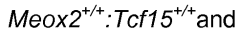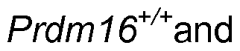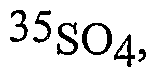Endothelial heterogeneity derived from different vascular beds: implications for vascular OoC models
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Endothelial Heterogeneity Background and Research Objectives
The field of vascular biology has evolved significantly over the past decades, with increasing recognition of the remarkable heterogeneity exhibited by endothelial cells across different vascular beds. Historically, endothelial cells were considered primarily as passive lining cells forming a homogeneous barrier between blood and tissues. However, research since the 1980s has progressively revealed that endothelial cells display organ-specific phenotypes and functions, challenging this simplistic view.
Endothelial heterogeneity manifests in multiple dimensions, including morphological differences, molecular expression patterns, and functional specializations. For instance, continuous endothelium in the brain forms the highly selective blood-brain barrier, while fenestrated endothelium in kidneys and endocrine glands allows for selective permeability. Discontinuous endothelium in liver sinusoids permits extensive metabolic exchange between blood and hepatocytes.
This heterogeneity is not merely structural but extends to molecular signatures, with organ-specific expression of surface markers, transporters, and adhesion molecules. Recent advances in single-cell RNA sequencing have further expanded our understanding of this diversity, revealing previously unrecognized endothelial subpopulations within the same organ.
The technological evolution of Organ-on-Chip (OoC) platforms represents a promising approach to model tissue-specific vascular functions in vitro. These microfluidic devices aim to recapitulate the physiological microenvironment of specific organs, including their vascular components. However, the endothelial heterogeneity presents both a challenge and an opportunity for these models.
Current research objectives focus on characterizing the molecular determinants of endothelial specialization across different vascular beds and understanding how these specializations contribute to organ-specific functions. This includes investigating the role of microenvironmental factors, genetic programming, and epigenetic modifications in establishing and maintaining endothelial heterogeneity.
A critical goal is to develop improved vascular Organ-on-Chip models that accurately reflect the heterogeneity of endothelial cells in different organs. This requires identifying appropriate sources of organ-specific endothelial cells, optimizing culture conditions to maintain their phenotypic characteristics, and validating these models against in vivo benchmarks.
Additionally, research aims to leverage endothelial heterogeneity for therapeutic applications, such as targeted drug delivery to specific vascular beds and development of organ-specific vascular disease models. Understanding the mechanisms underlying endothelial specialization may also provide insights into pathological conditions where this specialization is disrupted, including cancer, inflammatory disorders, and vascular malformations.
Endothelial heterogeneity manifests in multiple dimensions, including morphological differences, molecular expression patterns, and functional specializations. For instance, continuous endothelium in the brain forms the highly selective blood-brain barrier, while fenestrated endothelium in kidneys and endocrine glands allows for selective permeability. Discontinuous endothelium in liver sinusoids permits extensive metabolic exchange between blood and hepatocytes.
This heterogeneity is not merely structural but extends to molecular signatures, with organ-specific expression of surface markers, transporters, and adhesion molecules. Recent advances in single-cell RNA sequencing have further expanded our understanding of this diversity, revealing previously unrecognized endothelial subpopulations within the same organ.
The technological evolution of Organ-on-Chip (OoC) platforms represents a promising approach to model tissue-specific vascular functions in vitro. These microfluidic devices aim to recapitulate the physiological microenvironment of specific organs, including their vascular components. However, the endothelial heterogeneity presents both a challenge and an opportunity for these models.
Current research objectives focus on characterizing the molecular determinants of endothelial specialization across different vascular beds and understanding how these specializations contribute to organ-specific functions. This includes investigating the role of microenvironmental factors, genetic programming, and epigenetic modifications in establishing and maintaining endothelial heterogeneity.
A critical goal is to develop improved vascular Organ-on-Chip models that accurately reflect the heterogeneity of endothelial cells in different organs. This requires identifying appropriate sources of organ-specific endothelial cells, optimizing culture conditions to maintain their phenotypic characteristics, and validating these models against in vivo benchmarks.
Additionally, research aims to leverage endothelial heterogeneity for therapeutic applications, such as targeted drug delivery to specific vascular beds and development of organ-specific vascular disease models. Understanding the mechanisms underlying endothelial specialization may also provide insights into pathological conditions where this specialization is disrupted, including cancer, inflammatory disorders, and vascular malformations.
Clinical Demand Analysis for Vascular Organ-on-Chip Models
The clinical demand for vascular Organ-on-Chip (OoC) models has grown exponentially in recent years, driven by the limitations of traditional drug development approaches. Conventional methods rely heavily on animal models that often fail to accurately predict human responses, resulting in high attrition rates during clinical trials. According to industry reports, approximately 90% of drug candidates that succeed in preclinical animal testing fail in human clinical trials, with cardiovascular toxicity being a leading cause of these failures.
Vascular OoC models that incorporate endothelial heterogeneity from different vascular beds address critical unmet needs in several clinical domains. In cardiovascular disease research, these models enable more accurate testing of therapeutics targeting specific vascular pathologies such as atherosclerosis, thrombosis, and hypertension. The ability to replicate tissue-specific vascular responses provides researchers with platforms that more faithfully recapitulate human pathophysiology.
In oncology, there is substantial demand for advanced vascular models that can simulate tumor-specific vasculature. The interaction between cancer cells and their surrounding blood vessels is crucial for understanding tumor progression and developing effective anti-angiogenic therapies. Vascular OoC models that account for endothelial heterogeneity can better predict drug efficacy and potential side effects in different tumor microenvironments.
The pharmaceutical industry has demonstrated increasing interest in these technologies, with major companies establishing dedicated OoC research divisions. This trend is supported by regulatory agencies like the FDA and EMA, which are developing frameworks to incorporate OoC data in regulatory submissions. The potential to reduce animal testing while improving predictive accuracy aligns with both ethical considerations and cost-efficiency goals.
Neurological disorders represent another significant area of clinical demand. The blood-brain barrier (BBB) presents unique challenges for drug delivery, and OoC models that accurately represent the specialized endothelial cells of cerebral vasculature can help overcome these barriers. Researchers are particularly interested in models that can simulate BBB dysfunction in conditions like Alzheimer's disease, multiple sclerosis, and stroke.
Personalized medicine initiatives are driving demand for patient-specific vascular models. By incorporating patient-derived cells into OoC systems, clinicians can potentially test treatment responses before administration, optimizing therapeutic strategies and minimizing adverse effects. This approach is especially valuable for rare diseases and complex vascular disorders where treatment options are limited and outcomes highly variable.
Market analysis indicates that the global OoC market is projected to grow significantly, with vascular models representing a substantial segment of this growth. Healthcare providers, academic institutions, and pharmaceutical companies are all contributing to increased adoption, recognizing the potential for these technologies to transform clinical research and therapeutic development.
Vascular OoC models that incorporate endothelial heterogeneity from different vascular beds address critical unmet needs in several clinical domains. In cardiovascular disease research, these models enable more accurate testing of therapeutics targeting specific vascular pathologies such as atherosclerosis, thrombosis, and hypertension. The ability to replicate tissue-specific vascular responses provides researchers with platforms that more faithfully recapitulate human pathophysiology.
In oncology, there is substantial demand for advanced vascular models that can simulate tumor-specific vasculature. The interaction between cancer cells and their surrounding blood vessels is crucial for understanding tumor progression and developing effective anti-angiogenic therapies. Vascular OoC models that account for endothelial heterogeneity can better predict drug efficacy and potential side effects in different tumor microenvironments.
The pharmaceutical industry has demonstrated increasing interest in these technologies, with major companies establishing dedicated OoC research divisions. This trend is supported by regulatory agencies like the FDA and EMA, which are developing frameworks to incorporate OoC data in regulatory submissions. The potential to reduce animal testing while improving predictive accuracy aligns with both ethical considerations and cost-efficiency goals.
Neurological disorders represent another significant area of clinical demand. The blood-brain barrier (BBB) presents unique challenges for drug delivery, and OoC models that accurately represent the specialized endothelial cells of cerebral vasculature can help overcome these barriers. Researchers are particularly interested in models that can simulate BBB dysfunction in conditions like Alzheimer's disease, multiple sclerosis, and stroke.
Personalized medicine initiatives are driving demand for patient-specific vascular models. By incorporating patient-derived cells into OoC systems, clinicians can potentially test treatment responses before administration, optimizing therapeutic strategies and minimizing adverse effects. This approach is especially valuable for rare diseases and complex vascular disorders where treatment options are limited and outcomes highly variable.
Market analysis indicates that the global OoC market is projected to grow significantly, with vascular models representing a substantial segment of this growth. Healthcare providers, academic institutions, and pharmaceutical companies are all contributing to increased adoption, recognizing the potential for these technologies to transform clinical research and therapeutic development.
Current Status and Challenges in Endothelial Cell Modeling
The global landscape of endothelial cell modeling has evolved significantly over the past decade, with substantial advancements in both in vitro and in silico approaches. Currently, traditional two-dimensional cell culture systems remain the most widely used platforms for endothelial research, despite their limited ability to recapitulate the complex three-dimensional microenvironment of native vasculature. These conventional models fail to account for the heterogeneity of endothelial cells derived from different vascular beds, which exhibit distinct molecular signatures and functional properties.
Recent technological innovations have led to the development of more sophisticated three-dimensional models, including spheroids, hydrogel-based constructs, and microfluidic systems. These advanced platforms have demonstrated improved capabilities in mimicking physiological conditions, particularly in terms of fluid dynamics and cell-cell interactions. However, they still struggle to fully capture the organ-specific characteristics of endothelial cells from different vascular origins.
A significant challenge in the field is the limited availability of primary human endothelial cells from diverse vascular beds. While human umbilical vein endothelial cells (HUVECs) are readily accessible and extensively characterized, they represent only one specific vascular phenotype and may not accurately reflect the behavior of endothelial cells in other organs. This discrepancy becomes particularly problematic when developing organ-specific vascular models.
The integration of endothelial cells into Organ-on-Chip (OoC) platforms represents another frontier in the field. Current OoC models incorporating vascular components have shown promise in replicating certain aspects of tissue-specific vasculature, but they often utilize generic endothelial cell lines that lack the distinctive properties of organ-specific endothelium. This limitation undermines the physiological relevance of these models, particularly for drug development and disease modeling applications.
Computational modeling approaches have emerged as complementary tools for understanding endothelial heterogeneity. These in silico methods can incorporate multi-omics data to predict cell behavior under various conditions. However, they are constrained by the quality and comprehensiveness of available experimental data, which remains insufficient for many vascular beds.
The standardization of protocols for isolating, characterizing, and maintaining organ-specific endothelial cells presents another significant hurdle. Variations in methodology across research groups complicate cross-study comparisons and limit reproducibility. Additionally, the dynamic nature of endothelial phenotypes, which can change in response to environmental cues, poses challenges for maintaining stable, representative models over extended periods.
Addressing these challenges requires interdisciplinary collaboration among bioengineers, cell biologists, computational scientists, and clinicians to develop more accurate and physiologically relevant models that account for the inherent heterogeneity of the vascular endothelium across different organ systems.
Recent technological innovations have led to the development of more sophisticated three-dimensional models, including spheroids, hydrogel-based constructs, and microfluidic systems. These advanced platforms have demonstrated improved capabilities in mimicking physiological conditions, particularly in terms of fluid dynamics and cell-cell interactions. However, they still struggle to fully capture the organ-specific characteristics of endothelial cells from different vascular origins.
A significant challenge in the field is the limited availability of primary human endothelial cells from diverse vascular beds. While human umbilical vein endothelial cells (HUVECs) are readily accessible and extensively characterized, they represent only one specific vascular phenotype and may not accurately reflect the behavior of endothelial cells in other organs. This discrepancy becomes particularly problematic when developing organ-specific vascular models.
The integration of endothelial cells into Organ-on-Chip (OoC) platforms represents another frontier in the field. Current OoC models incorporating vascular components have shown promise in replicating certain aspects of tissue-specific vasculature, but they often utilize generic endothelial cell lines that lack the distinctive properties of organ-specific endothelium. This limitation undermines the physiological relevance of these models, particularly for drug development and disease modeling applications.
Computational modeling approaches have emerged as complementary tools for understanding endothelial heterogeneity. These in silico methods can incorporate multi-omics data to predict cell behavior under various conditions. However, they are constrained by the quality and comprehensiveness of available experimental data, which remains insufficient for many vascular beds.
The standardization of protocols for isolating, characterizing, and maintaining organ-specific endothelial cells presents another significant hurdle. Variations in methodology across research groups complicate cross-study comparisons and limit reproducibility. Additionally, the dynamic nature of endothelial phenotypes, which can change in response to environmental cues, poses challenges for maintaining stable, representative models over extended periods.
Addressing these challenges requires interdisciplinary collaboration among bioengineers, cell biologists, computational scientists, and clinicians to develop more accurate and physiologically relevant models that account for the inherent heterogeneity of the vascular endothelium across different organ systems.
Current Methodologies for Modeling Vascular Bed Differences
01 Molecular markers of endothelial heterogeneity
Endothelial cells from different vascular beds express distinct molecular markers that contribute to their heterogeneity. These markers include specific proteins, receptors, and transcription factors that are differentially expressed depending on the vascular location. The identification and characterization of these molecular signatures help in understanding the functional differences between endothelial cells from various organs and tissues, providing insights into vascular bed-specific characteristics.- Molecular markers of endothelial heterogeneity: Endothelial cells from different vascular beds express distinct molecular markers that contribute to their heterogeneity. These markers include specific proteins, receptors, and transcription factors that are differentially expressed depending on the vascular location. The identification and characterization of these molecular signatures help in understanding the functional differences between endothelial cells from various organs and tissues, providing insights into vascular bed-specific characteristics.
- Organ-specific endothelial cell functions: Endothelial cells exhibit specialized functions depending on their location within different organs. These specialized functions include organ-specific barrier properties, transport mechanisms, and interactions with surrounding tissues. For example, brain endothelial cells form the blood-brain barrier with tight junctions, while liver sinusoidal endothelial cells have fenestrations that facilitate exchange of materials. Understanding these organ-specific functions is crucial for developing targeted therapies for vascular diseases.
- Genetic regulation of vascular bed-specific characteristics: The heterogeneity of endothelial cells across different vascular beds is regulated by specific genetic programs. These genetic programs control the expression of genes responsible for the development and maintenance of vascular bed-specific characteristics. Transcription factors and epigenetic modifications play crucial roles in establishing and maintaining these distinct endothelial phenotypes. Research in this area helps to understand how endothelial heterogeneity is established during development and maintained throughout life.
- Endothelial response to pathological conditions: Endothelial cells from different vascular beds respond differently to pathological conditions such as inflammation, hypoxia, and oxidative stress. These differential responses contribute to the organ-specific manifestations of vascular diseases. For instance, coronary endothelial cells may be more susceptible to atherosclerosis, while brain endothelial cells may respond differently to inflammatory stimuli. Understanding these vascular bed-specific responses is important for developing targeted therapeutic approaches for vascular disorders.
- Therapeutic targeting of endothelial heterogeneity: The recognition of endothelial heterogeneity has led to the development of therapeutic strategies that target specific vascular beds. These approaches exploit the unique characteristics of endothelial cells in different organs to deliver treatments with enhanced specificity and reduced side effects. Examples include organ-specific drug delivery systems, gene therapies targeting specific endothelial populations, and bioengineered vascular grafts that mimic the properties of native vessels. These targeted therapies hold promise for treating vascular diseases with improved efficacy and safety profiles.
02 Organ-specific endothelial cell functions
Endothelial cells exhibit specialized functions depending on their location within different organs. These organ-specific functions include selective permeability, regulation of immune cell trafficking, and adaptation to local metabolic demands. For example, brain endothelial cells form the blood-brain barrier with tight junctions, while liver sinusoidal endothelial cells have fenestrations to facilitate metabolite exchange. These functional adaptations are critical for maintaining organ homeostasis and responding to physiological challenges.Expand Specific Solutions03 Developmental origins of endothelial heterogeneity
The heterogeneity of endothelial cells across different vascular beds is partly determined by their developmental origins. During embryogenesis, endothelial cells derive from distinct progenitor populations and are exposed to different microenvironmental cues that shape their phenotype. These developmental processes establish the foundation for vascular bed-specific characteristics that persist into adulthood, influencing how endothelial cells respond to physiological and pathological stimuli.Expand Specific Solutions04 Microenvironmental regulation of endothelial phenotype
The local microenvironment plays a crucial role in regulating endothelial cell phenotype and function in different vascular beds. Factors such as shear stress, oxygen tension, and interactions with surrounding cells (including pericytes, smooth muscle cells, and tissue-specific cells) influence endothelial behavior. These microenvironmental signals activate specific signaling pathways and gene expression programs that maintain vascular bed-specific characteristics and allow endothelial cells to adapt to changing conditions.Expand Specific Solutions05 Pathological implications of endothelial heterogeneity
Endothelial heterogeneity has significant implications for vascular pathologies that affect specific organs or vascular beds. Diseases such as atherosclerosis, diabetic retinopathy, and tumor angiogenesis manifest differently across vascular beds due to the unique properties of local endothelial cells. Understanding these vascular bed-specific characteristics is essential for developing targeted therapeutic approaches that address the underlying mechanisms of endothelial dysfunction in different pathological contexts.Expand Specific Solutions
Leading Research Institutions and Industry Stakeholders
The field of endothelial heterogeneity in vascular Organ-on-Chip models is in its early growth phase, with an estimated market size of $300-500 million and projected annual growth of 25-30%. The competitive landscape features established players like Emulate, Inc. leading commercial development alongside academic powerhouses such as MIT and Harvard. Pharmaceutical giants including Roche and Genentech are investing heavily in this technology for drug development applications. The technology is approaching early commercial maturity, with companies developing specialized platforms that account for vascular bed-specific endothelial differences. Research institutions in North America, Europe, and Asia are actively advancing the fundamental science, creating a globally distributed innovation ecosystem with increasing cross-sector collaborations.
Emulate, Inc.
Technical Solution: Emulate has developed advanced Organ-on-Chip technology specifically addressing endothelial heterogeneity across different vascular beds. Their platform incorporates microfluidic channels lined with human endothelial cells sourced from specific organs, allowing researchers to recreate the unique microenvironments of different vascular beds. The company's Organ-Chips feature two parallel microchannels separated by a porous membrane, where one channel is lined with organ-specific endothelial cells and the other with tissue-specific cells, enabling the study of vascular-parenchymal interactions[1]. Emulate's technology applies mechanical forces (stretching and fluid flow) that mimic physiological conditions, inducing endothelial cells to express organ-specific markers and functions. Their chips incorporate real-time imaging capabilities and sampling ports for continuous monitoring of endothelial responses across different vascular beds, facilitating comparative studies of drug effects on various vascular territories simultaneously[3].
Strengths: Industry-leading microfluidic technology with precise control over mechanical forces and fluid dynamics; commercially available standardized platform with validated protocols for multiple organ systems. Weaknesses: Relatively high cost compared to traditional cell culture methods; requires specialized equipment and expertise; challenges in achieving full physiological complexity of in vivo vascular networks.
Genentech, Inc.
Technical Solution: Genentech has developed a sophisticated Organ-on-Chip platform specifically designed to address endothelial heterogeneity across different vascular beds for drug development applications. Their approach utilizes primary human endothelial cells isolated from multiple organs (brain, lung, kidney, liver) cultured under organ-specific conditions to maintain their native phenotypes[3]. The company's technology incorporates customized microfluidic devices with precisely controlled shear stress parameters calibrated to match the hemodynamic conditions of each vascular bed. Genentech's platform features integrated sensors for real-time monitoring of barrier function through transendothelial electrical resistance (TEER) measurements, enabling quantitative assessment of drug effects on vascular permeability across different organ systems[6]. Their chips incorporate co-culture capabilities with organ-specific parenchymal cells and pericytes to recreate the complex cellular interactions that influence endothelial phenotypes. Genentech has validated their platform through comparative pharmacokinetic studies demonstrating organ-specific drug responses that correlate with clinical observations, particularly for biologics with differential tissue distribution profiles[7].
Strengths: Robust validation with pharmaceutical compounds; seamless integration with drug discovery pipeline; advanced analytical capabilities for quantitative assessment of drug-endothelium interactions. Weaknesses: Proprietary system with limited accessibility to external researchers; challenges in scaling to high-throughput applications; difficulty in modeling complex pathological states like inflammation or thrombosis.
Key Scientific Breakthroughs in Endothelial Heterogeneity
Vascular bed-specific endothelial cells
PatentWO2014006228A1
Innovation
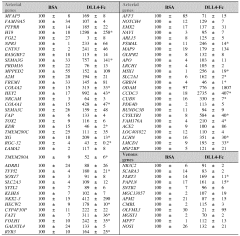
- A method is developed to generate vascular bed-specific ECs by transducing or transfecting stem/progenitor cells with specific transcription factors such as Meox2, Tcf15, Ppary, Wt1, and others to differentiate into arterial or microvascular ECs, allowing for tailored therapies and targeted drug delivery.
NF-HEV compositions and methods of use
PatentWO2004056868A2
Innovation
- Characterization and utilization of nuclear factor NF-HEV, specifically expressed in human endothelial cells from chronically inflamed tissues, as a target for therapeutic intervention to modulate endothelial cell differentiation and inflammation, using NF-HEV polypeptides and recombinant vectors to inhibit HEV-like vessel development and reduce lymphocyte adhesion.
Translational Potential for Personalized Medicine
The recognition of endothelial heterogeneity across different vascular beds presents a transformative opportunity for personalized medicine approaches. By accurately modeling patient-specific vascular characteristics in Organ-on-Chip (OoC) platforms, clinicians can develop tailored therapeutic strategies that account for individual vascular phenotypes and disease manifestations.
Vascular OoC models incorporating bed-specific endothelial heterogeneity enable precision drug screening that considers patient-specific vascular responses. This approach allows for the identification of optimal therapeutic compounds and dosages based on an individual's unique vascular profile, potentially reducing adverse effects while maximizing efficacy. The ability to recreate patient-derived vascular microenvironments provides a platform for testing personalized treatment regimens before clinical implementation.
These advanced models also facilitate companion diagnostic development by correlating vascular biomarkers with treatment outcomes. By analyzing how specific endothelial phenotypes respond to various interventions, researchers can identify predictive biomarkers that guide treatment selection. This creates opportunities for developing diagnostic tools that match patients with the most appropriate therapies based on their vascular characteristics.
For rare vascular disorders, which often lack adequate treatment options due to limited research populations, personalized OoC models offer particular value. By recreating patient-specific vascular abnormalities, these platforms enable therapeutic testing even for conditions with small patient cohorts, potentially accelerating orphan drug development and validation for underserved patient populations.
The integration of patient-derived cells into vascular OoC systems creates powerful tools for stratifying patients into treatment-responsive subgroups. This approach supports the development of targeted therapies for specific endothelial phenotypes associated with particular disease manifestations, moving beyond one-size-fits-all treatment paradigms toward truly personalized vascular medicine.
Looking forward, the combination of vascular OoC technology with emerging computational approaches and artificial intelligence presents opportunities for predictive personalized medicine. Machine learning algorithms analyzing data from patient-specific vascular models could forecast individual treatment responses and disease progression, enabling proactive rather than reactive healthcare interventions tailored to each patient's unique vascular biology.
Vascular OoC models incorporating bed-specific endothelial heterogeneity enable precision drug screening that considers patient-specific vascular responses. This approach allows for the identification of optimal therapeutic compounds and dosages based on an individual's unique vascular profile, potentially reducing adverse effects while maximizing efficacy. The ability to recreate patient-derived vascular microenvironments provides a platform for testing personalized treatment regimens before clinical implementation.
These advanced models also facilitate companion diagnostic development by correlating vascular biomarkers with treatment outcomes. By analyzing how specific endothelial phenotypes respond to various interventions, researchers can identify predictive biomarkers that guide treatment selection. This creates opportunities for developing diagnostic tools that match patients with the most appropriate therapies based on their vascular characteristics.
For rare vascular disorders, which often lack adequate treatment options due to limited research populations, personalized OoC models offer particular value. By recreating patient-specific vascular abnormalities, these platforms enable therapeutic testing even for conditions with small patient cohorts, potentially accelerating orphan drug development and validation for underserved patient populations.
The integration of patient-derived cells into vascular OoC systems creates powerful tools for stratifying patients into treatment-responsive subgroups. This approach supports the development of targeted therapies for specific endothelial phenotypes associated with particular disease manifestations, moving beyond one-size-fits-all treatment paradigms toward truly personalized vascular medicine.
Looking forward, the combination of vascular OoC technology with emerging computational approaches and artificial intelligence presents opportunities for predictive personalized medicine. Machine learning algorithms analyzing data from patient-specific vascular models could forecast individual treatment responses and disease progression, enabling proactive rather than reactive healthcare interventions tailored to each patient's unique vascular biology.
Standardization and Validation Requirements
The standardization and validation of vascular Organ-on-Chip (OoC) models represent critical challenges in translating these promising technologies from research tools to reliable platforms for drug development and disease modeling. Given the significant endothelial heterogeneity across different vascular beds, establishing robust standardization protocols becomes even more complex yet essential.
Standardization efforts must address multiple dimensions of vascular OoC models, beginning with cell sourcing and characterization. Protocols for isolating, expanding, and characterizing endothelial cells from specific vascular beds need precise documentation, including passage number limitations and phenotypic stability assessments. The microfluidic device design parameters, including channel dimensions, surface treatments, and material properties, require standardization to ensure reproducibility across laboratories.
Culture conditions represent another critical standardization domain, encompassing media composition, flow rates, and shear stress parameters that must be tailored to specific vascular bed characteristics. These parameters significantly influence endothelial phenotype maintenance and should reflect physiological conditions of the target vascular bed.
Validation frameworks must incorporate multi-level assessment approaches. At the molecular level, expression profiles of endothelial markers specific to different vascular beds (such as VCAM-1, ICAM-1, VE-cadherin) should be quantitatively evaluated. Functional validation requires standardized assays for barrier integrity, response to inflammatory stimuli, and interaction with circulating cells, with metrics tailored to the specific vascular bed being modeled.
Physiological validation necessitates comparison with in vivo data from the corresponding vascular bed, establishing acceptance criteria that acknowledge inherent biological variability while ensuring model reliability. This may include response to known vasoactive compounds, permeability characteristics, and tissue-specific endothelial functions.
Inter-laboratory reproducibility studies represent a crucial yet often overlooked validation requirement. Round-robin testing protocols should be established to assess model robustness across different research environments, identifying critical variables that impact reproducibility.
Regulatory considerations must also be addressed, particularly for models intended for drug development applications. This includes developing standardized reporting formats documenting model specifications, validation data, and limitations, aligned with emerging regulatory frameworks for microphysiological systems.
The development of reference standards—well-characterized endothelial cell lines or primary cell populations from specific vascular beds—would significantly advance standardization efforts, providing benchmarks against which new models can be validated.
Standardization efforts must address multiple dimensions of vascular OoC models, beginning with cell sourcing and characterization. Protocols for isolating, expanding, and characterizing endothelial cells from specific vascular beds need precise documentation, including passage number limitations and phenotypic stability assessments. The microfluidic device design parameters, including channel dimensions, surface treatments, and material properties, require standardization to ensure reproducibility across laboratories.
Culture conditions represent another critical standardization domain, encompassing media composition, flow rates, and shear stress parameters that must be tailored to specific vascular bed characteristics. These parameters significantly influence endothelial phenotype maintenance and should reflect physiological conditions of the target vascular bed.
Validation frameworks must incorporate multi-level assessment approaches. At the molecular level, expression profiles of endothelial markers specific to different vascular beds (such as VCAM-1, ICAM-1, VE-cadherin) should be quantitatively evaluated. Functional validation requires standardized assays for barrier integrity, response to inflammatory stimuli, and interaction with circulating cells, with metrics tailored to the specific vascular bed being modeled.
Physiological validation necessitates comparison with in vivo data from the corresponding vascular bed, establishing acceptance criteria that acknowledge inherent biological variability while ensuring model reliability. This may include response to known vasoactive compounds, permeability characteristics, and tissue-specific endothelial functions.
Inter-laboratory reproducibility studies represent a crucial yet often overlooked validation requirement. Round-robin testing protocols should be established to assess model robustness across different research environments, identifying critical variables that impact reproducibility.
Regulatory considerations must also be addressed, particularly for models intended for drug development applications. This includes developing standardized reporting formats documenting model specifications, validation data, and limitations, aligned with emerging regulatory frameworks for microphysiological systems.
The development of reference standards—well-characterized endothelial cell lines or primary cell populations from specific vascular beds—would significantly advance standardization efforts, providing benchmarks against which new models can be validated.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!