMS. These materials provide tunable mechanical properties while maintaining the flexibility needed for cyclic actuation.
Material fatigue represents a significant consideration for pneumatic systems designed to replicate physiological strain cycles. Cardiac tissues typically require actuation at 1-2 Hz continuously for extended periods, while lung tissues may experience slower but larger strain magnitudes. Materials must maintain consistent mechanical properties over millions of cycles without significant hysteresis or permanent deformation.
Surface modifications of pneumatic chamber materials can significantly impact performance. Plasma treatment, chemical functionalization, and coating strategies can improve wettability, reduce protein adsorption, and enhance biocompatibility without compromising mechanical properties essential for actuation.
The interface between different materials in multi-layer pneumatic systems demands careful consideration. Bonding techniques must create robust seals while maintaining the elastic properties required for actuation. Oxygen plasma treatment, chemical bonding agents, and mechanical clamping each present different advantages depending on the specific design requirements.
Manufacturing precision significantly impacts pneumatic actuation performance. Variations in membrane thickness as small as 10-20 μm can lead to substantial differences in strain transfer efficiency and spatial strain patterns. Advanced fabrication techniques including soft lithography, 3D printing, and precision molding must be optimized to ensure reproducible material properties across production batches.
Polydimethylsiloxane (PDMS) remains the gold standard material for pneumatic actuation components in cardiac and lung chips due to its excellent elasticity, optical transparency, and gas permeability. The elastic modulus of PDMS can be tuned between 0.1-3 MPa by adjusting the base-to-curing agent ratio, allowing customization for specific strain requirements. However, PDMS presents challenges including hydrophobicity, potential for protein adsorption, and absorption of small hydrophobic molecules that may affect cell behavior.
Alternative elastomeric materials gaining traction include thermoplastic polyurethanes (TPUs) and styrenic block copolymers, which offer improved manufacturing scalability and batch-to-batch consistency compared to PDMS. These materials provide tunable mechanical properties while maintaining the flexibility needed for cyclic actuation.
Material fatigue represents a significant consideration for pneumatic systems designed to replicate physiological strain cycles. Cardiac tissues typically require actuation at 1-2 Hz continuously for extended periods, while lung tissues may experience slower but larger strain magnitudes. Materials must maintain consistent mechanical properties over millions of cycles without significant hysteresis or permanent deformation.
Surface modifications of pneumatic chamber materials can significantly impact performance. Plasma treatment, chemical functionalization, and coating strategies can improve wettability, reduce protein adsorption, and enhance biocompatibility without compromising mechanical properties essential for actuation.
The interface between different materials in multi-layer pneumatic systems demands careful consideration. Bonding techniques must create robust seals while maintaining the elastic properties required for actuation. Oxygen plasma treatment, chemical bonding agents, and mechanical clamping each present different advantages depending on the specific design requirements.
Manufacturing precision significantly impacts pneumatic actuation performance. Variations in membrane thickness as small as 10-20 μm can lead to substantial differences in strain transfer efficiency and spatial strain patterns. Advanced fabrication techniques including soft lithography, 3D printing, and precision molding must be optimized to ensure reproducible material properties across production batches.
Validation Protocols for Physiological Strain Parameters
To ensure the validity and reliability of pneumatic actuation systems in organ-on-chip devices, comprehensive validation protocols for physiological strain parameters must be established. These protocols should verify that the mechanical strains generated by pneumatic actuation accurately replicate the physiological conditions experienced by cardiac and lung tissues in vivo.
Strain measurement techniques form the foundation of these validation protocols. Digital image correlation (DIC) offers non-contact full-field displacement and strain mapping capabilities, allowing researchers to visualize strain distributions across the entire membrane surface. Laser displacement sensors provide high-precision point measurements for real-time monitoring of membrane deformation during actuation cycles. Embedded strain gauges, while more invasive, deliver continuous electrical signals proportional to applied strain.
Calibration procedures are essential for ensuring measurement accuracy. Standard reference materials with known mechanical properties should be used to calibrate strain measurement systems before each experimental series. Multi-point calibration across the expected strain range (typically 5-20% for lung tissue and 10-15% for cardiac tissue) ensures linearity and accuracy throughout the physiological spectrum.
Temporal validation protocols must address the dynamic nature of physiological strains. Frequency response testing should verify system performance across breathing rates (12-20 cycles/minute) and cardiac beating frequencies (60-180 beats/minute). Strain waveform analysis using Fourier transforms can quantify how closely the actuated strain patterns match physiological profiles, with particular attention to rise/fall times and dwell periods.
Spatial uniformity assessment is critical for ensuring consistent cellular mechanostimulation. Heat maps of strain distribution should be generated to identify potential hotspots or dead zones. Coefficient of variation calculations across the cell culture area should target values below 15% to ensure experimental reproducibility. Edge effect characterization is particularly important as strain patterns often deviate near membrane boundaries.
Long-term stability testing protocols must verify system performance over extended experimental timeframes. Drift analysis should quantify changes in strain parameters during continuous operation (24-72 hours), with acceptable drift typically below 5% of the target strain value. Environmental sensitivity testing should evaluate system performance under varying temperature and humidity conditions typical of incubator environments.
Documentation standards for validation results should include comprehensive strain characterization reports with statistical analyses of spatial and temporal strain parameters. These protocols collectively ensure that pneumatic actuation systems deliver physiologically relevant mechanical stimulation to cells in organ-on-chip platforms.
Strain measurement techniques form the foundation of these validation protocols. Digital image correlation (DIC) offers non-contact full-field displacement and strain mapping capabilities, allowing researchers to visualize strain distributions across the entire membrane surface. Laser displacement sensors provide high-precision point measurements for real-time monitoring of membrane deformation during actuation cycles. Embedded strain gauges, while more invasive, deliver continuous electrical signals proportional to applied strain.
Calibration procedures are essential for ensuring measurement accuracy. Standard reference materials with known mechanical properties should be used to calibrate strain measurement systems before each experimental series. Multi-point calibration across the expected strain range (typically 5-20% for lung tissue and 10-15% for cardiac tissue) ensures linearity and accuracy throughout the physiological spectrum.
Temporal validation protocols must address the dynamic nature of physiological strains. Frequency response testing should verify system performance across breathing rates (12-20 cycles/minute) and cardiac beating frequencies (60-180 beats/minute). Strain waveform analysis using Fourier transforms can quantify how closely the actuated strain patterns match physiological profiles, with particular attention to rise/fall times and dwell periods.
Spatial uniformity assessment is critical for ensuring consistent cellular mechanostimulation. Heat maps of strain distribution should be generated to identify potential hotspots or dead zones. Coefficient of variation calculations across the cell culture area should target values below 15% to ensure experimental reproducibility. Edge effect characterization is particularly important as strain patterns often deviate near membrane boundaries.
Long-term stability testing protocols must verify system performance over extended experimental timeframes. Drift analysis should quantify changes in strain parameters during continuous operation (24-72 hours), with acceptable drift typically below 5% of the target strain value. Environmental sensitivity testing should evaluate system performance under varying temperature and humidity conditions typical of incubator environments.
Documentation standards for validation results should include comprehensive strain characterization reports with statistical analyses of spatial and temporal strain parameters. These protocols collectively ensure that pneumatic actuation systems deliver physiologically relevant mechanical stimulation to cells in organ-on-chip platforms.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!