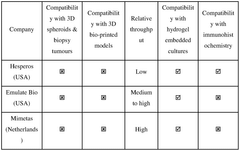
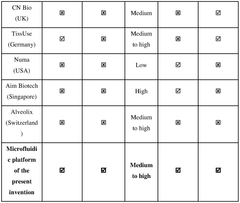
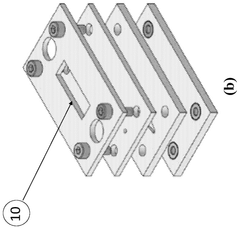
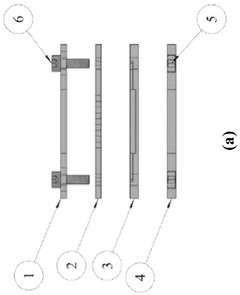
A modular microfluidic interface for rapid assembly of multi-organ microphysiological systems
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic Interface Technology Background and Objectives
Microfluidic technology has evolved significantly over the past three decades, transitioning from simple channel designs to sophisticated integrated systems capable of mimicking complex biological environments. The development of modular microfluidic interfaces represents a critical advancement in this field, enabling the interconnection of multiple organ models to create comprehensive microphysiological systems (MPS). These systems aim to replicate human physiological responses more accurately than traditional cell culture or animal models.
The evolution of microfluidic technology began in the 1990s with simple proof-of-concept devices, progressing through various stages of refinement to today's advanced organ-on-chip platforms. Early systems faced significant challenges in scalability, reproducibility, and integration capabilities. The introduction of standardized interfaces has been a game-changing development, allowing for plug-and-play functionality between different microfluidic components.
Current technological objectives in modular microfluidic interfaces focus on creating standardized, user-friendly systems that can be rapidly assembled without specialized expertise. These interfaces must maintain sterility, prevent cross-contamination, and ensure consistent fluid flow between connected organ modules. Additionally, they should accommodate various sensing technologies for real-time monitoring of cellular responses and metabolic activities.
The field is moving toward developing interfaces that can support long-term culture conditions, enabling the study of chronic disease states and drug effects over extended periods. This requires materials that are biocompatible, minimize non-specific protein adsorption, and maintain stable mechanical properties throughout experimental timeframes.
Another key objective is achieving physiologically relevant scaling between different organ modules to accurately model multi-organ interactions. This includes appropriate residence times of circulating factors and maintaining proper fluid-to-tissue ratios that reflect human physiology. The ultimate goal is to create systems that can recapitulate complex organ-organ interactions, including metabolic transformations of compounds as they pass through different tissue types.
Recent technological trends indicate a move toward incorporating automation and digital control systems, reducing user variability and enhancing reproducibility. There is also growing interest in developing interfaces compatible with high-throughput screening platforms to accelerate drug discovery and toxicology testing applications.
The convergence of microfluidics with advanced manufacturing techniques, such as 3D printing and microfabrication, is enabling more complex and customizable interface designs. These manufacturing approaches are helping to address previous limitations in scalability and mass production, potentially making multi-organ systems more accessible to researchers across various disciplines.
The evolution of microfluidic technology began in the 1990s with simple proof-of-concept devices, progressing through various stages of refinement to today's advanced organ-on-chip platforms. Early systems faced significant challenges in scalability, reproducibility, and integration capabilities. The introduction of standardized interfaces has been a game-changing development, allowing for plug-and-play functionality between different microfluidic components.
Current technological objectives in modular microfluidic interfaces focus on creating standardized, user-friendly systems that can be rapidly assembled without specialized expertise. These interfaces must maintain sterility, prevent cross-contamination, and ensure consistent fluid flow between connected organ modules. Additionally, they should accommodate various sensing technologies for real-time monitoring of cellular responses and metabolic activities.
The field is moving toward developing interfaces that can support long-term culture conditions, enabling the study of chronic disease states and drug effects over extended periods. This requires materials that are biocompatible, minimize non-specific protein adsorption, and maintain stable mechanical properties throughout experimental timeframes.
Another key objective is achieving physiologically relevant scaling between different organ modules to accurately model multi-organ interactions. This includes appropriate residence times of circulating factors and maintaining proper fluid-to-tissue ratios that reflect human physiology. The ultimate goal is to create systems that can recapitulate complex organ-organ interactions, including metabolic transformations of compounds as they pass through different tissue types.
Recent technological trends indicate a move toward incorporating automation and digital control systems, reducing user variability and enhancing reproducibility. There is also growing interest in developing interfaces compatible with high-throughput screening platforms to accelerate drug discovery and toxicology testing applications.
The convergence of microfluidics with advanced manufacturing techniques, such as 3D printing and microfabrication, is enabling more complex and customizable interface designs. These manufacturing approaches are helping to address previous limitations in scalability and mass production, potentially making multi-organ systems more accessible to researchers across various disciplines.
Market Analysis for Multi-Organ Microphysiological Systems
The multi-organ microphysiological systems (MPS) market is experiencing significant growth driven by increasing demand for more physiologically relevant drug testing platforms. Current global market valuation stands at approximately $1.2 billion with projections indicating a compound annual growth rate of 17-20% over the next five years, potentially reaching $3 billion by 2028.
Pharmaceutical and biotechnology companies represent the largest market segment, accounting for nearly 65% of the current demand. These organizations are increasingly adopting MPS technologies to reduce drug development costs, which currently average $2.6 billion per approved drug, with late-stage clinical failures due to toxicity or efficacy issues being a major contributor to these expenses.
Academic research institutions constitute the second-largest market segment at 25%, while contract research organizations represent approximately 10% of the market. Geographically, North America leads with 45% market share, followed by Europe (30%), Asia-Pacific (20%), and rest of the world (5%). The Asia-Pacific region, particularly China and South Korea, is expected to show the fastest growth rate due to increasing R&D investments.
Key market drivers include the push to reduce animal testing, regulatory support for alternative testing methods, and the need for more predictive human-relevant models. The FDA Modernization Act 2.0, which removes the requirement for animal testing in drug development, has created a favorable regulatory environment for MPS adoption.
Customer needs analysis reveals several critical requirements: physiological relevance, ease of use, reproducibility, compatibility with existing laboratory workflows, and cost-effectiveness. The modular microfluidic interface technology addresses these needs by enabling rapid assembly of multi-organ systems without specialized expertise.
Market barriers include high initial investment costs, technical complexity, and the need for validation against established testing methods. Additionally, standardization remains a challenge, with various proprietary systems using different protocols and readouts, complicating cross-platform comparisons.
The competitive landscape features established players like Emulate, TissUse, and CN Bio Innovations alongside emerging startups. Recent market consolidation has occurred through strategic acquisitions, such as Insphero's acquisition by Perkin Elmer, indicating growing interest from larger life science instrumentation companies.
Pricing models vary widely, with complete systems ranging from $50,000 to $200,000, while consumables represent a significant recurring revenue stream. The total addressable market is expected to expand as these systems move beyond drug development into toxicology testing, personalized medicine, and disease modeling applications.
Pharmaceutical and biotechnology companies represent the largest market segment, accounting for nearly 65% of the current demand. These organizations are increasingly adopting MPS technologies to reduce drug development costs, which currently average $2.6 billion per approved drug, with late-stage clinical failures due to toxicity or efficacy issues being a major contributor to these expenses.
Academic research institutions constitute the second-largest market segment at 25%, while contract research organizations represent approximately 10% of the market. Geographically, North America leads with 45% market share, followed by Europe (30%), Asia-Pacific (20%), and rest of the world (5%). The Asia-Pacific region, particularly China and South Korea, is expected to show the fastest growth rate due to increasing R&D investments.
Key market drivers include the push to reduce animal testing, regulatory support for alternative testing methods, and the need for more predictive human-relevant models. The FDA Modernization Act 2.0, which removes the requirement for animal testing in drug development, has created a favorable regulatory environment for MPS adoption.
Customer needs analysis reveals several critical requirements: physiological relevance, ease of use, reproducibility, compatibility with existing laboratory workflows, and cost-effectiveness. The modular microfluidic interface technology addresses these needs by enabling rapid assembly of multi-organ systems without specialized expertise.
Market barriers include high initial investment costs, technical complexity, and the need for validation against established testing methods. Additionally, standardization remains a challenge, with various proprietary systems using different protocols and readouts, complicating cross-platform comparisons.
The competitive landscape features established players like Emulate, TissUse, and CN Bio Innovations alongside emerging startups. Recent market consolidation has occurred through strategic acquisitions, such as Insphero's acquisition by Perkin Elmer, indicating growing interest from larger life science instrumentation companies.
Pricing models vary widely, with complete systems ranging from $50,000 to $200,000, while consumables represent a significant recurring revenue stream. The total addressable market is expected to expand as these systems move beyond drug development into toxicology testing, personalized medicine, and disease modeling applications.
Current Challenges in Microfluidic Interface Development
Despite significant advancements in microfluidic technology for organ-on-chip applications, several critical challenges persist in developing effective interfaces for multi-organ microphysiological systems. The primary obstacle remains achieving reliable, leak-free connections between different organ modules while maintaining sterility and preventing cross-contamination. Current connection methods often involve complex manual assembly processes that introduce variability and compromise experimental reproducibility.
Scaling remains problematic as researchers attempt to integrate increasing numbers of organ modules. Traditional microfluidic interfaces become unwieldy when connecting more than three or four organ compartments, creating spatial constraints and fluid distribution challenges. The complexity increases exponentially with each additional organ module, making truly comprehensive human-on-chip systems difficult to realize with current interface technologies.
Material compatibility presents another significant hurdle. Different organ modules may require specific materials for optimal cell function, yet these materials must also be compatible with the interface components. PDMS (polydimethylsiloxane), while widely used, exhibits limitations including drug absorption, protein adsorption, and hydrophobicity changes over time that can affect experimental outcomes. Alternative materials often introduce new compatibility issues with biological samples or manufacturing processes.
Flow control and pressure regulation across multiple organ modules represent sophisticated engineering challenges. Maintaining physiologically relevant fluid dynamics becomes increasingly difficult as system complexity grows. Current interface technologies struggle to accommodate the different flow rates and pressure requirements of various organ modules, potentially creating non-physiological conditions that compromise biological relevance.
Standardization remains elusive in the field, with most research groups developing proprietary connection systems that lack interoperability. This fragmentation impedes progress as researchers cannot easily combine innovations from different laboratories. The absence of standardized interfaces also creates barriers to commercial translation and widespread adoption of organ-on-chip technology in pharmaceutical and clinical settings.
Real-time monitoring capabilities are frequently compromised by current interface designs. Integrating sensors at connection points without disrupting fluid flow or cellular environments presents significant technical difficulties. Most existing interfaces require trade-offs between monitoring capabilities and system integrity, limiting the comprehensive data collection needed for advanced physiological modeling.
Manufacturing scalability represents a final major challenge. Many current microfluidic interfaces rely on fabrication techniques that are difficult to scale for mass production, involving complex multi-step processes or specialized equipment. This limitation restricts broader implementation and increases costs, preventing wider adoption of multi-organ microphysiological systems in research and industrial applications.
Scaling remains problematic as researchers attempt to integrate increasing numbers of organ modules. Traditional microfluidic interfaces become unwieldy when connecting more than three or four organ compartments, creating spatial constraints and fluid distribution challenges. The complexity increases exponentially with each additional organ module, making truly comprehensive human-on-chip systems difficult to realize with current interface technologies.
Material compatibility presents another significant hurdle. Different organ modules may require specific materials for optimal cell function, yet these materials must also be compatible with the interface components. PDMS (polydimethylsiloxane), while widely used, exhibits limitations including drug absorption, protein adsorption, and hydrophobicity changes over time that can affect experimental outcomes. Alternative materials often introduce new compatibility issues with biological samples or manufacturing processes.
Flow control and pressure regulation across multiple organ modules represent sophisticated engineering challenges. Maintaining physiologically relevant fluid dynamics becomes increasingly difficult as system complexity grows. Current interface technologies struggle to accommodate the different flow rates and pressure requirements of various organ modules, potentially creating non-physiological conditions that compromise biological relevance.
Standardization remains elusive in the field, with most research groups developing proprietary connection systems that lack interoperability. This fragmentation impedes progress as researchers cannot easily combine innovations from different laboratories. The absence of standardized interfaces also creates barriers to commercial translation and widespread adoption of organ-on-chip technology in pharmaceutical and clinical settings.
Real-time monitoring capabilities are frequently compromised by current interface designs. Integrating sensors at connection points without disrupting fluid flow or cellular environments presents significant technical difficulties. Most existing interfaces require trade-offs between monitoring capabilities and system integrity, limiting the comprehensive data collection needed for advanced physiological modeling.
Manufacturing scalability represents a final major challenge. Many current microfluidic interfaces rely on fabrication techniques that are difficult to scale for mass production, involving complex multi-step processes or specialized equipment. This limitation restricts broader implementation and increases costs, preventing wider adoption of multi-organ microphysiological systems in research and industrial applications.
Current Modular Interface Design Solutions
01 Modular microfluidic connectors for rapid assembly
Specialized connectors designed for microfluidic systems that allow for quick and tool-free assembly and disassembly. These connectors feature standardized interfaces that enable different microfluidic modules to be connected together without leakage. The modular design allows researchers to rapidly reconfigure microfluidic systems for different experiments without requiring complete redesign or fabrication of new components.- Modular microfluidic components for rapid assembly: Modular microfluidic systems that feature standardized components designed for quick and easy assembly without specialized tools. These systems include interlocking parts, snap-fit connections, and pre-fabricated modules that can be combined in various configurations to create customized microfluidic devices. The modular approach allows researchers to rapidly prototype and modify designs without the need for complete system redesign.
- Interface connectors for microfluidic systems: Specialized interface connectors that enable seamless integration between different microfluidic components. These connectors provide leak-proof junctions while maintaining fluid flow integrity across module boundaries. The interfaces include standardized ports, gaskets, and alignment features that ensure proper connection between modules and external equipment such as pumps, sensors, or analytical instruments.
- Plug-and-play microfluidic platforms: Plug-and-play microfluidic platforms that allow users to quickly reconfigure system components without specialized knowledge. These platforms feature standardized connection protocols, automated alignment mechanisms, and universal interfaces that accommodate various module types. The systems often include software interfaces that automatically recognize connected components and adjust operational parameters accordingly.
- Rapid prototyping methods for microfluidic devices: Methods and technologies for rapidly prototyping microfluidic devices, including 3D printing, laser cutting, and injection molding techniques adapted for quick turnaround. These approaches enable researchers to quickly iterate designs, test concepts, and produce functional microfluidic systems in hours rather than days or weeks. The methods often incorporate standardized connection features to ensure compatibility with existing modular systems.
- Automated assembly systems for microfluidic devices: Automated systems that facilitate the rapid assembly of microfluidic devices through robotic handling, precision alignment, and quality control mechanisms. These systems reduce human error in assembly, increase reproducibility, and enable high-throughput production of complex microfluidic devices. The automation includes component recognition, positioning verification, and testing protocols to ensure proper functionality of the assembled devices.
02 Plug-and-play microfluidic platforms
Integrated microfluidic platforms that utilize a plug-and-play approach for rapid assembly of complex systems. These platforms feature standardized interfaces and connection mechanisms that allow various functional modules to be easily combined. The systems often include alignment features, sealing mechanisms, and fluid routing capabilities that enable users to quickly assemble customized microfluidic devices without specialized equipment.Expand Specific Solutions03 Automated assembly systems for microfluidic devices
Automated systems designed specifically for the assembly of microfluidic components. These systems utilize robotic handling, precision alignment mechanisms, and automated testing to rapidly assemble microfluidic interfaces. The automation reduces human error, increases throughput, and ensures consistent quality in the assembly process, making it suitable for both research and production environments.Expand Specific Solutions04 Reconfigurable microfluidic interface systems
Microfluidic systems with interfaces designed for rapid reconfiguration and adaptation. These systems feature modular components that can be rearranged in different configurations to achieve various functionalities. The interfaces include features for fluid transfer, electrical connections, and optical access, allowing users to quickly modify experimental setups without fabricating new devices.Expand Specific Solutions05 Standardized microfluidic interface protocols
Standardized protocols and designs for microfluidic interfaces that facilitate rapid assembly across different platforms. These standards define connection geometries, sealing mechanisms, and operational parameters to ensure compatibility between components from different sources. The standardization enables researchers to mix and match components, accelerating development cycles and promoting collaboration across different laboratories and industries.Expand Specific Solutions
Leading Organizations in Microphysiological Systems Research
The microfluidic multi-organ microphysiological systems market is in its growth phase, characterized by increasing adoption across pharmaceutical and academic research sectors. The global market size for organ-on-chip technologies is expanding rapidly, projected to reach several billion dollars by 2030. Technologically, the field shows moderate maturity with established players like Corning and Agilent Technologies providing foundational microfluidic solutions, while academic institutions (MIT, Cornell, National University of Singapore) drive innovation. Emerging companies like Nutcracker Therapeutics and Fluicell are advancing specialized applications. The competitive landscape features collaboration between industry leaders and research institutions, with pharmaceutical companies like Sinopec increasingly investing in this technology for drug development applications. The modular approach represents a significant advancement in addressing standardization challenges that have previously limited widespread commercial adoption.
The Regents of the University of Michigan
Technical Solution: The University of Michigan has developed a sophisticated microfluidic interface system called "MicroJoint" for connecting multiple organ-on-chip platforms. Their technology utilizes a universal connector design that accommodates different chip geometries and materials through standardized ports. The system incorporates a novel gasket-based sealing mechanism that ensures leak-free connections while minimizing dead volumes between modules. Michigan's platform features integrated bubble traps at each interface to prevent air bubbles from disrupting cellular environments or blocking microchannels. The interface includes automated flow control systems that maintain physiologically relevant fluid residence times across connected organs. Their technology has been validated with interconnected liver-heart-lung models demonstrating appropriate metabolite exchange and drug response patterns. The platform also incorporates sampling ports that allow for non-disruptive collection of media from specific points in the multi-organ circuit for detailed metabolomic analysis.
Strengths: The universal connector design enables integration of organ chips from different manufacturers, promoting collaboration and standardization in the field. The automated flow control system ensures consistent conditions throughout extended experiments. Weaknesses: The complex interface components may increase the overall footprint of the system, potentially limiting the number of organ modules that can be practically connected in space-constrained laboratory settings.
Cornell University
Technical Solution: Cornell University has pioneered a microfluidic interface technology called "HuBiX" (Human Body-on-Interconnected-Chips) that addresses the challenge of connecting multiple organ-on-chip systems. Their approach utilizes a central routing manifold with programmable valves that enable dynamic reconfiguration of fluidic pathways between organ modules. The system incorporates gravity-driven flow to minimize shear stress on cellular components while maintaining physiological conditions. Cornell's technology features removable organ modules that can be independently cultured before assembly into the integrated system, allowing for optimal tissue maturation. The interface includes integrated optical windows for real-time imaging and monitoring of cellular responses. Their platform has been validated with liver-kidney-intestine-lung interconnected systems demonstrating physiologically relevant drug metabolism and toxicity responses across multiple tissue types.
Strengths: The gravity-driven flow system eliminates the need for external pumps, simplifying operation and reducing potential points of failure. The ability to pre-culture organ modules independently before assembly allows for optimal tissue development. Weaknesses: The reliance on gravity flow may limit the range of flow rates achievable, potentially restricting applications requiring precise control over shear stress or pulsatile flow conditions.
Key Technical Innovations in Rapid Assembly Interfaces
Modular microfluidic assay system
PatentWO2018226163A1
Innovation
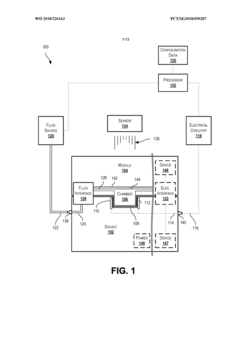
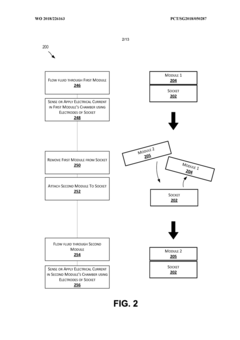
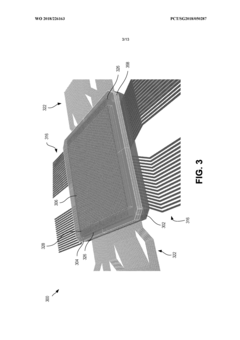
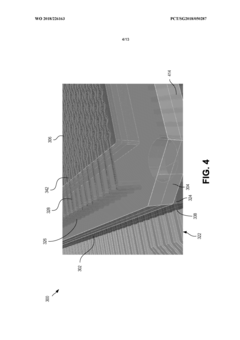
- A modular microfluidic system where electrodes are located in a reusable socket and microfluidics are in a disposable module, allowing for easy swapping and configuration through a standardized fluid and electrical interface, enabling rapid deployment of different assays without the need for re-outfitting existing chips.
A versatile microfluidic apparatus and an attachment thereof
PatentWO2024246945A9
Innovation
- A versatile microfluidic apparatus with a multi-compartment design and attachment-based concept that allows for the culture of various 3D cell models, such as spheroids, biopsy tissues, and organoids, without the need for continuous membranes, enabling multi-cellular interactions and mimicking organ-specific micro-physiological environments.
Standardization Efforts in Microfluidic Modules
Standardization efforts in the microfluidic field have gained significant momentum in recent years, particularly for modular systems that enable multi-organ microphysiological platforms. The lack of standardized interfaces between microfluidic modules has historically hindered broader adoption and commercialization of these technologies. Several international consortia and research groups have been working to establish common specifications for physical connections, flow rates, and communication protocols between microfluidic building blocks.
The EU-funded ORCHID project represents one of the most comprehensive standardization initiatives, focusing on establishing uniform connection standards for organ-on-chip modules. Similarly, the U.S.-based Tissue Chip Consortium has proposed dimensional standards for microfluidic connectors that ensure compatibility across different manufacturer platforms. These efforts aim to create an ecosystem where researchers can mix and match modules from various sources without compatibility concerns.
ISO Technical Committee 276 on Biotechnology has recently formed a working group specifically addressing standardization needs for microfluidic interfaces in biological applications. Their draft guidelines emphasize the importance of standardized fluidic ports, electrical connections, and optical access points to facilitate module integration. The committee has proposed a dimensional standard based on a 9mm grid pattern that allows for scalable assembly of multiple modules.
Industry players have also contributed to standardization efforts, with companies like Emulate, TissUse, and Mimetas working together to develop cross-compatible connection systems. The "Microfluidic Open Interface Standard" (MOIS) proposed by this industry consortium defines specifications for fluid handling, electrical connections, and mechanical alignment features. This standard has been implemented in several commercial platforms and is gaining traction in academic research settings.
Academic research groups have developed and published open-source standards for microfluidic connections. The "Plug-and-Play Microfluidics" initiative from MIT and the "Universal Microfluidic Connector" from Stanford represent significant contributions that have been adopted by multiple laboratories worldwide. These open standards typically include detailed CAD files and fabrication protocols to ensure reproducibility.
Challenges remain in achieving widespread adoption of these standards, particularly in reconciling the diverse requirements of different organ models. Flow rates, media composition, and sensing requirements can vary dramatically between liver, kidney, and brain models, complicating standardization efforts. Future standardization work must balance the need for uniformity with the flexibility required to accommodate biological complexity in multi-organ systems.
The EU-funded ORCHID project represents one of the most comprehensive standardization initiatives, focusing on establishing uniform connection standards for organ-on-chip modules. Similarly, the U.S.-based Tissue Chip Consortium has proposed dimensional standards for microfluidic connectors that ensure compatibility across different manufacturer platforms. These efforts aim to create an ecosystem where researchers can mix and match modules from various sources without compatibility concerns.
ISO Technical Committee 276 on Biotechnology has recently formed a working group specifically addressing standardization needs for microfluidic interfaces in biological applications. Their draft guidelines emphasize the importance of standardized fluidic ports, electrical connections, and optical access points to facilitate module integration. The committee has proposed a dimensional standard based on a 9mm grid pattern that allows for scalable assembly of multiple modules.
Industry players have also contributed to standardization efforts, with companies like Emulate, TissUse, and Mimetas working together to develop cross-compatible connection systems. The "Microfluidic Open Interface Standard" (MOIS) proposed by this industry consortium defines specifications for fluid handling, electrical connections, and mechanical alignment features. This standard has been implemented in several commercial platforms and is gaining traction in academic research settings.
Academic research groups have developed and published open-source standards for microfluidic connections. The "Plug-and-Play Microfluidics" initiative from MIT and the "Universal Microfluidic Connector" from Stanford represent significant contributions that have been adopted by multiple laboratories worldwide. These open standards typically include detailed CAD files and fabrication protocols to ensure reproducibility.
Challenges remain in achieving widespread adoption of these standards, particularly in reconciling the diverse requirements of different organ models. Flow rates, media composition, and sensing requirements can vary dramatically between liver, kidney, and brain models, complicating standardization efforts. Future standardization work must balance the need for uniformity with the flexibility required to accommodate biological complexity in multi-organ systems.
Translational Applications in Drug Discovery and Toxicology
Modular microfluidic multi-organ systems represent a revolutionary approach in drug discovery and toxicology, offering unprecedented capabilities for predicting human responses to pharmaceutical compounds. These systems bridge the critical translational gap between traditional cell culture models and clinical trials by simulating complex organ interactions within a controlled environment.
The pharmaceutical industry faces significant challenges with high attrition rates during drug development, with approximately 90% of candidates failing in clinical trials despite promising preclinical results. Multi-organ microphysiological systems (MPS) address this issue by providing more physiologically relevant models that can detect toxicity and efficacy issues earlier in the development pipeline, potentially saving billions in development costs.
In toxicology applications, these systems enable assessment of both acute and chronic exposure effects across multiple organ systems simultaneously. This capability is particularly valuable for evaluating compounds that may produce metabolites in one organ that affect others - a phenomenon traditional single-organ models cannot capture. The liver-kidney-intestine connections, for example, provide crucial insights into ADME (absorption, distribution, metabolism, excretion) properties that determine a compound's safety profile.
Pharmaceutical companies have begun implementing these platforms in decision-making processes, with several reporting improved predictive accuracy compared to conventional methods. Case studies demonstrate successful identification of hepatotoxicity, cardiotoxicity, and nephrotoxicity that animal models had missed. The FDA and EMA have recognized the potential of these systems, initiating qualification programs for their integration into regulatory frameworks.
The economic impact extends beyond drug development efficiency. These systems also support the 3Rs principle (replacement, reduction, refinement) in animal testing, addressing both ethical concerns and regulatory pressures to minimize animal experimentation. Companies adopting these technologies report significant reductions in animal usage while maintaining or improving predictive capabilities.
For personalized medicine applications, patient-derived cells can be incorporated into these platforms, enabling testing of drug responses on individual genetic backgrounds. This approach has shown particular promise in oncology, where treatment efficacy varies significantly between patients. Several academic-industry partnerships are currently exploring the integration of these systems with AI-driven predictive algorithms to further enhance their translational value.
As the technology matures, standardization efforts are underway to ensure reproducibility across different laboratories and platforms, a critical step toward broader adoption in regulated environments. The combination of microfluidic engineering precision with advanced tissue engineering techniques positions these systems as transformative tools in the future landscape of drug discovery and safety assessment.
The pharmaceutical industry faces significant challenges with high attrition rates during drug development, with approximately 90% of candidates failing in clinical trials despite promising preclinical results. Multi-organ microphysiological systems (MPS) address this issue by providing more physiologically relevant models that can detect toxicity and efficacy issues earlier in the development pipeline, potentially saving billions in development costs.
In toxicology applications, these systems enable assessment of both acute and chronic exposure effects across multiple organ systems simultaneously. This capability is particularly valuable for evaluating compounds that may produce metabolites in one organ that affect others - a phenomenon traditional single-organ models cannot capture. The liver-kidney-intestine connections, for example, provide crucial insights into ADME (absorption, distribution, metabolism, excretion) properties that determine a compound's safety profile.
Pharmaceutical companies have begun implementing these platforms in decision-making processes, with several reporting improved predictive accuracy compared to conventional methods. Case studies demonstrate successful identification of hepatotoxicity, cardiotoxicity, and nephrotoxicity that animal models had missed. The FDA and EMA have recognized the potential of these systems, initiating qualification programs for their integration into regulatory frameworks.
The economic impact extends beyond drug development efficiency. These systems also support the 3Rs principle (replacement, reduction, refinement) in animal testing, addressing both ethical concerns and regulatory pressures to minimize animal experimentation. Companies adopting these technologies report significant reductions in animal usage while maintaining or improving predictive capabilities.
For personalized medicine applications, patient-derived cells can be incorporated into these platforms, enabling testing of drug responses on individual genetic backgrounds. This approach has shown particular promise in oncology, where treatment efficacy varies significantly between patients. Several academic-industry partnerships are currently exploring the integration of these systems with AI-driven predictive algorithms to further enhance their translational value.
As the technology matures, standardization efforts are underway to ensure reproducibility across different laboratories and platforms, a critical step toward broader adoption in regulated environments. The combination of microfluidic engineering precision with advanced tissue engineering techniques positions these systems as transformative tools in the future landscape of drug discovery and safety assessment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!