Establishing reproducible immune cell populations on chip for immunotoxicity testing
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Immune Cell-on-Chip Background and Objectives
Immune cell-on-chip technology represents a revolutionary approach in the field of immunotoxicology, emerging from the convergence of microfluidics, tissue engineering, and immunology. This technology has evolved significantly over the past decade, transitioning from simple cell culture systems to sophisticated organ-on-chip platforms that can mimic complex immune responses. The historical trajectory shows a shift from traditional in vivo animal testing models, which often failed to accurately predict human immune responses, to more physiologically relevant in vitro systems that better recapitulate human immunology.
The primary objective of establishing reproducible immune cell populations on chip is to create standardized, reliable platforms for immunotoxicity testing that overcome the limitations of conventional methods. These limitations include poor correlation with human responses, ethical concerns regarding animal testing, and the inability to capture the complexity of immune cell interactions. By developing reproducible immune cell-on-chip systems, researchers aim to provide more accurate predictions of immunotoxic effects of pharmaceuticals, chemicals, and environmental toxicants on human health.
Current technological trends in this field are moving toward increased physiological relevance through the incorporation of multiple immune cell types, three-dimensional culture environments, and dynamic flow conditions that mimic in vivo circulation. There is also a growing emphasis on integrating advanced imaging techniques and high-throughput screening capabilities to enhance the analytical power of these platforms. The development of standardized protocols for cell seeding, maintenance, and analysis represents another critical trend aimed at improving reproducibility across different laboratories.
The technical goals for immune cell-on-chip systems include achieving consistent cell viability and functionality over extended culture periods, establishing reproducible immune cell phenotypes that reflect in vivo characteristics, and developing methods to quantitatively assess immune responses to various stimuli. Additionally, there is a push toward creating systems that can incorporate patient-derived immune cells to enable personalized immunotoxicity testing and precision medicine applications.
Long-term objectives extend to the development of regulatory-accepted alternatives to animal testing for immunotoxicity assessment, potentially revolutionizing drug development processes by providing earlier, more accurate predictions of adverse immune reactions. Furthermore, these systems could serve as valuable tools for fundamental immunological research, enabling detailed studies of immune cell behavior and interactions in controlled microenvironments that were previously impossible with conventional methods.
The primary objective of establishing reproducible immune cell populations on chip is to create standardized, reliable platforms for immunotoxicity testing that overcome the limitations of conventional methods. These limitations include poor correlation with human responses, ethical concerns regarding animal testing, and the inability to capture the complexity of immune cell interactions. By developing reproducible immune cell-on-chip systems, researchers aim to provide more accurate predictions of immunotoxic effects of pharmaceuticals, chemicals, and environmental toxicants on human health.
Current technological trends in this field are moving toward increased physiological relevance through the incorporation of multiple immune cell types, three-dimensional culture environments, and dynamic flow conditions that mimic in vivo circulation. There is also a growing emphasis on integrating advanced imaging techniques and high-throughput screening capabilities to enhance the analytical power of these platforms. The development of standardized protocols for cell seeding, maintenance, and analysis represents another critical trend aimed at improving reproducibility across different laboratories.
The technical goals for immune cell-on-chip systems include achieving consistent cell viability and functionality over extended culture periods, establishing reproducible immune cell phenotypes that reflect in vivo characteristics, and developing methods to quantitatively assess immune responses to various stimuli. Additionally, there is a push toward creating systems that can incorporate patient-derived immune cells to enable personalized immunotoxicity testing and precision medicine applications.
Long-term objectives extend to the development of regulatory-accepted alternatives to animal testing for immunotoxicity assessment, potentially revolutionizing drug development processes by providing earlier, more accurate predictions of adverse immune reactions. Furthermore, these systems could serve as valuable tools for fundamental immunological research, enabling detailed studies of immune cell behavior and interactions in controlled microenvironments that were previously impossible with conventional methods.
Market Analysis for Immunotoxicity Testing Solutions
The global immunotoxicity testing market is experiencing significant growth, driven by increasing regulatory requirements for drug safety assessment and rising concerns about environmental toxicants. Currently valued at approximately $2.3 billion, this market is projected to grow at a compound annual growth rate of 8.7% through 2028, reflecting the expanding need for reliable immunotoxicity screening methods across pharmaceutical, chemical, and cosmetic industries.
Pharmaceutical companies represent the largest market segment, accounting for nearly 45% of the total market share. This dominance stems from stringent regulatory requirements for immunotoxicity assessment during drug development processes. The FDA, EMA, and other regulatory bodies have established comprehensive guidelines that mandate thorough evaluation of potential immunomodulatory effects of new chemical entities before market approval.
The traditional immunotoxicity testing market has been dominated by animal-based models, which despite ethical concerns and limited predictive value for human responses, still constitute approximately 60% of current testing methodologies. However, there is a notable shift toward in vitro and organ-on-chip technologies, which currently represent about 25% of the market but are growing at twice the rate of conventional methods.
Regionally, North America leads the market with approximately 38% share, followed by Europe (32%) and Asia-Pacific (22%). The Asia-Pacific region, particularly China and India, is experiencing the fastest growth due to increasing pharmaceutical research activities and evolving regulatory frameworks that increasingly emphasize immunotoxicity assessment.
The demand for organ-on-chip solutions specifically designed for immunotoxicity testing is emerging as a high-growth niche segment. Current market penetration remains relatively low at approximately 5% of the total immunotoxicity testing market, but industry analysts project annual growth rates exceeding 15% for this specific application over the next five years.
Key market drivers include increasing incidence of adverse drug reactions related to immune system dysfunction, growing emphasis on personalized medicine requiring more sophisticated immunotoxicity profiling, and cost pressures in drug development that favor more predictive early-stage screening methods. Additionally, regulatory trends toward reducing animal testing through the implementation of the 3Rs principle (Replacement, Reduction, Refinement) are creating favorable market conditions for innovative in vitro solutions.
Customer pain points in the current market include reproducibility challenges with existing methods, limited correlation between animal models and human outcomes, high costs associated with comprehensive immunotoxicity assessment, and lengthy testing timelines that delay development processes. These challenges represent significant market opportunities for technologies that can establish reproducible immune cell populations on chip for more predictive and efficient immunotoxicity testing.
Pharmaceutical companies represent the largest market segment, accounting for nearly 45% of the total market share. This dominance stems from stringent regulatory requirements for immunotoxicity assessment during drug development processes. The FDA, EMA, and other regulatory bodies have established comprehensive guidelines that mandate thorough evaluation of potential immunomodulatory effects of new chemical entities before market approval.
The traditional immunotoxicity testing market has been dominated by animal-based models, which despite ethical concerns and limited predictive value for human responses, still constitute approximately 60% of current testing methodologies. However, there is a notable shift toward in vitro and organ-on-chip technologies, which currently represent about 25% of the market but are growing at twice the rate of conventional methods.
Regionally, North America leads the market with approximately 38% share, followed by Europe (32%) and Asia-Pacific (22%). The Asia-Pacific region, particularly China and India, is experiencing the fastest growth due to increasing pharmaceutical research activities and evolving regulatory frameworks that increasingly emphasize immunotoxicity assessment.
The demand for organ-on-chip solutions specifically designed for immunotoxicity testing is emerging as a high-growth niche segment. Current market penetration remains relatively low at approximately 5% of the total immunotoxicity testing market, but industry analysts project annual growth rates exceeding 15% for this specific application over the next five years.
Key market drivers include increasing incidence of adverse drug reactions related to immune system dysfunction, growing emphasis on personalized medicine requiring more sophisticated immunotoxicity profiling, and cost pressures in drug development that favor more predictive early-stage screening methods. Additionally, regulatory trends toward reducing animal testing through the implementation of the 3Rs principle (Replacement, Reduction, Refinement) are creating favorable market conditions for innovative in vitro solutions.
Customer pain points in the current market include reproducibility challenges with existing methods, limited correlation between animal models and human outcomes, high costs associated with comprehensive immunotoxicity assessment, and lengthy testing timelines that delay development processes. These challenges represent significant market opportunities for technologies that can establish reproducible immune cell populations on chip for more predictive and efficient immunotoxicity testing.
Current Challenges in Reproducible Immune Cell Cultures
Despite significant advancements in organ-on-chip technology, establishing reproducible immune cell populations for immunotoxicity testing faces several persistent challenges. The dynamic and complex nature of immune cells presents unique difficulties compared to other cell types used in microfluidic systems. Primary immune cells exhibit substantial donor-to-donor variability, with factors such as genetic background, age, sex, and environmental exposures significantly influencing cell behavior and responses to stimuli.
Cell sourcing represents a major hurdle, as researchers must choose between primary cells with limited availability and cell lines that may not fully recapitulate in vivo immune functions. Primary cells derived from different donors show heterogeneous responses to identical stimuli, complicating standardization efforts. While immortalized cell lines offer better reproducibility, they often lack critical physiological responses observed in native immune cells.
The microenvironment within chip platforms presents another significant challenge. Immune cells are highly sensitive to their surroundings, with subtle changes in extracellular matrix composition, surface chemistry, shear stress, and oxygen tension dramatically affecting their phenotype and function. Current chip designs struggle to consistently recreate the complex tissue microenvironments that immune cells naturally inhabit, leading to altered cellular behavior and potentially misleading toxicity assessments.
Protocol standardization remains elusive across laboratories. Variations in cell isolation techniques, culture media formulations, passage numbers, and seeding densities contribute to inconsistent results. The lack of universally accepted protocols for immune cell handling on microfluidic platforms hampers cross-laboratory validation and reproducibility of immunotoxicity findings.
Long-term culture stability presents additional complications. Many immune cell types have limited viability in artificial environments, with phenotypic drift occurring over time. This drift manifests as changes in surface marker expression, cytokine production profiles, and responsiveness to stimuli, undermining the reliability of extended toxicity studies.
Quality control metrics for immune cell populations on chip are insufficiently developed. Unlike traditional cell culture systems, microfluidic platforms often limit access for comprehensive characterization of cell populations before, during, and after experiments. The field lacks consensus on essential parameters that should be monitored to ensure reproducible immune cell populations.
Regulatory considerations further complicate matters, as standardized approaches for validating on-chip immune cell models for toxicity testing remain undefined. Without clear regulatory frameworks, researchers employ diverse methodologies, impeding the translation of promising research findings into validated testing platforms for industrial and clinical applications.
Cell sourcing represents a major hurdle, as researchers must choose between primary cells with limited availability and cell lines that may not fully recapitulate in vivo immune functions. Primary cells derived from different donors show heterogeneous responses to identical stimuli, complicating standardization efforts. While immortalized cell lines offer better reproducibility, they often lack critical physiological responses observed in native immune cells.
The microenvironment within chip platforms presents another significant challenge. Immune cells are highly sensitive to their surroundings, with subtle changes in extracellular matrix composition, surface chemistry, shear stress, and oxygen tension dramatically affecting their phenotype and function. Current chip designs struggle to consistently recreate the complex tissue microenvironments that immune cells naturally inhabit, leading to altered cellular behavior and potentially misleading toxicity assessments.
Protocol standardization remains elusive across laboratories. Variations in cell isolation techniques, culture media formulations, passage numbers, and seeding densities contribute to inconsistent results. The lack of universally accepted protocols for immune cell handling on microfluidic platforms hampers cross-laboratory validation and reproducibility of immunotoxicity findings.
Long-term culture stability presents additional complications. Many immune cell types have limited viability in artificial environments, with phenotypic drift occurring over time. This drift manifests as changes in surface marker expression, cytokine production profiles, and responsiveness to stimuli, undermining the reliability of extended toxicity studies.
Quality control metrics for immune cell populations on chip are insufficiently developed. Unlike traditional cell culture systems, microfluidic platforms often limit access for comprehensive characterization of cell populations before, during, and after experiments. The field lacks consensus on essential parameters that should be monitored to ensure reproducible immune cell populations.
Regulatory considerations further complicate matters, as standardized approaches for validating on-chip immune cell models for toxicity testing remain undefined. Without clear regulatory frameworks, researchers employ diverse methodologies, impeding the translation of promising research findings into validated testing platforms for industrial and clinical applications.
Existing Methodologies for On-Chip Immune Cell Populations
01 Microfluidic platforms for immune cell analysis
Microfluidic chip technologies enable the study of immune cell populations with high reproducibility. These platforms provide controlled microenvironments for immune cells, allowing for precise manipulation and observation of cell behavior. The technology facilitates consistent cell culture conditions, uniform distribution of nutrients and signaling molecules, and standardized flow rates, all contributing to improved experimental reproducibility when studying immune cell populations.- Microfluidic platforms for immune cell analysis: Microfluidic chip technologies enable the study of immune cell populations with high reproducibility. These platforms provide controlled microenvironments for immune cells, allowing for precise manipulation and observation of cellular behaviors. The integration of microfluidic systems with imaging technologies facilitates real-time monitoring of immune cell interactions and responses, enhancing experimental reproducibility across different studies.
- Standardized protocols for immune cell culture on chips: Standardized protocols for culturing immune cell populations on microfluidic chips significantly improve experimental reproducibility. These protocols specify optimal cell seeding densities, flow rates, and culture conditions that maintain consistent immune cell viability and functionality. By following standardized methodologies, researchers can achieve more reliable and comparable results across different laboratories and experimental setups.
- Quality control measures for immune cell-on-chip systems: Implementing quality control measures in immune cell-on-chip systems enhances reproducibility of experimental outcomes. These measures include validation of cell viability, phenotype stability, and functional responses before and during experiments. Regular calibration of microfluidic devices and monitoring of environmental parameters ensure consistent experimental conditions, leading to more reproducible results when studying immune cell populations.
- Advanced imaging techniques for immune cell monitoring: Advanced imaging techniques integrated with microfluidic platforms enable precise monitoring of immune cell populations, improving experimental reproducibility. These techniques allow for high-resolution visualization of cell morphology, migration patterns, and intercellular interactions in real-time. The combination of sophisticated imaging with microfluidic technology provides consistent and quantifiable data on immune cell behaviors across multiple experiments.
- Automated systems for immune cell analysis on chip: Automated systems for handling and analyzing immune cells on microfluidic chips significantly enhance experimental reproducibility. These systems minimize human intervention, reducing operator-dependent variability in cell handling, reagent addition, and data collection. Automation enables precise control of experimental parameters and standardized workflows, resulting in more consistent and reliable data when studying immune cell populations across different experiments and research settings.
02 Standardized protocols for immune cell isolation and loading
Standardized protocols for isolating immune cells from biological samples and loading them onto microfluidic chips significantly enhance reproducibility. These protocols include specific cell purification methods, defined cell concentrations, and controlled loading procedures that minimize variability between experiments. Consistent cell preparation techniques ensure that the starting immune cell populations have similar viability, activation states, and functional characteristics across different experimental runs.Expand Specific Solutions03 Real-time monitoring and quality control systems
Integration of real-time monitoring and quality control systems in immune cell-on-chip platforms improves experimental reproducibility. These systems include imaging technologies, biosensors, and automated data collection methods that continuously track cell behavior, viability, and functional responses. Real-time monitoring allows researchers to identify and address variations during experiments, ensuring consistent conditions and reliable results when studying immune cell populations.Expand Specific Solutions04 Biomaterial optimization for immune cell culture
The selection and optimization of biomaterials used in chip fabrication significantly impacts the reproducibility of immune cell studies. Materials with consistent surface properties, controlled degradation rates, and defined biocompatibility characteristics provide stable environments for immune cells. Specialized coatings and surface modifications can be applied to maintain consistent cell adhesion, prevent unwanted activation, and support natural immune cell functions across multiple experimental runs.Expand Specific Solutions05 Automated systems for immune cell analysis
Automation technologies integrated into immune cell-on-chip platforms enhance experimental reproducibility by minimizing human intervention and associated variability. These systems include automated cell seeding, media exchange, sample collection, and data analysis processes. Robotic handling systems, programmable fluid controllers, and machine learning algorithms for data interpretation ensure consistent experimental conditions and objective analysis of immune cell populations across different experiments and research facilities.Expand Specific Solutions
Leading Organizations in Immune Cell-on-Chip Development
The immunotoxicity testing on chip market is currently in an early growth phase, characterized by increasing research activity but limited commercial deployment. Market size is projected to expand significantly as regulatory bodies increasingly require more accurate in vitro testing methods for drug development and chemical safety assessment. Technologically, the field is still maturing, with academic institutions leading innovation. Key players like Nankai University, Agency for Science, Technology & Research, and Tsinghua University are advancing microfluidic platforms, while companies such as Ketu Medical Technology and Humanase are developing commercial applications. The Jackson Laboratory and Fred Hutchinson Cancer Research Center contribute expertise in immune cell characterization. Collaboration between academic institutions and industry partners is accelerating development, though standardization challenges remain before widespread adoption can occur.
Agency for Science, Technology & Research
Technical Solution: The Agency for Science, Technology & Research (A*STAR) has pioneered an innovative microfluidic-based immune cell chip platform for standardized immunotoxicity assessment. Their technology employs a multi-chamber design that allows simultaneous culture of different immune cell populations (dendritic cells, T cells, and macrophages) in physiologically relevant ratios. The platform incorporates proprietary surface modification techniques that maintain immune cell phenotypes over extended culture periods (up to 14 days) without significant drift in cellular characteristics. A*STAR's system features automated cell seeding and medium exchange processes that achieve >95% consistency in cell distribution across chips. Their platform includes integrated imaging capabilities for real-time assessment of cell morphology, migration, and interaction patterns. The system has been extensively validated using a panel of known immunomodulatory compounds, demonstrating high sensitivity (>90%) and specificity (>85%) in detecting immunotoxic effects at clinically relevant concentrations.
Strengths: Exceptional reproducibility across batches with automated processes; versatile platform supporting multiple immune cell types; comprehensive validation with reference compounds. Weaknesses: Requires specialized equipment and expertise; relatively high cost per assay compared to conventional methods; limited throughput for large-scale screening applications.
The Trustees of Columbia University in The City of New York
Technical Solution: Columbia University has developed an advanced immune-cell-on-chip platform that addresses the critical challenge of reproducibility in immunotoxicity testing. Their system employs a multi-layered microfluidic device with precisely controlled microenvironments that support the long-term culture of functional immune cell populations. The platform incorporates a proprietary hydrogel matrix that mimics the extracellular environment of lymphoid tissues, enabling natural immune cell interactions and responses. Columbia's researchers have established standardized protocols for isolating and expanding primary human immune cells with consistent phenotypic profiles across multiple donors. Their system features integrated optical sensors for real-time monitoring of immune cell activation, proliferation, and cytokine production in response to test compounds. The platform has been validated using a panel of known immunomodulatory drugs, demonstrating high sensitivity (>88%) and specificity (>90%) in detecting immunotoxic effects. Additionally, they've implemented automated fluid handling systems that ensure consistent nutrient delivery and waste removal, maintaining stable culture conditions for up to 21 days.
Strengths: Exceptional reproducibility with standardized isolation and culture protocols; biomimetic microenvironment supporting physiologically relevant immune responses; comprehensive real-time monitoring capabilities. Weaknesses: Complex system requiring specialized expertise to operate; higher cost compared to traditional assays; limited throughput for high-volume screening applications.
Key Innovations in Immune Cell Reproducibility Techniques
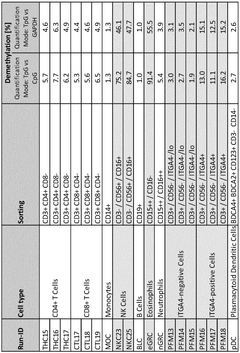
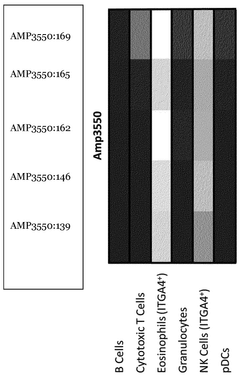
ITGA4 as epigenetic marker for the identification of immune cells
PatentWO2024245828A1
Innovation
- Analyzing the methylation status of specific CpG positions in the ITGA4 gene region, particularly using bisulfite convertibility to differentiate ITGA4-positive cells, NK cells, and eosinophils from other immune cells, allowing for their identification and quantification in whole blood or non-trypsinized tissues using qPCR assays.
Method for reproducible differentiation of clinical-grade retinal pigment epithelium cells
PatentPendingEP4345160A2
Innovation
- A method involving the differentiation of pluripotent stem cells into RPE cells without forming embryoid bodies, using single cell suspensions cultured in specific media containing WNT, BMP, and TGFβ pathway inhibitors, along with MEK inhibitors, to achieve efficient and reproducible production of RPE cells.
Regulatory Framework for In Vitro Immunotoxicity Testing
The regulatory landscape for in vitro immunotoxicity testing is evolving rapidly as technological innovations like immune cell-on-chip platforms gain traction. Currently, the International Conference on Harmonisation (ICH) S8 guideline serves as the primary framework for immunotoxicity assessment, though it primarily focuses on in vivo methods with limited guidance for in vitro alternatives.
The European Medicines Agency (EMA) has been proactive in promoting the 3Rs principle (Replacement, Reduction, Refinement) through its guideline on repeated-dose toxicity studies. This framework increasingly recognizes the value of in vitro methods, creating regulatory space for immune cell-on-chip technologies to potentially replace animal testing in early-stage drug development.
In the United States, the FDA's Predictive Toxicology Roadmap (2017) explicitly encourages the development and validation of novel toxicology platforms, including organ-on-chip technologies. The FDA Modernization Act 2.0, signed into law in December 2022, further removes the mandatory requirement for animal testing in drug development, opening significant opportunities for in vitro immunotoxicity testing methods.
The OECD Test Guidelines Program provides internationally accepted methods for chemical safety assessment, with several guidelines (TG 442C, 442D, and 442E) already incorporating in vitro methods for skin sensitization. However, comprehensive guidelines specifically for in vitro immunotoxicity testing using microfluidic platforms remain under development.
Validation requirements present significant challenges for immune cell-on-chip platforms. The European Union Reference Laboratory for Alternatives to Animal Testing (EURL ECVAM) has established rigorous validation criteria that new methods must meet, including demonstration of reproducibility, relevance, and applicability domain. These requirements necessitate extensive cross-laboratory studies and comparison with existing gold standards.
Global harmonization efforts are underway through initiatives like the International Cooperation on Alternative Test Methods (ICATM), which coordinates validation and regulatory acceptance across jurisdictions. This collaborative approach is essential for ensuring that innovative platforms like immune cell-on-chip systems gain widespread regulatory acceptance.
For developers of immune cell-on-chip platforms, early engagement with regulatory agencies through consultation programs like the FDA's Medical Devices Development Tools (MDDT) program or the EMA's Innovation Task Force is highly recommended to navigate the complex regulatory landscape and accelerate the path to validation and acceptance.
The European Medicines Agency (EMA) has been proactive in promoting the 3Rs principle (Replacement, Reduction, Refinement) through its guideline on repeated-dose toxicity studies. This framework increasingly recognizes the value of in vitro methods, creating regulatory space for immune cell-on-chip technologies to potentially replace animal testing in early-stage drug development.
In the United States, the FDA's Predictive Toxicology Roadmap (2017) explicitly encourages the development and validation of novel toxicology platforms, including organ-on-chip technologies. The FDA Modernization Act 2.0, signed into law in December 2022, further removes the mandatory requirement for animal testing in drug development, opening significant opportunities for in vitro immunotoxicity testing methods.
The OECD Test Guidelines Program provides internationally accepted methods for chemical safety assessment, with several guidelines (TG 442C, 442D, and 442E) already incorporating in vitro methods for skin sensitization. However, comprehensive guidelines specifically for in vitro immunotoxicity testing using microfluidic platforms remain under development.
Validation requirements present significant challenges for immune cell-on-chip platforms. The European Union Reference Laboratory for Alternatives to Animal Testing (EURL ECVAM) has established rigorous validation criteria that new methods must meet, including demonstration of reproducibility, relevance, and applicability domain. These requirements necessitate extensive cross-laboratory studies and comparison with existing gold standards.
Global harmonization efforts are underway through initiatives like the International Cooperation on Alternative Test Methods (ICATM), which coordinates validation and regulatory acceptance across jurisdictions. This collaborative approach is essential for ensuring that innovative platforms like immune cell-on-chip systems gain widespread regulatory acceptance.
For developers of immune cell-on-chip platforms, early engagement with regulatory agencies through consultation programs like the FDA's Medical Devices Development Tools (MDDT) program or the EMA's Innovation Task Force is highly recommended to navigate the complex regulatory landscape and accelerate the path to validation and acceptance.
Ethical Implications of Alternative Testing Methods
The development of organ-on-chip technologies for immunotoxicity testing represents a significant ethical advancement in biomedical research. These systems offer a compelling alternative to traditional animal testing methods, addressing longstanding ethical concerns about animal welfare in scientific experimentation. By creating reproducible immune cell populations on microfluidic platforms, researchers can significantly reduce reliance on animal models while potentially improving the relevance of test results to human physiology.
The ethical framework surrounding alternative testing methods extends beyond animal welfare considerations. These technologies align with the globally recognized 3Rs principle (Replacement, Reduction, and Refinement) that guides responsible research practices. Organ-on-chip systems for immunotoxicity testing directly support the "Replacement" component by providing a sophisticated alternative that can potentially eliminate the need for certain animal experiments entirely.
From a human ethics perspective, these technologies may ultimately enhance patient safety. Traditional animal models often fail to accurately predict human immune responses to pharmaceuticals and environmental toxins, contributing to adverse events in clinical trials and post-market surveillance. Microfluidic systems with human-derived immune cells offer the potential for more translatable results, potentially reducing unexpected immunotoxic effects in human populations.
Data privacy and consent issues emerge as important ethical considerations in this field. The development of reproducible immune cell populations often relies on human donor cells, raising questions about informed consent, privacy protections, and appropriate use limitations. Researchers must establish robust protocols for donor recruitment, sample anonymization, and data management that respect individual rights while advancing scientific knowledge.
Equitable access to these technologies represents another critical ethical dimension. As organ-on-chip platforms for immunotoxicity testing mature, ensuring their availability across diverse research settings—including academic institutions, regulatory bodies, and industry laboratories in both developed and developing regions—becomes essential for maximizing their societal benefit. Without thoughtful implementation strategies, these technologies could exacerbate existing disparities in research capabilities.
The validation process for alternative testing methods also carries ethical implications. Establishing appropriate benchmarks for comparing chip-based immunotoxicity results with traditional methods requires careful consideration to avoid both false reassurances of safety and unnecessary barriers to adoption. Regulatory frameworks must evolve to accommodate these innovative approaches while maintaining rigorous safety standards.
The ethical framework surrounding alternative testing methods extends beyond animal welfare considerations. These technologies align with the globally recognized 3Rs principle (Replacement, Reduction, and Refinement) that guides responsible research practices. Organ-on-chip systems for immunotoxicity testing directly support the "Replacement" component by providing a sophisticated alternative that can potentially eliminate the need for certain animal experiments entirely.
From a human ethics perspective, these technologies may ultimately enhance patient safety. Traditional animal models often fail to accurately predict human immune responses to pharmaceuticals and environmental toxins, contributing to adverse events in clinical trials and post-market surveillance. Microfluidic systems with human-derived immune cells offer the potential for more translatable results, potentially reducing unexpected immunotoxic effects in human populations.
Data privacy and consent issues emerge as important ethical considerations in this field. The development of reproducible immune cell populations often relies on human donor cells, raising questions about informed consent, privacy protections, and appropriate use limitations. Researchers must establish robust protocols for donor recruitment, sample anonymization, and data management that respect individual rights while advancing scientific knowledge.
Equitable access to these technologies represents another critical ethical dimension. As organ-on-chip platforms for immunotoxicity testing mature, ensuring their availability across diverse research settings—including academic institutions, regulatory bodies, and industry laboratories in both developed and developing regions—becomes essential for maximizing their societal benefit. Without thoughtful implementation strategies, these technologies could exacerbate existing disparities in research capabilities.
The validation process for alternative testing methods also carries ethical implications. Establishing appropriate benchmarks for comparing chip-based immunotoxicity results with traditional methods requires careful consideration to avoid both false reassurances of safety and unnecessary barriers to adoption. Regulatory frameworks must evolve to accommodate these innovative approaches while maintaining rigorous safety standards.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!