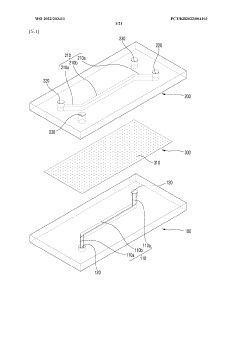
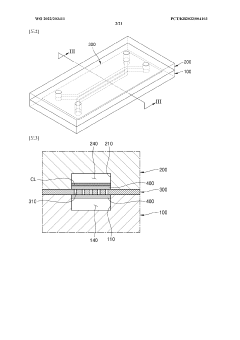
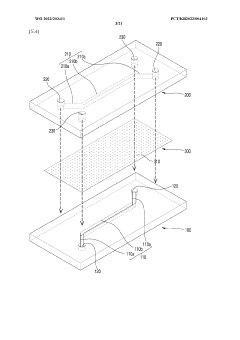
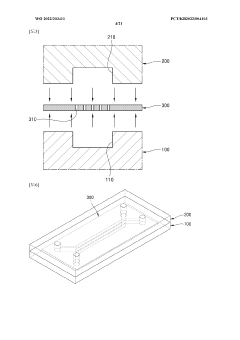
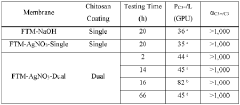
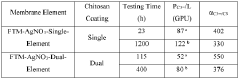
Engineering porous membrane materials to tune paracellular transport in barrier chips
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Porous Membrane Engineering Background and Objectives
The engineering of porous membrane materials for barrier chips has evolved significantly over the past decades, transitioning from simple filtration applications to sophisticated biomimetic platforms. Initially developed in the 1950s for basic separation processes, membrane technology has undergone revolutionary advancements with the integration of microfabrication techniques in the 1990s and nanotechnology in the early 2000s. This technological progression has enabled unprecedented control over membrane properties at the micro and nanoscale.
The fundamental objective of engineering porous membranes for barrier chips is to recreate the selective permeability characteristics of biological barriers found in living organisms. These barriers, such as the blood-brain barrier, gut epithelium, and lung alveolar interfaces, exhibit complex transport mechanisms that regulate the passage of molecules between compartments. By mimicking these natural barriers, researchers aim to develop in vitro models that accurately represent physiological conditions for drug development, toxicology studies, and fundamental biological research.
Current technological trends in this field focus on precise control over pore size distribution, surface chemistry modification, and mechanical properties of membranes. The integration of stimuli-responsive materials represents a cutting-edge approach, allowing dynamic adjustment of membrane properties in response to environmental cues. Additionally, the incorporation of bioactive components into membrane structures is gaining traction as a means to enhance cellular interaction and tissue-specific functions.
The convergence of materials science, microfluidics, and tissue engineering has accelerated innovation in this domain. Particularly noteworthy is the shift from traditional polymeric membranes toward hybrid materials that combine synthetic polymers with biological components. This hybrid approach aims to better recapitulate the complex microenvironment of native tissues while maintaining the reproducibility and tunability of engineered systems.
The technical objectives for advancing porous membrane engineering include achieving sub-micron control over pore geometry and distribution, developing membranes with gradient properties to mimic transitional zones in biological barriers, and creating materials with programmable permeability that can respond to physiological signals. Furthermore, there is a growing emphasis on developing manufacturing processes that enable scalable production of these sophisticated membrane systems while maintaining batch-to-batch consistency.
Ultimately, the goal is to establish a versatile platform technology that can be customized for specific barrier models, thereby accelerating drug discovery processes and reducing reliance on animal testing. This requires interdisciplinary collaboration between materials scientists, bioengineers, and pharmacologists to translate fundamental membrane engineering principles into functional barrier chip systems with predictive capabilities for human physiology.
The fundamental objective of engineering porous membranes for barrier chips is to recreate the selective permeability characteristics of biological barriers found in living organisms. These barriers, such as the blood-brain barrier, gut epithelium, and lung alveolar interfaces, exhibit complex transport mechanisms that regulate the passage of molecules between compartments. By mimicking these natural barriers, researchers aim to develop in vitro models that accurately represent physiological conditions for drug development, toxicology studies, and fundamental biological research.
Current technological trends in this field focus on precise control over pore size distribution, surface chemistry modification, and mechanical properties of membranes. The integration of stimuli-responsive materials represents a cutting-edge approach, allowing dynamic adjustment of membrane properties in response to environmental cues. Additionally, the incorporation of bioactive components into membrane structures is gaining traction as a means to enhance cellular interaction and tissue-specific functions.
The convergence of materials science, microfluidics, and tissue engineering has accelerated innovation in this domain. Particularly noteworthy is the shift from traditional polymeric membranes toward hybrid materials that combine synthetic polymers with biological components. This hybrid approach aims to better recapitulate the complex microenvironment of native tissues while maintaining the reproducibility and tunability of engineered systems.
The technical objectives for advancing porous membrane engineering include achieving sub-micron control over pore geometry and distribution, developing membranes with gradient properties to mimic transitional zones in biological barriers, and creating materials with programmable permeability that can respond to physiological signals. Furthermore, there is a growing emphasis on developing manufacturing processes that enable scalable production of these sophisticated membrane systems while maintaining batch-to-batch consistency.
Ultimately, the goal is to establish a versatile platform technology that can be customized for specific barrier models, thereby accelerating drug discovery processes and reducing reliance on animal testing. This requires interdisciplinary collaboration between materials scientists, bioengineers, and pharmacologists to translate fundamental membrane engineering principles into functional barrier chip systems with predictive capabilities for human physiology.
Market Analysis for Barrier Chip Applications
The global market for barrier chip applications is experiencing robust growth, driven by increasing demand for advanced drug development tools and personalized medicine solutions. The barrier chip market, valued at approximately $86 million in 2022, is projected to reach $225 million by 2028, representing a compound annual growth rate of 17.3% during the forecast period. This growth trajectory is primarily fueled by the pharmaceutical and biotechnology sectors seeking more physiologically relevant in vitro models for drug testing and development.
The pharmaceutical industry remains the largest end-user segment for barrier chip technologies, accounting for nearly 45% of the market share. This dominance stems from the critical need to reduce drug development costs and timelines while improving predictive accuracy. With the average cost to develop a new drug exceeding $2.6 billion and failure rates in clinical trials remaining high, pharmaceutical companies are increasingly turning to organ-on-chip technologies, including barrier chips, to better predict drug efficacy and toxicity before entering costly clinical trials.
Academic research institutions represent the second-largest market segment, contributing approximately 30% of market demand. These institutions play a crucial role in advancing fundamental research on barrier functions and developing novel applications for barrier chip technologies. The remaining market share is distributed among contract research organizations, cosmetics companies, and food safety testing laboratories.
Geographically, North America leads the market with approximately 40% share, followed by Europe (35%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and South Korea, is expected to witness the fastest growth rate of 22% annually, driven by increasing R&D investments and government initiatives supporting biotechnology development.
By application type, drug development and toxicity testing currently dominate the market, accounting for 55% of applications. Disease modeling represents 25% of the market, while personalized medicine applications constitute 15%. The remaining 5% encompasses various emerging applications including environmental toxicity assessment and cosmetics testing alternatives.
The market for engineered porous membranes specifically designed for barrier chips is estimated at $32 million in 2022, with projected growth to reach $95 million by 2028. This sub-segment is experiencing particularly strong demand due to the critical role these membranes play in accurately replicating physiological barriers and enabling precise control over paracellular transport - a key factor in developing more predictive in vitro models for drug absorption, distribution, metabolism, and excretion studies.
The pharmaceutical industry remains the largest end-user segment for barrier chip technologies, accounting for nearly 45% of the market share. This dominance stems from the critical need to reduce drug development costs and timelines while improving predictive accuracy. With the average cost to develop a new drug exceeding $2.6 billion and failure rates in clinical trials remaining high, pharmaceutical companies are increasingly turning to organ-on-chip technologies, including barrier chips, to better predict drug efficacy and toxicity before entering costly clinical trials.
Academic research institutions represent the second-largest market segment, contributing approximately 30% of market demand. These institutions play a crucial role in advancing fundamental research on barrier functions and developing novel applications for barrier chip technologies. The remaining market share is distributed among contract research organizations, cosmetics companies, and food safety testing laboratories.
Geographically, North America leads the market with approximately 40% share, followed by Europe (35%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and South Korea, is expected to witness the fastest growth rate of 22% annually, driven by increasing R&D investments and government initiatives supporting biotechnology development.
By application type, drug development and toxicity testing currently dominate the market, accounting for 55% of applications. Disease modeling represents 25% of the market, while personalized medicine applications constitute 15%. The remaining 5% encompasses various emerging applications including environmental toxicity assessment and cosmetics testing alternatives.
The market for engineered porous membranes specifically designed for barrier chips is estimated at $32 million in 2022, with projected growth to reach $95 million by 2028. This sub-segment is experiencing particularly strong demand due to the critical role these membranes play in accurately replicating physiological barriers and enabling precise control over paracellular transport - a key factor in developing more predictive in vitro models for drug absorption, distribution, metabolism, and excretion studies.
Current Challenges in Paracellular Transport Modulation
Despite significant advancements in barrier chip technology, modulating paracellular transport remains a formidable challenge. Current membrane materials often fail to accurately replicate the selective permeability characteristics of biological barriers, creating a significant gap between in vitro models and in vivo reality. The primary technical hurdle lies in engineering membranes with precisely controlled pore size distributions that can selectively regulate the passage of specific molecules while maintaining barrier integrity.
Conventional track-etched membranes used in barrier chips typically feature randomly distributed pores with limited control over pore geometry and surface properties. This randomness introduces variability in experimental results and limits the reproducibility of paracellular transport studies. Additionally, current manufacturing techniques struggle to create membranes thin enough to mimic physiological barriers while maintaining sufficient mechanical stability for long-term cell culture applications.
The integration of engineered membranes with microfluidic systems presents another significant challenge. Interface issues between the membrane and the surrounding chip structure often lead to leakage problems and non-specific transport pathways that compromise barrier integrity. These technical limitations severely restrict the ability to accurately model complex biological barriers such as the blood-brain barrier, gut epithelium, or lung alveolar interfaces.
Material biocompatibility represents a critical constraint in current membrane technologies. Many high-performance materials with desirable mechanical and transport properties exhibit poor cell adhesion characteristics or release compounds that affect cellular behavior. This forces researchers to make compromises between optimal transport properties and cellular compatibility, limiting the physiological relevance of barrier chip models.
Dynamic regulation of paracellular transport remains largely unachieved in current systems. Biological barriers naturally respond to various stimuli by modulating tight junction permeability, but existing membrane technologies lack this adaptive capability. The development of stimuli-responsive materials that can dynamically alter pore characteristics in response to biochemical or physical cues represents an unsolved technical challenge.
Scaling and manufacturing constraints further complicate widespread adoption of advanced membrane technologies. Novel approaches using nanofabrication techniques or advanced materials often face significant barriers to scale-up, limiting their practical application in commercial barrier chip platforms. The high cost and technical complexity of producing precisely engineered membranes at scale remain substantial obstacles to broader implementation.
Standardization issues across different research groups and commercial platforms create additional challenges for comparative studies. The lack of standardized methods for characterizing membrane properties and paracellular transport metrics makes it difficult to benchmark performance improvements and establish reliable design principles for next-generation barrier chip membranes.
Conventional track-etched membranes used in barrier chips typically feature randomly distributed pores with limited control over pore geometry and surface properties. This randomness introduces variability in experimental results and limits the reproducibility of paracellular transport studies. Additionally, current manufacturing techniques struggle to create membranes thin enough to mimic physiological barriers while maintaining sufficient mechanical stability for long-term cell culture applications.
The integration of engineered membranes with microfluidic systems presents another significant challenge. Interface issues between the membrane and the surrounding chip structure often lead to leakage problems and non-specific transport pathways that compromise barrier integrity. These technical limitations severely restrict the ability to accurately model complex biological barriers such as the blood-brain barrier, gut epithelium, or lung alveolar interfaces.
Material biocompatibility represents a critical constraint in current membrane technologies. Many high-performance materials with desirable mechanical and transport properties exhibit poor cell adhesion characteristics or release compounds that affect cellular behavior. This forces researchers to make compromises between optimal transport properties and cellular compatibility, limiting the physiological relevance of barrier chip models.
Dynamic regulation of paracellular transport remains largely unachieved in current systems. Biological barriers naturally respond to various stimuli by modulating tight junction permeability, but existing membrane technologies lack this adaptive capability. The development of stimuli-responsive materials that can dynamically alter pore characteristics in response to biochemical or physical cues represents an unsolved technical challenge.
Scaling and manufacturing constraints further complicate widespread adoption of advanced membrane technologies. Novel approaches using nanofabrication techniques or advanced materials often face significant barriers to scale-up, limiting their practical application in commercial barrier chip platforms. The high cost and technical complexity of producing precisely engineered membranes at scale remain substantial obstacles to broader implementation.
Standardization issues across different research groups and commercial platforms create additional challenges for comparative studies. The lack of standardized methods for characterizing membrane properties and paracellular transport metrics makes it difficult to benchmark performance improvements and establish reliable design principles for next-generation barrier chip membranes.
Current Approaches to Engineer Membrane Porosity
01 Porous membrane materials for controlled paracellular transport
Porous membrane materials can be designed with specific pore sizes and distributions to control paracellular transport pathways. These membranes can mimic biological barriers by regulating the passage of molecules between cells. The controlled porosity allows for selective permeability, making these materials useful for drug delivery systems and tissue engineering applications where precise regulation of molecular transport is required.- Nanoporous membrane materials for controlled paracellular transport: Nanoporous membrane materials with precisely controlled pore sizes can regulate paracellular transport across biological barriers. These membranes mimic the selective permeability of tight junctions in epithelial and endothelial tissues, allowing for controlled passage of molecules based on size, charge, and other physicochemical properties. The nanoporous structure can be engineered to allow specific molecules to pass through while blocking others, making them useful for drug delivery applications and studying transport mechanisms.
- Polymer-based membranes for enhanced paracellular permeability: Polymer-based porous membranes can be designed to enhance paracellular transport by incorporating specific functional groups that interact with tight junction proteins. These membranes can temporarily open tight junctions to allow for increased permeability of therapeutic agents across biological barriers. Various polymers including chitosan derivatives, polyethylene glycol, and other biocompatible materials can be used to create membranes with tunable properties for controlled paracellular transport in drug delivery systems.
- Biomimetic membrane systems for studying paracellular transport: Biomimetic membrane systems that replicate the structure and function of biological barriers are valuable tools for studying paracellular transport mechanisms. These systems incorporate porous membrane materials with cultured epithelial or endothelial cells to create in vitro models of biological barriers. Such models allow researchers to investigate how different compounds cross cell layers through the paracellular pathway and evaluate the effects of potential permeability enhancers on tight junction function.
- Composite membrane materials with selective permeability: Composite membrane materials combining different materials or layers can achieve selective permeability for paracellular transport applications. These membranes often incorporate both hydrophilic and hydrophobic components to control the passage of molecules with different properties. By engineering the composition, structure, and surface properties of these composite membranes, researchers can create materials that selectively enhance paracellular transport of specific therapeutic agents while maintaining barrier integrity for other molecules.
- Stimuli-responsive porous membranes for regulated paracellular transport: Stimuli-responsive porous membranes can dynamically regulate paracellular transport in response to specific triggers such as pH, temperature, or electrical signals. These smart membrane materials can change their permeability characteristics when exposed to certain conditions, allowing for on-demand control of molecular transport across biological barriers. This technology enables targeted delivery of therapeutic agents by enhancing paracellular permeability only at specific sites or under specific conditions, improving treatment efficacy while reducing side effects.
02 Polymer-based membranes for enhanced paracellular permeability
Polymer-based porous membranes can be formulated to enhance paracellular transport through specific chemical modifications. These membranes incorporate polymers that can interact with tight junction proteins to temporarily open paracellular pathways. The polymer composition can be tailored to achieve desired permeability characteristics while maintaining biocompatibility, making them suitable for pharmaceutical applications and controlled release systems.Expand Specific Solutions03 Nanostructured membranes for selective molecular transport
Nanostructured porous membranes offer precise control over paracellular transport at the molecular level. These membranes feature nanoscale pores or channels that can selectively allow passage of specific molecules based on size, charge, or other physicochemical properties. The nanoscale architecture mimics biological tight junctions and can be engineered to achieve targeted delivery of therapeutic agents across biological barriers.Expand Specific Solutions04 Biomimetic membranes for paracellular transport studies
Biomimetic porous membranes are designed to replicate the structure and function of biological barriers for studying paracellular transport mechanisms. These membranes incorporate features of epithelial or endothelial barriers, including tight junction-like structures. They serve as in vitro models for investigating drug permeation, toxicity screening, and disease mechanisms related to paracellular pathway dysfunction, providing alternatives to animal testing in pharmaceutical research.Expand Specific Solutions05 Composite membranes with enhanced transport properties
Composite porous membranes combine multiple materials to achieve enhanced paracellular transport properties. These membranes typically feature a supportive substrate with a functional layer that regulates molecular passage. The composite structure allows for independent optimization of mechanical stability and transport characteristics. Applications include separation technologies, controlled release systems, and biomedical devices where both structural integrity and selective permeability are required.Expand Specific Solutions
Leading Organizations in Barrier Chip Development
The engineering of porous membrane materials for tuning paracellular transport in barrier chips is currently in an early growth phase, with a rapidly expanding market driven by pharmaceutical and biomedical research needs. The global market for organ-on-chip technologies, including barrier chips, is projected to reach significant scale as demand for physiologically relevant in vitro models increases. Technologically, this field shows varying maturity levels across players. Academic institutions like MIT, UNIST, and Sun Yat-Sen University are pioneering fundamental research, while companies such as DexCom, Finnadvance, and Anecova are advancing commercial applications. Established corporations including 3M and Boeing are exploring specialized applications, leveraging their materials expertise. The convergence of microfluidics, materials science, and tissue engineering is accelerating development, with interdisciplinary collaboration emerging as a key success factor.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered advanced membrane engineering techniques for barrier chip applications, focusing on precisely controlled porous structures that mimic biological barriers. Their approach involves using stimuli-responsive polymers to create dynamic membranes with tunable porosity and permeability. These smart membranes can adjust their transport properties in response to external stimuli such as pH, temperature, or electrical signals, allowing for real-time control of paracellular transport. MIT researchers have developed a proprietary layer-by-layer assembly technique that enables nanometer-scale precision in membrane fabrication, creating well-defined pore architectures with sizes ranging from 1-100 nm. Their recent innovations include incorporating biodegradable components that allow for controlled membrane remodeling, mimicking the dynamic nature of biological barriers[1][3].
Strengths: Exceptional precision in pore size control and distribution; responsive membranes that can dynamically adjust to experimental conditions; integration with microfluidic systems for high-throughput applications. Weaknesses: Complex fabrication processes that may limit scalability; higher production costs compared to conventional membranes; potential challenges in long-term stability of responsive components.
The Regents of the University of California
Technical Solution: The University of California system has developed innovative approaches to engineered porous membranes for barrier chips, particularly focusing on biomimetic materials that replicate the selective permeability of biological barriers. Their technology utilizes a combination of electrospinning and phase separation techniques to create membranes with precisely controlled fiber architecture and porosity. UC researchers have pioneered the incorporation of extracellular matrix components directly into synthetic membranes, creating hybrid structures that provide both mechanical support and biological cues to cultured cells. Their proprietary surface modification techniques allow for spatial patterning of membrane properties, creating regions with different transport characteristics within a single barrier chip. Recent developments include membranes with embedded nanosensors that can monitor transport in real-time, providing immediate feedback on barrier integrity and function[2][5].
Strengths: Excellent biomimetic properties that closely replicate natural tissue barriers; versatile fabrication methods adaptable to different barrier types; strong integration with sensing capabilities for real-time monitoring. Weaknesses: Relatively complex manufacturing processes requiring specialized equipment; potential batch-to-batch variability in biologically-derived components; higher costs associated with incorporating sensing elements.
Key Innovations in Paracellular Transport Control
Functional membrane and microfluidic chip comprising same and method for manufacturing same
PatentWO2022203411A1
Innovation
- A functional membrane with pores of sufficient size for light transmission and cell separation, coated with a hydrogel or sol-state material to prevent initial cell migration, allowing for controlled cell movement under specific conditions using a microfluidic chip manufacturing method involving substrate patterning, membrane placement, and coating material application.
Methods of making high selectivity facilitated transport membranes
PatentWO2020068778A1
Innovation
- A new method for fabricating facilitated transport membranes using a hydrophilic, very small pore, nanoporous support membrane with a thin, nonporous hydrophilic polymer layer and incorporated metal salts, avoiding the use of basic NaOH solutions and water washes, to create spiral wound or hollow fiber modules with enhanced selectivity and permeance.
Biocompatibility and Cell-Material Interactions
Biocompatibility is a critical factor in the development of porous membrane materials for barrier chips, as these materials directly interface with living cells and tissues. The interaction between cells and membrane materials significantly influences cellular behavior, including adhesion, proliferation, and differentiation, which are essential for establishing functional tissue barriers in vitro.
The surface properties of porous membranes, including chemistry, topography, and stiffness, play decisive roles in determining cell-material interactions. Hydrophilicity, for instance, generally promotes cell adhesion by facilitating protein adsorption, while specific surface functional groups can enhance selective cell attachment. Recent advances in surface modification techniques have enabled precise control over these properties, allowing researchers to optimize membranes for specific cell types used in barrier models.
Extracellular matrix (ECM) protein coatings represent another significant approach to enhancing biocompatibility. Proteins such as collagen, fibronectin, and laminin provide natural binding sites for cell surface receptors, promoting physiologically relevant cell attachment and organization. The combination of engineered membrane topography with strategic ECM coating has proven particularly effective in recreating the complex microenvironments of biological barriers.
Inflammatory responses to membrane materials remain a challenge in barrier chip development. Materials that trigger inflammation can disrupt barrier formation and function, leading to experimental artifacts. Synthetic polymers like PDMS and PC offer good biocompatibility but may release uncured monomers or additives that affect cell behavior. Alternatively, natural materials such as cellulose derivatives and silk fibroin demonstrate excellent biocompatibility but present challenges in controlling pore size and distribution.
The mechanical properties of membrane materials significantly impact cellular mechanotransduction pathways. Substrate stiffness has been shown to influence cell morphology, migration, and barrier-forming capacity. Emerging research indicates that matching membrane mechanical properties to those of native tissues improves the physiological relevance of barrier models, particularly for mechanosensitive barriers like the blood-brain barrier and lung alveolar interfaces.
Long-term stability of cell-material interactions presents another consideration for barrier chips intended for extended studies. Materials must maintain their integrity and biocompatibility during prolonged culture periods, resisting degradation that could release cytotoxic byproducts or alter membrane properties. Recent developments in biostable polymers and hybrid organic-inorganic materials show promise for addressing these challenges while maintaining precise control over paracellular transport.
The surface properties of porous membranes, including chemistry, topography, and stiffness, play decisive roles in determining cell-material interactions. Hydrophilicity, for instance, generally promotes cell adhesion by facilitating protein adsorption, while specific surface functional groups can enhance selective cell attachment. Recent advances in surface modification techniques have enabled precise control over these properties, allowing researchers to optimize membranes for specific cell types used in barrier models.
Extracellular matrix (ECM) protein coatings represent another significant approach to enhancing biocompatibility. Proteins such as collagen, fibronectin, and laminin provide natural binding sites for cell surface receptors, promoting physiologically relevant cell attachment and organization. The combination of engineered membrane topography with strategic ECM coating has proven particularly effective in recreating the complex microenvironments of biological barriers.
Inflammatory responses to membrane materials remain a challenge in barrier chip development. Materials that trigger inflammation can disrupt barrier formation and function, leading to experimental artifacts. Synthetic polymers like PDMS and PC offer good biocompatibility but may release uncured monomers or additives that affect cell behavior. Alternatively, natural materials such as cellulose derivatives and silk fibroin demonstrate excellent biocompatibility but present challenges in controlling pore size and distribution.
The mechanical properties of membrane materials significantly impact cellular mechanotransduction pathways. Substrate stiffness has been shown to influence cell morphology, migration, and barrier-forming capacity. Emerging research indicates that matching membrane mechanical properties to those of native tissues improves the physiological relevance of barrier models, particularly for mechanosensitive barriers like the blood-brain barrier and lung alveolar interfaces.
Long-term stability of cell-material interactions presents another consideration for barrier chips intended for extended studies. Materials must maintain their integrity and biocompatibility during prolonged culture periods, resisting degradation that could release cytotoxic byproducts or alter membrane properties. Recent developments in biostable polymers and hybrid organic-inorganic materials show promise for addressing these challenges while maintaining precise control over paracellular transport.
Regulatory Considerations for Organ-on-Chip Technologies
The regulatory landscape for organ-on-chip (OOC) technologies, particularly those involving engineered porous membranes for paracellular transport in barrier chips, presents significant complexity for developers and manufacturers. These technologies occupy an intersection between medical devices, in vitro diagnostic tools, and research instruments, creating ambiguity in their regulatory classification.
In the United States, the Food and Drug Administration (FDA) has begun developing frameworks specifically for OOC technologies through its Emerging Technology Program. Engineered membrane materials in barrier chips may fall under different regulatory pathways depending on their intended use—whether for drug development, toxicity testing, or personalized medicine applications. The FDA's Center for Devices and Radiological Health (CDRH) typically oversees such technologies when they function as medical devices.
European regulations under the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) impose stringent requirements for validation and verification of OOC technologies. Specifically, engineered porous membranes must demonstrate consistent performance in facilitating paracellular transport that accurately mimics human physiological barriers. Documentation of material composition, manufacturing processes, and quality control measures for these membranes is essential for regulatory compliance.
International Standardization Organization (ISO) standards, particularly ISO 13485 for medical device quality management systems, provide important frameworks for manufacturers developing barrier chip technologies. However, the field currently lacks specific standards for membrane characterization in OOC applications, creating challenges for consistent evaluation across different platforms and manufacturers.
Regulatory considerations must also address the biological aspects of these technologies. When barrier chips incorporate human cells alongside engineered membranes, additional regulations regarding human tissue use, informed consent, and biological safety come into play. The source, handling, and disposal of biological materials used in conjunction with engineered membranes require careful documentation and compliance with biosafety regulations.
Data validation represents another critical regulatory consideration. Authorities increasingly require evidence that engineered membrane systems produce physiologically relevant and reproducible results. This includes validation of paracellular transport mechanisms against established in vivo models and demonstration of batch-to-batch consistency in membrane performance characteristics.
Looking forward, regulatory bodies are moving toward more collaborative approaches with technology developers. The FDA's Tissue Chip for Drug Screening program exemplifies this trend, working with researchers to establish appropriate validation methodologies for barrier chip technologies. Similarly, the European Medicines Agency has initiated dialogue with stakeholders to develop specific guidance for OOC technologies that incorporate engineered membrane systems.
In the United States, the Food and Drug Administration (FDA) has begun developing frameworks specifically for OOC technologies through its Emerging Technology Program. Engineered membrane materials in barrier chips may fall under different regulatory pathways depending on their intended use—whether for drug development, toxicity testing, or personalized medicine applications. The FDA's Center for Devices and Radiological Health (CDRH) typically oversees such technologies when they function as medical devices.
European regulations under the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) impose stringent requirements for validation and verification of OOC technologies. Specifically, engineered porous membranes must demonstrate consistent performance in facilitating paracellular transport that accurately mimics human physiological barriers. Documentation of material composition, manufacturing processes, and quality control measures for these membranes is essential for regulatory compliance.
International Standardization Organization (ISO) standards, particularly ISO 13485 for medical device quality management systems, provide important frameworks for manufacturers developing barrier chip technologies. However, the field currently lacks specific standards for membrane characterization in OOC applications, creating challenges for consistent evaluation across different platforms and manufacturers.
Regulatory considerations must also address the biological aspects of these technologies. When barrier chips incorporate human cells alongside engineered membranes, additional regulations regarding human tissue use, informed consent, and biological safety come into play. The source, handling, and disposal of biological materials used in conjunction with engineered membranes require careful documentation and compliance with biosafety regulations.
Data validation represents another critical regulatory consideration. Authorities increasingly require evidence that engineered membrane systems produce physiologically relevant and reproducible results. This includes validation of paracellular transport mechanisms against established in vivo models and demonstration of batch-to-batch consistency in membrane performance characteristics.
Looking forward, regulatory bodies are moving toward more collaborative approaches with technology developers. The FDA's Tissue Chip for Drug Screening program exemplifies this trend, working with researchers to establish appropriate validation methodologies for barrier chip technologies. Similarly, the European Medicines Agency has initiated dialogue with stakeholders to develop specific guidance for OOC technologies that incorporate engineered membrane systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!