Long-term maintenance and phenotype stability of pancreatic islets in microphysiological devices
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Pancreatic Islet Microphysiology Background and Objectives
Pancreatic islets, the endocrine units of the pancreas, have been a focal point in diabetes research for decades. These micro-organs, comprising multiple cell types including insulin-producing beta cells, play a critical role in glucose homeostasis. The evolution of research in this field has progressed from basic understanding of islet physiology to sophisticated approaches for maintaining and studying islets outside the body.
The technological trajectory in pancreatic islet research has been marked by significant milestones. Early studies in the 1960s and 1970s established fundamental knowledge about islet function, while the 1990s saw the emergence of islet transplantation as a potential therapeutic approach for type 1 diabetes. The 2000s witnessed the development of initial microfluidic systems for islet culture, and the 2010s brought advanced microphysiological systems (MPS) that better recapitulate the in vivo environment.
Current research trends are increasingly focused on creating physiologically relevant microenvironments that can maintain islet viability and functionality over extended periods. This represents a shift from traditional static culture methods, which often result in rapid deterioration of islet function and phenotype stability. The integration of biomaterials, microfluidics, and tissue engineering principles has opened new avenues for creating more sophisticated islet culture platforms.
The primary technical objective in this field is to develop microphysiological devices capable of maintaining pancreatic islet viability, functionality, and phenotypic stability for extended periods (weeks to months) that more accurately reflect in vivo conditions. This includes preserving the complex cellular architecture of islets, maintaining appropriate cell-cell interactions, and ensuring sustained hormone secretion patterns in response to glucose stimulation.
Secondary objectives include developing systems that allow for real-time monitoring of islet function, incorporating vascularization elements to enhance nutrient and oxygen delivery, and establishing standardized protocols for islet loading, maintenance, and analysis within these devices. Additionally, there is growing interest in creating patient-specific models using donor or stem cell-derived islets to enable personalized medicine approaches.
The ultimate goal of these technological advancements is multifaceted: to provide better platforms for basic research into islet biology, to create more predictive models for drug screening and toxicity testing, to develop improved methods for islet preservation prior to transplantation, and potentially to engineer bioartificial pancreas devices for therapeutic applications in diabetes management.
The technological trajectory in pancreatic islet research has been marked by significant milestones. Early studies in the 1960s and 1970s established fundamental knowledge about islet function, while the 1990s saw the emergence of islet transplantation as a potential therapeutic approach for type 1 diabetes. The 2000s witnessed the development of initial microfluidic systems for islet culture, and the 2010s brought advanced microphysiological systems (MPS) that better recapitulate the in vivo environment.
Current research trends are increasingly focused on creating physiologically relevant microenvironments that can maintain islet viability and functionality over extended periods. This represents a shift from traditional static culture methods, which often result in rapid deterioration of islet function and phenotype stability. The integration of biomaterials, microfluidics, and tissue engineering principles has opened new avenues for creating more sophisticated islet culture platforms.
The primary technical objective in this field is to develop microphysiological devices capable of maintaining pancreatic islet viability, functionality, and phenotypic stability for extended periods (weeks to months) that more accurately reflect in vivo conditions. This includes preserving the complex cellular architecture of islets, maintaining appropriate cell-cell interactions, and ensuring sustained hormone secretion patterns in response to glucose stimulation.
Secondary objectives include developing systems that allow for real-time monitoring of islet function, incorporating vascularization elements to enhance nutrient and oxygen delivery, and establishing standardized protocols for islet loading, maintenance, and analysis within these devices. Additionally, there is growing interest in creating patient-specific models using donor or stem cell-derived islets to enable personalized medicine approaches.
The ultimate goal of these technological advancements is multifaceted: to provide better platforms for basic research into islet biology, to create more predictive models for drug screening and toxicity testing, to develop improved methods for islet preservation prior to transplantation, and potentially to engineer bioartificial pancreas devices for therapeutic applications in diabetes management.
Market Analysis for Diabetes Research and Treatment Technologies
The global diabetes market represents a significant healthcare sector, with the International Diabetes Federation estimating that approximately 537 million adults were living with diabetes in 2021. This number is projected to rise to 643 million by 2030 and 783 million by 2045, creating an expanding market for diabetes research and treatment technologies.
The market for technologies related to pancreatic islet research and treatment is experiencing robust growth, driven by the increasing prevalence of diabetes and the limitations of current treatment options. The global market for diabetes devices was valued at $25.5 billion in 2020 and is expected to reach $39.8 billion by 2027, growing at a CAGR of 6.2%.
Microphysiological systems (MPS) for pancreatic islet research represent an emerging segment within this market. These advanced in vitro platforms that maintain long-term viability and functionality of pancreatic islets are gaining traction as they offer more physiologically relevant environments for studying islet biology and testing potential therapeutics.
The demand for improved diabetes management solutions is further fueled by the economic burden of the disease. The American Diabetes Association reported that the total cost of diagnosed diabetes in the United States reached $327 billion in 2017, including $237 billion in direct medical costs and $90 billion in reduced productivity.
Key market segments related to pancreatic islet research include islet isolation and purification technologies, culture systems, transplantation technologies, and artificial pancreas development. The islet transplantation market, though still relatively small, is growing as clinical trials demonstrate improved outcomes for patients with type 1 diabetes.
Regionally, North America dominates the market due to high diabetes prevalence, substantial research funding, and advanced healthcare infrastructure. Europe follows closely, with significant investments in regenerative medicine and tissue engineering. The Asia-Pacific region represents the fastest-growing market, driven by increasing diabetes prevalence, improving healthcare access, and growing research capabilities.
Venture capital investment in diabetes technology startups has been substantial, with over $7.5 billion invested between 2018 and 2021. Companies developing novel islet maintenance and delivery systems have attracted particular interest from investors seeking alternatives to traditional insulin therapy.
The market for technologies supporting long-term maintenance of pancreatic islets faces challenges including high development costs, regulatory hurdles, and competition from established diabetes management approaches. However, the potential to revolutionize diabetes treatment through improved islet transplantation or bioartificial pancreas development continues to drive market growth and innovation.
The market for technologies related to pancreatic islet research and treatment is experiencing robust growth, driven by the increasing prevalence of diabetes and the limitations of current treatment options. The global market for diabetes devices was valued at $25.5 billion in 2020 and is expected to reach $39.8 billion by 2027, growing at a CAGR of 6.2%.
Microphysiological systems (MPS) for pancreatic islet research represent an emerging segment within this market. These advanced in vitro platforms that maintain long-term viability and functionality of pancreatic islets are gaining traction as they offer more physiologically relevant environments for studying islet biology and testing potential therapeutics.
The demand for improved diabetes management solutions is further fueled by the economic burden of the disease. The American Diabetes Association reported that the total cost of diagnosed diabetes in the United States reached $327 billion in 2017, including $237 billion in direct medical costs and $90 billion in reduced productivity.
Key market segments related to pancreatic islet research include islet isolation and purification technologies, culture systems, transplantation technologies, and artificial pancreas development. The islet transplantation market, though still relatively small, is growing as clinical trials demonstrate improved outcomes for patients with type 1 diabetes.
Regionally, North America dominates the market due to high diabetes prevalence, substantial research funding, and advanced healthcare infrastructure. Europe follows closely, with significant investments in regenerative medicine and tissue engineering. The Asia-Pacific region represents the fastest-growing market, driven by increasing diabetes prevalence, improving healthcare access, and growing research capabilities.
Venture capital investment in diabetes technology startups has been substantial, with over $7.5 billion invested between 2018 and 2021. Companies developing novel islet maintenance and delivery systems have attracted particular interest from investors seeking alternatives to traditional insulin therapy.
The market for technologies supporting long-term maintenance of pancreatic islets faces challenges including high development costs, regulatory hurdles, and competition from established diabetes management approaches. However, the potential to revolutionize diabetes treatment through improved islet transplantation or bioartificial pancreas development continues to drive market growth and innovation.
Current Challenges in Pancreatic Islet Maintenance
The maintenance of pancreatic islets in microphysiological devices faces significant challenges that hinder long-term viability and functional stability. Traditional culture methods typically support islet survival for only 7-14 days before significant deterioration occurs, severely limiting research applications and therapeutic development. This short lifespan represents a critical bottleneck in diabetes research, drug screening, and personalized medicine approaches.
Oxygen and nutrient diffusion limitations constitute primary obstacles in islet maintenance. The three-dimensional structure of islets, typically 100-500 μm in diameter, creates diffusion gradients that lead to central necrosis in static culture systems. Current microphysiological devices struggle to provide adequate perfusion that mimics the highly vascularized native pancreatic environment, where islets receive approximately 10% of pancreatic blood flow despite comprising only 1-2% of pancreatic mass.
Extracellular matrix (ECM) interactions present another significant challenge. Native islets exist within a complex ECM microenvironment that provides both mechanical support and biochemical signaling. Current devices often utilize simplified matrix components that fail to recapitulate the full complexity of native ECM, leading to gradual dedifferentiation of islet cells and loss of function over time.
The heterogeneous cellular composition of islets further complicates maintenance efforts. Beyond the insulin-producing beta cells, islets contain alpha, delta, PP, and epsilon cells that collectively maintain glucose homeostasis through paracrine signaling networks. Current systems struggle to preserve this delicate cellular balance, with non-beta cells often declining more rapidly than beta cells, disrupting normal islet function.
Mechanical stress represents another significant challenge. Islets in microphysiological devices experience shear forces from fluid flow that can damage surface cells and trigger inflammatory responses. Additionally, many devices constrain islets in configurations that prevent normal expansion and contraction, further compromising viability.
Immune and inflammatory responses also undermine long-term islet maintenance. Even in the absence of specific immune cells, islets release damage-associated molecular patterns (DAMPs) when stressed, triggering inflammatory cascades that compromise function. Current systems lack effective means to monitor and modulate these inflammatory processes.
Phenotypic drift over time presents perhaps the most insidious challenge. Extended culture periods lead to gradual changes in gene expression profiles, hormone secretion patterns, and glucose responsiveness. This drift compromises the physiological relevance of studies conducted using these systems and limits their predictive value for in vivo outcomes.
Oxygen and nutrient diffusion limitations constitute primary obstacles in islet maintenance. The three-dimensional structure of islets, typically 100-500 μm in diameter, creates diffusion gradients that lead to central necrosis in static culture systems. Current microphysiological devices struggle to provide adequate perfusion that mimics the highly vascularized native pancreatic environment, where islets receive approximately 10% of pancreatic blood flow despite comprising only 1-2% of pancreatic mass.
Extracellular matrix (ECM) interactions present another significant challenge. Native islets exist within a complex ECM microenvironment that provides both mechanical support and biochemical signaling. Current devices often utilize simplified matrix components that fail to recapitulate the full complexity of native ECM, leading to gradual dedifferentiation of islet cells and loss of function over time.
The heterogeneous cellular composition of islets further complicates maintenance efforts. Beyond the insulin-producing beta cells, islets contain alpha, delta, PP, and epsilon cells that collectively maintain glucose homeostasis through paracrine signaling networks. Current systems struggle to preserve this delicate cellular balance, with non-beta cells often declining more rapidly than beta cells, disrupting normal islet function.
Mechanical stress represents another significant challenge. Islets in microphysiological devices experience shear forces from fluid flow that can damage surface cells and trigger inflammatory responses. Additionally, many devices constrain islets in configurations that prevent normal expansion and contraction, further compromising viability.
Immune and inflammatory responses also undermine long-term islet maintenance. Even in the absence of specific immune cells, islets release damage-associated molecular patterns (DAMPs) when stressed, triggering inflammatory cascades that compromise function. Current systems lack effective means to monitor and modulate these inflammatory processes.
Phenotypic drift over time presents perhaps the most insidious challenge. Extended culture periods lead to gradual changes in gene expression profiles, hormone secretion patterns, and glucose responsiveness. This drift compromises the physiological relevance of studies conducted using these systems and limits their predictive value for in vivo outcomes.
Established Approaches for Islet Longevity in Microdevices
01 Microfluidic devices for pancreatic islet culture
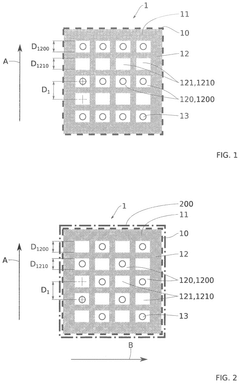
Microfluidic devices provide controlled environments for pancreatic islet culture, allowing for precise regulation of nutrient flow, oxygen levels, and waste removal. These devices mimic physiological conditions more accurately than traditional culture methods, supporting long-term maintenance of islet function and phenotypic stability. The dynamic flow conditions in these systems help maintain islet architecture and cellular interactions critical for proper insulin secretion and glucose responsiveness.- Microfluidic systems for pancreatic islet culture: Microfluidic devices provide controlled environments for maintaining pancreatic islets with stable phenotypes. These systems allow for precise control of nutrient delivery, oxygen levels, and waste removal, creating physiologically relevant conditions. The continuous perfusion in these devices helps maintain islet viability and functionality over extended periods, preserving their insulin-secreting capabilities and cellular architecture.
- Extracellular matrix components for islet stabilization: Incorporating specific extracellular matrix components in microphysiological devices helps maintain pancreatic islet structure and function. These components provide mechanical support and biochemical cues that mimic the native pancreatic environment. Materials such as collagen, laminin, and fibronectin promote cell adhesion, prevent islet disaggregation, and support long-term phenotypic stability by preserving cell-cell and cell-matrix interactions essential for proper islet function.
- Oxygen delivery systems for islet preservation: Specialized oxygen delivery systems in microphysiological devices address the high metabolic demands of pancreatic islets. These systems ensure adequate oxygenation throughout the islet structure, preventing central necrosis that commonly occurs in conventional culture systems. Enhanced oxygen delivery maintains islet viability, preserves cellular function, and supports stable glucose-responsive insulin secretion, which is critical for maintaining the physiological phenotype of pancreatic islets in long-term culture.
- Growth factors and supplements for phenotype maintenance: Specific growth factors and supplements in culture media help maintain pancreatic islet phenotype stability in microphysiological devices. These bioactive molecules support islet cell survival, prevent dedifferentiation, and preserve specialized cellular functions. Formulations containing insulin-like growth factor, glucagon-like peptide-1, nicotinamide, and other factors have been shown to maintain beta-cell identity and insulin-producing capacity over extended culture periods, ensuring functional stability of the islets.
- Co-culture systems for enhanced islet function: Co-culturing pancreatic islets with supporting cell types in microphysiological devices enhances their functional stability. Endothelial cells, mesenchymal stem cells, or other supporting cells provide paracrine factors that maintain islet health and function. These co-culture systems better recapitulate the in vivo pancreatic microenvironment, promoting vascularization, reducing stress responses, and preserving the specialized phenotype of islet cells, resulting in improved glucose responsiveness and hormone secretion profiles.
02 Extracellular matrix components for islet preservation
Incorporating specific extracellular matrix (ECM) components in culture systems enhances pancreatic islet survival and functional stability. These ECM components provide structural support and biochemical cues that maintain islet architecture and cell-cell interactions. Materials such as collagen, laminin, and fibronectin create a more physiologically relevant microenvironment, preventing islet disintegration and preserving their three-dimensional structure, which is crucial for maintaining proper hormone secretion patterns and cellular phenotype.Expand Specific Solutions03 Supplementation with growth factors and hormones
The addition of specific growth factors and hormones to culture media significantly improves pancreatic islet viability and functional stability in microphysiological devices. Factors such as glucagon-like peptide-1 (GLP-1), insulin-like growth factor (IGF), and hepatocyte growth factor (HGF) help maintain islet cell differentiation states and prevent dedifferentiation. These bioactive molecules support cellular metabolism, reduce stress responses, and promote survival signaling pathways that are essential for preserving islet phenotype during extended culture periods.Expand Specific Solutions04 Oxygen delivery systems for islet maintenance
Effective oxygen delivery systems are critical for maintaining pancreatic islet viability and function in microphysiological devices. Islets have high metabolic demands and require adequate oxygenation to prevent central necrosis and maintain cellular function. Advanced oxygen delivery strategies, including perfluorocarbon-based carriers, oxygen-permeable membranes, and controlled perfusion systems, help ensure sufficient oxygen reaches all cells within the islet structure, preserving their viability, glucose responsiveness, and hormone secretion capabilities.Expand Specific Solutions05 Co-culture systems for enhanced islet stability
Co-culturing pancreatic islets with supporting cell types enhances their long-term stability and functional maintenance in microphysiological devices. Endothelial cells, mesenchymal stem cells, and other supporting cells provide paracrine factors and cell-cell interactions that help preserve islet architecture and function. These co-culture systems better recapitulate the native pancreatic microenvironment, promoting vascularization, reducing inflammatory responses, and supporting the maintenance of differentiated β-cell phenotype and insulin secretion capacity.Expand Specific Solutions
Leading Organizations in Islet Preservation Research
The pancreatic islet microphysiological device market is in its early growth phase, characterized by increasing research activity but limited commercial deployment. The global market size is estimated to reach $300-400 million by 2025, driven by rising diabetes prevalence and demand for better treatment options. Technical challenges in long-term islet maintenance have slowed widespread adoption. Companies like Abbott Diabetes Care and F. Hoffmann-La Roche lead in commercial applications, while Bionime, Medtrum Technologies, and Beta-O2 Technologies are developing innovative solutions for islet preservation. Academic institutions including Tohoku University and University of Florida collaborate with industry partners to advance technology maturity, though significant hurdles remain in achieving stable long-term phenotype preservation in microphysiological environments.
University of Florida
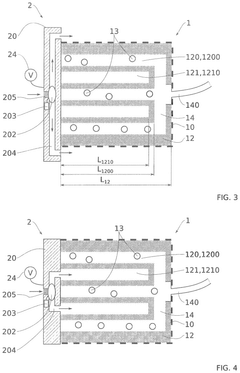
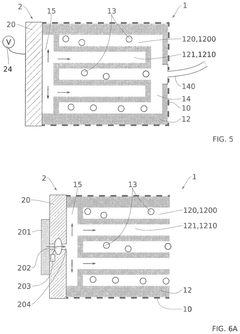
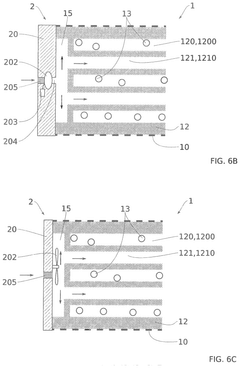
Technical Solution: The University of Florida has developed a silicone-based microfluidic device specifically designed for long-term pancreatic islet culture and monitoring. Their system features a dual-chamber design that separates the islet culture compartment from the perfusion channel via a semipermeable membrane, allowing controlled nutrient and oxygen exchange while protecting islets from shear stress[7]. The device incorporates oxygen-permeable polydimethylsiloxane (PDMS) materials that enhance oxygen delivery to the cultured islets. A distinguishing feature is their implementation of integrated electrochemical sensors that enable continuous, non-invasive monitoring of insulin secretion and metabolic activity. The University of Florida team has demonstrated sustained islet viability and function for over 60 days in their device, with preserved glucose-stimulated insulin secretion and maintained expression of key beta-cell markers including PDX1 and NKX6.1[8]. Their technology also includes a modular design that allows for scalability and potential adaptation for high-throughput drug screening applications. Recent advancements include the integration of controlled temperature gradients to optimize islet microenvironment conditions.
Strengths: The dual-chamber design with semipermeable membrane provides excellent protection from shear stress while ensuring adequate nutrient delivery. The integrated electrochemical sensors enable valuable real-time functional assessment without disrupting the culture. Weaknesses: The PDMS-based fabrication may present challenges with protein adsorption and potential absorption of hydrophobic compounds, potentially affecting long-term culture conditions. The system requires specialized equipment for continuous perfusion and monitoring.
Regents of The University of Minnesota
Technical Solution: The University of Minnesota has pioneered advanced microfluidic devices for pancreatic islet maintenance that incorporate continuous medium perfusion systems. Their technology utilizes a multi-layered microfluidic platform with integrated sensing capabilities to monitor islet health parameters in real-time, including oxygen consumption, insulin secretion, and metabolic activity[3]. The system features specialized microwell structures coated with extracellular matrix components that mimic the native pancreatic microenvironment, promoting islet attachment while maintaining their three-dimensional architecture. A key innovation is their implementation of controlled gradient generation of soluble factors that support islet phenotype stability. The university's research team has demonstrated sustained islet functionality for over 30 days in these devices, with preserved glucose-stimulated insulin secretion profiles comparable to freshly isolated islets[4]. Their technology also incorporates on-chip cryopreservation capabilities, allowing for long-term storage and subsequent revival of functional islets within the same device.
Strengths: The integrated sensing capabilities provide valuable real-time data on islet health and function, enabling dynamic adjustment of culture conditions. The biomimetic microenvironment supports long-term maintenance of islet architecture and function. Weaknesses: The complex microfluidic system requires specialized expertise for operation and maintenance. The technology may face challenges in scaling up for clinical applications due to its intricate design and fabrication requirements.
Critical Technologies for Phenotype Stability Maintenance
Pancreatic cell receiving matrix and improved artificial pancreas device
PatentPendingUS20240325603A1
Innovation
- A matrix for receiving pancreatic cells is designed with a semi-permeable wall and a porous body comprising alternating cavities with and without cells, allowing controlled diffusion of nutrients and gases, reducing islet mortality and enhancing insulin delivery.
Transplantation device using chemically crosslinked alginic acid
PatentWO2022137345A1
Innovation
- A chemically crosslinked alginic acid-based implantation device is developed, where insulin-secreting cells or pancreatic islets are encapsulated in a hydrogel that maintains stability and biocompatibility, preventing immune cell interaction while allowing nutrient and insulin permeability, and is coated with a semipermeable membrane for enhanced performance.
Regulatory Framework for Microphysiological Devices
The regulatory landscape for microphysiological devices (MPDs) used for pancreatic islet maintenance presents a complex framework that continues to evolve as these technologies advance. Currently, these devices exist in a regulatory gray area between research tools and potential therapeutic applications, creating challenges for developers and clinical researchers alike.
In the United States, the Food and Drug Administration (FDA) has established a multi-tiered approach for MPDs based on their intended use. Devices designed purely for research purposes face less stringent oversight, while those intended for diagnostic applications or therapeutic decision-making fall under more comprehensive regulatory scrutiny. The FDA's Center for Devices and Radiological Health (CDRH) has recently developed specific guidance documents addressing the unique challenges of organ-on-chip technologies, including those designed for pancreatic islet maintenance.
European regulatory frameworks, governed by the European Medicines Agency (EMA), have implemented the Medical Device Regulation (MDR) which includes specific provisions for advanced tissue models. These regulations emphasize validation protocols and quality control measures that demonstrate reproducibility and reliability of islet function within microphysiological environments over extended periods.
International harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) have begun addressing standardization of MPD validation protocols. These initiatives aim to establish consensus on acceptable performance metrics for long-term islet viability and functionality, including glucose responsiveness and insulin secretion stability over time.
Quality system requirements represent another critical regulatory component. Manufacturers must implement robust quality management systems that ensure consistency in device production and performance. For pancreatic islet MPDs, this includes validation of materials biocompatibility, sterility maintenance protocols, and demonstration of consistent cellular microenvironment parameters over extended culture periods.
Data integrity and documentation standards have emerged as particularly important for longitudinal studies involving pancreatic islets. Regulatory bodies increasingly require comprehensive documentation of environmental parameters, media composition changes, and cellular phenotype markers throughout the maintenance period to support claims of long-term stability.
Emerging regulatory considerations include the development of reference standards for phenotypic stability assessment and the establishment of acceptable thresholds for functional drift in maintained islets. Several international working groups are currently developing consensus documents to address these gaps, with particular focus on defining minimum performance requirements for devices intended to maintain islet function beyond traditional culture timeframes.
In the United States, the Food and Drug Administration (FDA) has established a multi-tiered approach for MPDs based on their intended use. Devices designed purely for research purposes face less stringent oversight, while those intended for diagnostic applications or therapeutic decision-making fall under more comprehensive regulatory scrutiny. The FDA's Center for Devices and Radiological Health (CDRH) has recently developed specific guidance documents addressing the unique challenges of organ-on-chip technologies, including those designed for pancreatic islet maintenance.
European regulatory frameworks, governed by the European Medicines Agency (EMA), have implemented the Medical Device Regulation (MDR) which includes specific provisions for advanced tissue models. These regulations emphasize validation protocols and quality control measures that demonstrate reproducibility and reliability of islet function within microphysiological environments over extended periods.
International harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) have begun addressing standardization of MPD validation protocols. These initiatives aim to establish consensus on acceptable performance metrics for long-term islet viability and functionality, including glucose responsiveness and insulin secretion stability over time.
Quality system requirements represent another critical regulatory component. Manufacturers must implement robust quality management systems that ensure consistency in device production and performance. For pancreatic islet MPDs, this includes validation of materials biocompatibility, sterility maintenance protocols, and demonstration of consistent cellular microenvironment parameters over extended culture periods.
Data integrity and documentation standards have emerged as particularly important for longitudinal studies involving pancreatic islets. Regulatory bodies increasingly require comprehensive documentation of environmental parameters, media composition changes, and cellular phenotype markers throughout the maintenance period to support claims of long-term stability.
Emerging regulatory considerations include the development of reference standards for phenotypic stability assessment and the establishment of acceptable thresholds for functional drift in maintained islets. Several international working groups are currently developing consensus documents to address these gaps, with particular focus on defining minimum performance requirements for devices intended to maintain islet function beyond traditional culture timeframes.
Translational Potential to Clinical Applications
The translational pathway from microphysiological devices supporting pancreatic islets to clinical applications represents a critical frontier in diabetes treatment. Current clinical islet transplantation faces significant challenges including limited donor availability, immunosuppression requirements, and poor long-term islet survival. Microphysiological systems demonstrating successful long-term maintenance of islet phenotype stability offer promising alternatives that could revolutionize diabetes management.
These advanced systems have potential applications in personalized medicine, where patient-derived cells could be maintained in microphysiological environments to test drug efficacy and toxicity profiles. This approach would enable clinicians to determine optimal therapeutic regimens for individual patients, significantly improving treatment outcomes while reducing adverse effects. The ability to maintain functional islets for extended periods provides a platform for personalized drug screening that current methods cannot match.
Beyond personalized medicine, these systems could transform cell-based therapies for diabetes. The technology could serve as a bridge to transplantation by maintaining islet viability and functionality until suitable recipients are identified. Furthermore, microphysiological devices could function as implantable bio-artificial pancreas systems, potentially eliminating the need for exogenous insulin administration in diabetic patients.
Regulatory pathways for clinical translation present significant challenges. These devices would likely be classified as combination products by regulatory agencies, requiring extensive preclinical validation and clinical trials demonstrating safety and efficacy. Standardization of manufacturing processes, quality control measures, and sterilization protocols must be established to ensure consistent performance across production batches.
Cost-effectiveness represents another critical consideration for clinical translation. While initial development and implementation costs may be substantial, the potential reduction in long-term healthcare expenditures for diabetes management could justify these investments. Economic analyses suggest that successful implementation could significantly reduce costs associated with diabetes complications, hospitalization rates, and lifetime insulin therapy.
Scalability and accessibility must be addressed to ensure widespread clinical adoption. Current laboratory-scale devices must be adapted for mass production while maintaining quality and functionality. Partnerships between academic institutions, biotechnology companies, and healthcare providers will be essential to navigate the complex landscape of clinical implementation and ensure these promising technologies reach patients who need them most.
These advanced systems have potential applications in personalized medicine, where patient-derived cells could be maintained in microphysiological environments to test drug efficacy and toxicity profiles. This approach would enable clinicians to determine optimal therapeutic regimens for individual patients, significantly improving treatment outcomes while reducing adverse effects. The ability to maintain functional islets for extended periods provides a platform for personalized drug screening that current methods cannot match.
Beyond personalized medicine, these systems could transform cell-based therapies for diabetes. The technology could serve as a bridge to transplantation by maintaining islet viability and functionality until suitable recipients are identified. Furthermore, microphysiological devices could function as implantable bio-artificial pancreas systems, potentially eliminating the need for exogenous insulin administration in diabetic patients.
Regulatory pathways for clinical translation present significant challenges. These devices would likely be classified as combination products by regulatory agencies, requiring extensive preclinical validation and clinical trials demonstrating safety and efficacy. Standardization of manufacturing processes, quality control measures, and sterilization protocols must be established to ensure consistent performance across production batches.
Cost-effectiveness represents another critical consideration for clinical translation. While initial development and implementation costs may be substantial, the potential reduction in long-term healthcare expenditures for diabetes management could justify these investments. Economic analyses suggest that successful implementation could significantly reduce costs associated with diabetes complications, hospitalization rates, and lifetime insulin therapy.
Scalability and accessibility must be addressed to ensure widespread clinical adoption. Current laboratory-scale devices must be adapted for mass production while maintaining quality and functionality. Partnerships between academic institutions, biotechnology companies, and healthcare providers will be essential to navigate the complex landscape of clinical implementation and ensure these promising technologies reach patients who need them most.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!