Applying organ-on-chip models to prioritize oncology drug candidates by tumor penetration and microenvironment response
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Organ-on-Chip Technology Evolution and Objectives
Organ-on-chip (OoC) technology has evolved significantly over the past decade, transforming from simple microfluidic cell culture systems to sophisticated 3D models that recapitulate complex organ functionalities. The journey began in the early 2000s with rudimentary microfluidic devices that could maintain cell cultures in controlled environments. By 2010, researchers had developed the first lung-on-chip model at Harvard's Wyss Institute, marking a pivotal moment in the field's development.
The evolution accelerated between 2012-2016, with various organ models being developed including liver, kidney, heart, and brain chips. These early models primarily focused on single organ functionality and basic physiological responses. The technological progression continued with the integration of multiple cell types to better mimic tissue heterogeneity, incorporation of mechanical forces to simulate physiological stresses, and implementation of real-time monitoring capabilities.
From 2017 onwards, the field witnessed a significant shift towards interconnected multi-organ systems, often referred to as "body-on-chip" platforms. These advanced systems enable the study of organ interactions and systemic responses, crucial for understanding complex disease mechanisms and drug effects. Concurrently, the integration of patient-derived cells has moved the technology towards personalized medicine applications.
In oncology specifically, tumor-on-chip models have evolved to incorporate key elements of the tumor microenvironment (TME), including cancer-associated fibroblasts, immune cells, and extracellular matrix components. Recent advancements have focused on recreating tumor-specific features such as hypoxic gradients, acidic microenvironments, and abnormal vasculature that significantly influence drug penetration and efficacy.
The primary objective of applying OoC technology to oncology drug prioritization is to establish more predictive preclinical models that can accurately assess drug penetration through tumor tissue and evaluate responses within the complex TME. This aims to address the high failure rate of oncology drugs in clinical trials, often attributed to inadequate preclinical models that fail to capture tumor complexity.
Additional objectives include: developing standardized, reproducible tumor models for consistent drug screening; enabling high-throughput assessment of drug candidates to accelerate development timelines; providing mechanistic insights into drug resistance mechanisms related to poor tissue penetration or TME-mediated protection; and ultimately reducing reliance on animal models while improving translation to human outcomes through the use of patient-derived cells.
The technology continues to evolve toward greater physiological relevance, with emerging trends focusing on vascularized tumor models, integration of immune components, and incorporation of advanced imaging techniques for real-time monitoring of drug distribution and cellular responses.
The evolution accelerated between 2012-2016, with various organ models being developed including liver, kidney, heart, and brain chips. These early models primarily focused on single organ functionality and basic physiological responses. The technological progression continued with the integration of multiple cell types to better mimic tissue heterogeneity, incorporation of mechanical forces to simulate physiological stresses, and implementation of real-time monitoring capabilities.
From 2017 onwards, the field witnessed a significant shift towards interconnected multi-organ systems, often referred to as "body-on-chip" platforms. These advanced systems enable the study of organ interactions and systemic responses, crucial for understanding complex disease mechanisms and drug effects. Concurrently, the integration of patient-derived cells has moved the technology towards personalized medicine applications.
In oncology specifically, tumor-on-chip models have evolved to incorporate key elements of the tumor microenvironment (TME), including cancer-associated fibroblasts, immune cells, and extracellular matrix components. Recent advancements have focused on recreating tumor-specific features such as hypoxic gradients, acidic microenvironments, and abnormal vasculature that significantly influence drug penetration and efficacy.
The primary objective of applying OoC technology to oncology drug prioritization is to establish more predictive preclinical models that can accurately assess drug penetration through tumor tissue and evaluate responses within the complex TME. This aims to address the high failure rate of oncology drugs in clinical trials, often attributed to inadequate preclinical models that fail to capture tumor complexity.
Additional objectives include: developing standardized, reproducible tumor models for consistent drug screening; enabling high-throughput assessment of drug candidates to accelerate development timelines; providing mechanistic insights into drug resistance mechanisms related to poor tissue penetration or TME-mediated protection; and ultimately reducing reliance on animal models while improving translation to human outcomes through the use of patient-derived cells.
The technology continues to evolve toward greater physiological relevance, with emerging trends focusing on vascularized tumor models, integration of immune components, and incorporation of advanced imaging techniques for real-time monitoring of drug distribution and cellular responses.
Market Analysis for Oncology Drug Screening Platforms
The global oncology drug screening market is experiencing robust growth, valued at approximately $2.5 billion in 2022 and projected to reach $4.7 billion by 2028, with a compound annual growth rate of 11.2%. This growth is primarily driven by increasing cancer incidence worldwide, with over 19.3 million new cases reported annually according to the World Health Organization.
Traditional drug screening platforms face significant limitations in predicting clinical efficacy, with over 90% of oncology drug candidates failing in clinical trials despite promising preclinical results. This high failure rate costs the pharmaceutical industry billions annually and significantly delays bringing effective treatments to market. The disconnect between traditional screening methods and human physiological responses represents a critical market gap.
Organ-on-chip technology addresses this gap by providing more physiologically relevant models that can recapitulate tumor microenvironments. The market for these advanced screening platforms is growing at 15.7% annually, outpacing conventional methods. Pharmaceutical companies are increasingly allocating R&D budgets toward these technologies, with major players investing between $50-100 million in advanced screening platform partnerships.
The market segmentation reveals distinct customer groups: large pharmaceutical companies seeking to reduce late-stage clinical failures; biotechnology startups requiring cost-effective screening solutions; and academic research institutions focusing on fundamental cancer biology. Each segment has different requirements regarding throughput, cost structure, and data integration capabilities.
Regional analysis shows North America dominating with 42% market share, followed by Europe at 28% and Asia-Pacific at 22%, with the latter showing the fastest growth rate of 17.3% annually. China and South Korea are emerging as significant markets due to increasing government investment in precision medicine initiatives.
Key market drivers include the push for personalized medicine approaches, regulatory pressure to reduce animal testing, and the need for better predictive models of drug efficacy and toxicity. The integration of artificial intelligence with organ-on-chip platforms for data analysis represents a particularly high-growth subsegment, expanding at 19.8% annually.
Market barriers include high initial investment costs, technical challenges in developing complex multi-organ systems, and the need for standardization across platforms to ensure reproducibility. Despite these challenges, the compelling value proposition of reducing late-stage clinical failures makes organ-on-chip technology increasingly attractive for oncology drug development pipelines.
Traditional drug screening platforms face significant limitations in predicting clinical efficacy, with over 90% of oncology drug candidates failing in clinical trials despite promising preclinical results. This high failure rate costs the pharmaceutical industry billions annually and significantly delays bringing effective treatments to market. The disconnect between traditional screening methods and human physiological responses represents a critical market gap.
Organ-on-chip technology addresses this gap by providing more physiologically relevant models that can recapitulate tumor microenvironments. The market for these advanced screening platforms is growing at 15.7% annually, outpacing conventional methods. Pharmaceutical companies are increasingly allocating R&D budgets toward these technologies, with major players investing between $50-100 million in advanced screening platform partnerships.
The market segmentation reveals distinct customer groups: large pharmaceutical companies seeking to reduce late-stage clinical failures; biotechnology startups requiring cost-effective screening solutions; and academic research institutions focusing on fundamental cancer biology. Each segment has different requirements regarding throughput, cost structure, and data integration capabilities.
Regional analysis shows North America dominating with 42% market share, followed by Europe at 28% and Asia-Pacific at 22%, with the latter showing the fastest growth rate of 17.3% annually. China and South Korea are emerging as significant markets due to increasing government investment in precision medicine initiatives.
Key market drivers include the push for personalized medicine approaches, regulatory pressure to reduce animal testing, and the need for better predictive models of drug efficacy and toxicity. The integration of artificial intelligence with organ-on-chip platforms for data analysis represents a particularly high-growth subsegment, expanding at 19.8% annually.
Market barriers include high initial investment costs, technical challenges in developing complex multi-organ systems, and the need for standardization across platforms to ensure reproducibility. Despite these challenges, the compelling value proposition of reducing late-stage clinical failures makes organ-on-chip technology increasingly attractive for oncology drug development pipelines.
Current Challenges in Tumor Microenvironment Modeling
Despite significant advancements in organ-on-chip (OOC) technology for modeling tumor microenvironments (TME), several critical challenges persist that limit their full potential in oncology drug development. The complexity of the TME, which comprises cancer cells, stromal cells, immune cells, extracellular matrix, and various biochemical factors, presents a formidable obstacle to accurate replication in vitro. Current OOC models struggle to fully recapitulate this heterogeneity, particularly the spatial organization and dynamic interactions between different cell types.
Vascularization remains one of the most significant technical hurdles. While some advanced models incorporate rudimentary vascular networks, they often fail to mimic the abnormal, leaky vasculature characteristic of tumors that significantly impacts drug delivery and efficacy. This limitation directly affects the ability to predict tumor penetration of drug candidates, a key parameter in prioritization efforts.
The integration of functional immune components presents another major challenge. The immune system plays a crucial role in tumor progression and response to therapies, yet most current OOC models either lack immune cells entirely or incorporate them in simplified, non-physiological ways. This deficiency is particularly problematic given the rising importance of immuno-oncology therapies.
Temporal dynamics pose additional complications. Cancer progression and treatment response occur over extended timeframes, while most OOC systems are limited to shorter experimental windows due to technical constraints in maintaining cell viability and functionality. This temporal mismatch hinders the assessment of long-term drug effects and resistance development.
Standardization and validation issues further complicate the field. The diversity of approaches to TME modeling has resulted in inconsistent methodologies and outcomes across different research groups. The absence of universally accepted benchmarks makes it difficult to compare results between studies or establish reliable predictive values for clinical outcomes.
Scaling and throughput limitations also restrict the application of OOC models in drug candidate screening. Most sophisticated TME models are labor-intensive and low-throughput, making them impractical for early-stage drug discovery where numerous compounds need evaluation.
Finally, there is a significant gap in correlating OOC model results with clinical outcomes. Without robust validation against human clinical data, the predictive power of these models remains uncertain, limiting their acceptance in regulatory decision-making and clinical trial design. This validation challenge is compounded by the inherent patient-to-patient variability in tumor biology that is difficult to capture in standardized models.
Vascularization remains one of the most significant technical hurdles. While some advanced models incorporate rudimentary vascular networks, they often fail to mimic the abnormal, leaky vasculature characteristic of tumors that significantly impacts drug delivery and efficacy. This limitation directly affects the ability to predict tumor penetration of drug candidates, a key parameter in prioritization efforts.
The integration of functional immune components presents another major challenge. The immune system plays a crucial role in tumor progression and response to therapies, yet most current OOC models either lack immune cells entirely or incorporate them in simplified, non-physiological ways. This deficiency is particularly problematic given the rising importance of immuno-oncology therapies.
Temporal dynamics pose additional complications. Cancer progression and treatment response occur over extended timeframes, while most OOC systems are limited to shorter experimental windows due to technical constraints in maintaining cell viability and functionality. This temporal mismatch hinders the assessment of long-term drug effects and resistance development.
Standardization and validation issues further complicate the field. The diversity of approaches to TME modeling has resulted in inconsistent methodologies and outcomes across different research groups. The absence of universally accepted benchmarks makes it difficult to compare results between studies or establish reliable predictive values for clinical outcomes.
Scaling and throughput limitations also restrict the application of OOC models in drug candidate screening. Most sophisticated TME models are labor-intensive and low-throughput, making them impractical for early-stage drug discovery where numerous compounds need evaluation.
Finally, there is a significant gap in correlating OOC model results with clinical outcomes. Without robust validation against human clinical data, the predictive power of these models remains uncertain, limiting their acceptance in regulatory decision-making and clinical trial design. This validation challenge is compounded by the inherent patient-to-patient variability in tumor biology that is difficult to capture in standardized models.
Existing Methodologies for Tumor Penetration Assessment
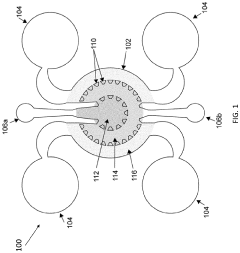
01 Microfluidic organ-on-chip platforms for tumor modeling
Microfluidic organ-on-chip platforms enable the creation of three-dimensional tumor models that mimic the in vivo tumor microenvironment. These platforms incorporate multiple cell types, extracellular matrix components, and fluid flow to recreate the complex interactions within tumors. The microfluidic design allows for controlled delivery of nutrients, drugs, and oxygen, facilitating the study of tumor penetration dynamics and cellular responses in a physiologically relevant context.- Microfluidic organ-on-chip platforms for tumor modeling: Microfluidic organ-on-chip platforms can be used to create three-dimensional models of tumors that mimic the in vivo microenvironment. These platforms allow for the study of tumor cell behavior, including invasion, migration, and response to therapeutic agents. The microfluidic nature of these devices enables precise control over fluid flow, nutrient delivery, and waste removal, creating physiologically relevant conditions for tumor growth and development.
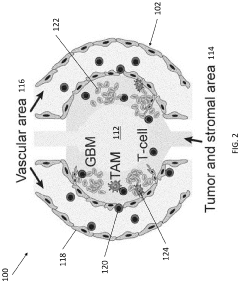
- Tumor microenvironment simulation and drug penetration studies: Organ-on-chip models can be designed to simulate the complex tumor microenvironment, including stromal components, immune cells, and extracellular matrix. These models allow researchers to study how drugs penetrate through tumor tissue and interact with different cell types within the tumor microenvironment. By recreating the heterogeneity of tumors, these models provide insights into barriers to drug delivery and potential strategies to overcome them.
- Integration of sensors for real-time monitoring of tumor responses: Advanced organ-on-chip models incorporate various sensors that enable real-time monitoring of tumor responses to treatments. These sensors can measure parameters such as oxygen consumption, pH changes, metabolite production, and cellular responses. This integration allows for dynamic assessment of how tumors respond to therapeutic interventions and how the microenvironment changes during treatment, providing valuable data for drug development and personalized medicine approaches.
- Co-culture systems for studying tumor-stroma interactions: Organ-on-chip platforms can be designed to support co-culture of multiple cell types, allowing for the study of interactions between tumor cells and stromal components. These co-culture systems enable investigation of how stromal cells influence tumor growth, invasion, and response to therapy. By incorporating endothelial cells, fibroblasts, immune cells, and other stromal elements, these models provide insights into the complex cellular interactions that occur within the tumor microenvironment.
- Vascularized tumor models for studying angiogenesis and metastasis: Vascularized organ-on-chip tumor models incorporate blood vessel-like structures to study angiogenesis, tumor cell intravasation, and metastasis. These models allow researchers to investigate how tumor cells interact with blood vessels, how they migrate through vessel walls, and how they establish metastatic sites. By recreating the vascular component of the tumor microenvironment, these models provide a platform for studying the complex processes involved in cancer progression and metastasis.
02 Tumor microenvironment simulation with integrated sensors
Advanced organ-on-chip models incorporate integrated sensing technologies to monitor real-time changes in the tumor microenvironment. These sensors can detect variations in pH, oxygen levels, metabolites, and cellular responses during drug treatment or environmental changes. The integration of sensing capabilities with tumor models enables researchers to understand how the microenvironment influences drug penetration, cellular behavior, and treatment efficacy in a dynamic and quantifiable manner.Expand Specific Solutions03 Multi-compartment systems for studying tumor-stroma interactions
Multi-compartment organ-on-chip systems allow for the co-culture of tumor cells with stromal components such as fibroblasts, immune cells, and endothelial cells in distinct but interconnected chambers. These systems enable the study of how stromal cells influence tumor behavior, drug resistance, and penetration barriers. By recreating the spatial organization of tumors and surrounding tissues, these models provide insights into how the tumor microenvironment affects therapeutic response and cancer progression.Expand Specific Solutions04 Vascularized tumor models for studying drug delivery
Organ-on-chip platforms with integrated vascular networks enable the study of drug delivery and penetration through blood vessels into tumor tissue. These models incorporate endothelial cells that form perfusable blood vessel-like structures, allowing researchers to investigate how drugs traverse from the circulation into the tumor mass. The vascularized models account for the abnormal blood vessel architecture in tumors, which creates barriers to effective drug delivery and influences the tumor microenvironment through irregular oxygen and nutrient distribution.Expand Specific Solutions05 Hypoxia and acidosis modeling in tumor microenvironments
Specialized organ-on-chip systems are designed to recreate the hypoxic and acidic conditions characteristic of solid tumors. These models incorporate oxygen gradient generators and pH control mechanisms to simulate how limited oxygen availability and increased acidity affect tumor cell behavior, gene expression, and drug resistance. By replicating these microenvironmental stressors, researchers can better understand how hypoxia and acidosis influence tumor progression and develop therapies that remain effective under these challenging conditions.Expand Specific Solutions
Leading Organizations in Oncology Organ-on-Chip Development
The organ-on-chip market for oncology drug prioritization is currently in an early growth phase, with significant expansion potential as pharmaceutical companies seek more predictive preclinical models. The global market is estimated at approximately $150 million, expected to grow at 25-30% annually as adoption increases. Technologically, the field is transitioning from early development to commercial application, with varying maturity levels among key players. Leading academic institutions (Memorial Sloan Kettering, Cornell University, Columbia University) are advancing fundamental research, while specialized companies like Emulate have developed commercial platforms. Pharmaceutical giants (F. Hoffmann-La Roche) are increasingly integrating these technologies into drug development pipelines. Research centers in Asia (KIST, Shanghai Institute of Microsystem & Information Technology) are rapidly advancing capabilities, creating a globally competitive landscape.
Memorial Sloan Kettering Cancer Center
Technical Solution: Memorial Sloan Kettering has pioneered patient-specific organ-on-chip models that incorporate actual tumor biopsies into microfluidic devices for personalized drug response prediction. Their platform maintains the native tumor architecture and heterogeneity while enabling controlled introduction of therapeutic agents. MSK's approach uniquely preserves tumor-infiltrating immune cells within the microenvironment, allowing assessment of immunotherapy responses in a physiologically relevant context[5]. The center has developed specialized imaging protocols that enable real-time visualization of drug penetration through patient tumor samples, correlating spatial distribution with cellular response. Their system incorporates multiple cell types from the tumor microenvironment, including cancer-associated fibroblasts and endothelial cells, to recreate the complex barriers to drug delivery present in solid tumors. MSK researchers have demonstrated that their organ-on-chip models can predict clinical responses to both standard chemotherapies and targeted agents with significantly higher accuracy than conventional patient-derived xenograft models, while providing results in a clinically relevant timeframe.
Strengths: Direct use of patient material preserves tumor heterogeneity; extensive clinical validation through correlation with patient outcomes; integration with comprehensive cancer center resources. Weaknesses: Limited scalability due to dependence on fresh patient samples; variability between patient-derived models complicates standardization; higher complexity in operation compared to cell line-based systems.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has pioneered patient-derived tumor organoid (PDTO) integration with microfluidic organ-on-chip systems to create advanced drug screening platforms. Their approach combines primary patient tumor samples with customized microenvironmental factors to create physiologically relevant testing conditions. The company has developed proprietary algorithms that analyze multiple parameters simultaneously, including drug penetration kinetics, stromal barrier effects, and immune cell interactions within the tumor microenvironment[2]. Roche's platform incorporates real-time imaging capabilities to track drug distribution through tumor tissue while monitoring cellular responses. Their system enables the assessment of both conventional chemotherapeutics and targeted biologics, with particular emphasis on antibody-drug conjugates and immunotherapeutics where tissue penetration is critical for efficacy. The technology has been validated across multiple solid tumor types including colorectal, pancreatic, and breast cancers, demonstrating superior predictive value compared to conventional 2D and static 3D models.
Strengths: Extensive pharmaceutical development expertise; integration with comprehensive genomic and proteomic analysis capabilities; validated correlation with clinical outcomes. Weaknesses: Proprietary system with limited accessibility to external researchers; longer development timeline for patient-derived models compared to cell line-based approaches.
Critical Patents in Tumor Microenvironment Modeling
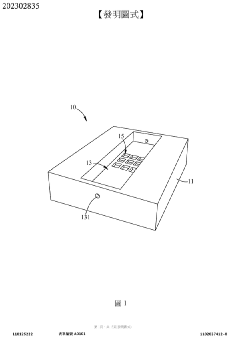
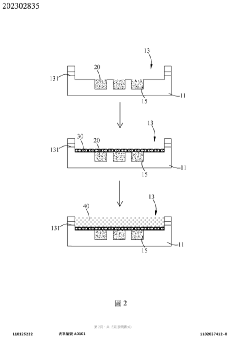
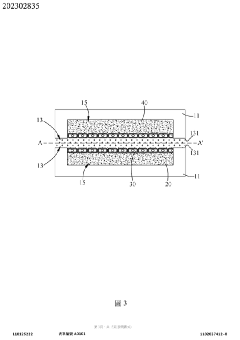
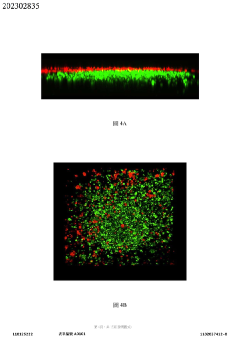
Tumor microenvironment on chip and production method thereof
PatentActiveTW202302835A
Innovation
- A biochip design with a carrier featuring recessed first and second cell tissue culture areas, allowing for the culture of cancer cells, endothelial cells, and autologous immune cells, utilizing 3D printing and bioprinting to simulate in vitro human tissue environments.
Tumor-on-a-chip
PatentPendingUS20230032623A1
Innovation
- A tumor-on-a-chip device is developed, featuring a cartridge with a central chamber partitioned by micropillars to mimic a tumor microenvironment, incorporating endothelial cells and tumor cells, and equipped with sensors for monitoring treatment responses, allowing for the replication of various cancer types and evaluation of anti-cancer therapies.
Regulatory Pathway for Organ-on-Chip Validation
The regulatory landscape for organ-on-chip (OOC) technology in oncology drug development requires careful navigation to achieve validation and acceptance by regulatory bodies. Currently, the FDA and EMA have established frameworks for biomarkers and alternative testing methods, but specific pathways for OOC validation in oncology remain under development.
A critical first step involves demonstrating reproducibility and reliability through standardized protocols. This includes establishing quality control parameters for tumor microenvironment representation, cellular response consistency, and drug penetration metrics. Leading research institutions and pharmaceutical companies are collaborating to develop these standards, with particular focus on tumor-specific microenvironmental factors that influence drug efficacy.
Qualification processes typically follow a three-phase approach. The exploratory phase establishes baseline performance metrics and correlation with existing in vitro and animal models. The validation phase requires multi-center studies comparing OOC predictions with clinical outcomes across diverse patient populations. The implementation phase involves regulatory submission with comprehensive validation data packages demonstrating the model's predictive value for specific oncology indications.
Regulatory bodies increasingly recognize the value of OOC technology in addressing the limitations of traditional models. The FDA's Predictive Toxicology Roadmap and the EMA's Innovation Task Force have both signaled support for advanced in vitro systems that better recapitulate human physiology. However, specific guidance for tumor microenvironment models remains limited, creating opportunities for pioneering companies to help shape regulatory frameworks.
Context of use (COU) documentation is particularly important for oncology OOC models. This must clearly define the intended application (e.g., early candidate screening, dose optimization, combination therapy assessment) and specify the model's limitations. Regulatory acceptance increases when the COU aligns with critical decision points in oncology drug development where traditional methods have demonstrated limitations.
Cross-validation with multiple methodologies strengthens regulatory submissions. Companies pursuing OOC validation should develop comprehensive data packages that include correlations with traditional methods, mechanistic explanations for observed differences, and evidence of improved predictive value for clinical outcomes. Patient-derived samples incorporated into OOC models can further enhance clinical relevance and regulatory acceptance.
Engagement with regulatory agencies through formal consultation processes is essential. The FDA's Drug Development Tool Qualification Program and similar EMA pathways provide mechanisms for obtaining feedback on validation approaches before significant resources are committed. Early and frequent communication with regulators has proven effective in accelerating the acceptance of novel methodologies in the oncology space.
A critical first step involves demonstrating reproducibility and reliability through standardized protocols. This includes establishing quality control parameters for tumor microenvironment representation, cellular response consistency, and drug penetration metrics. Leading research institutions and pharmaceutical companies are collaborating to develop these standards, with particular focus on tumor-specific microenvironmental factors that influence drug efficacy.
Qualification processes typically follow a three-phase approach. The exploratory phase establishes baseline performance metrics and correlation with existing in vitro and animal models. The validation phase requires multi-center studies comparing OOC predictions with clinical outcomes across diverse patient populations. The implementation phase involves regulatory submission with comprehensive validation data packages demonstrating the model's predictive value for specific oncology indications.
Regulatory bodies increasingly recognize the value of OOC technology in addressing the limitations of traditional models. The FDA's Predictive Toxicology Roadmap and the EMA's Innovation Task Force have both signaled support for advanced in vitro systems that better recapitulate human physiology. However, specific guidance for tumor microenvironment models remains limited, creating opportunities for pioneering companies to help shape regulatory frameworks.
Context of use (COU) documentation is particularly important for oncology OOC models. This must clearly define the intended application (e.g., early candidate screening, dose optimization, combination therapy assessment) and specify the model's limitations. Regulatory acceptance increases when the COU aligns with critical decision points in oncology drug development where traditional methods have demonstrated limitations.
Cross-validation with multiple methodologies strengthens regulatory submissions. Companies pursuing OOC validation should develop comprehensive data packages that include correlations with traditional methods, mechanistic explanations for observed differences, and evidence of improved predictive value for clinical outcomes. Patient-derived samples incorporated into OOC models can further enhance clinical relevance and regulatory acceptance.
Engagement with regulatory agencies through formal consultation processes is essential. The FDA's Drug Development Tool Qualification Program and similar EMA pathways provide mechanisms for obtaining feedback on validation approaches before significant resources are committed. Early and frequent communication with regulators has proven effective in accelerating the acceptance of novel methodologies in the oncology space.
Cost-Benefit Analysis of Implementation
Implementing organ-on-chip (OOC) models for oncology drug candidate prioritization requires substantial initial investment balanced against potential long-term benefits. The upfront costs include specialized equipment acquisition ranging from $50,000 to $500,000 depending on system complexity and throughput requirements. High-precision microfluidic platforms, advanced imaging systems, and cell culture facilities represent the bulk of this capital expenditure. Additionally, operational expenses encompass specialized consumables, maintenance contracts, and technical staff training, estimated at $100,000-$200,000 annually.
Personnel costs constitute another significant investment, requiring specialized expertise in microfluidics, tissue engineering, and oncology. A dedicated team typically includes 2-3 bioengineers, 1-2 oncology specialists, and supporting technical staff, representing an annual investment of $300,000-$500,000 depending on geographical location and expertise level.
Against these costs, the benefits of OOC implementation are substantial. Primary among these is the potential reduction in late-stage clinical trial failures, where each failure represents an average loss of $300 million. By improving prediction of tumor penetration and microenvironment response, OOC models can potentially increase phase II/III success rates by 10-15%, translating to significant cost avoidance.
Time-to-market acceleration represents another quantifiable benefit. Traditional drug development pathways require 10-15 years; OOC models can potentially reduce this timeline by 1-3 years through more efficient candidate selection. This acceleration translates to earlier market entry and extended patent protection periods, with each additional year of exclusivity worth $250-500 million for successful oncology drugs.
Resource optimization benefits include reducing animal testing requirements by 30-50%, with associated cost savings of $1-2 million per development program. Furthermore, the ability to test multiple microenvironmental conditions simultaneously increases experimental throughput while decreasing resource utilization.
Return on investment analysis indicates that despite high initial costs, OOC implementation typically achieves break-even within 2-3 development cycles. Organizations implementing these systems report 15-25% improvements in R&D efficiency and 20-30% reductions in candidate attrition rates, resulting in substantial long-term financial benefits that significantly outweigh implementation costs.
Personnel costs constitute another significant investment, requiring specialized expertise in microfluidics, tissue engineering, and oncology. A dedicated team typically includes 2-3 bioengineers, 1-2 oncology specialists, and supporting technical staff, representing an annual investment of $300,000-$500,000 depending on geographical location and expertise level.
Against these costs, the benefits of OOC implementation are substantial. Primary among these is the potential reduction in late-stage clinical trial failures, where each failure represents an average loss of $300 million. By improving prediction of tumor penetration and microenvironment response, OOC models can potentially increase phase II/III success rates by 10-15%, translating to significant cost avoidance.
Time-to-market acceleration represents another quantifiable benefit. Traditional drug development pathways require 10-15 years; OOC models can potentially reduce this timeline by 1-3 years through more efficient candidate selection. This acceleration translates to earlier market entry and extended patent protection periods, with each additional year of exclusivity worth $250-500 million for successful oncology drugs.
Resource optimization benefits include reducing animal testing requirements by 30-50%, with associated cost savings of $1-2 million per development program. Furthermore, the ability to test multiple microenvironmental conditions simultaneously increases experimental throughput while decreasing resource utilization.
Return on investment analysis indicates that despite high initial costs, OOC implementation typically achieves break-even within 2-3 development cycles. Organizations implementing these systems report 15-25% improvements in R&D efficiency and 20-30% reductions in candidate attrition rates, resulting in substantial long-term financial benefits that significantly outweigh implementation costs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!