Minimizing bubble formation in long-term microfluidic organ cultures: materials and engineering solutions
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic Organ Culture Background and Objectives
Microfluidic organ culture systems represent a revolutionary approach in biomedical research, offering unprecedented capabilities for maintaining and studying organ tissues in controlled environments that mimic physiological conditions. The evolution of this technology began in the early 2000s with simple microfluidic channels and has progressively advanced toward more sophisticated organ-on-chip platforms that incorporate multiple tissue types and physiological functions.
The trajectory of microfluidic organ culture development has been characterized by increasing complexity and functionality, moving from static culture systems to dynamic perfusion-based models that better replicate in vivo conditions. Recent technological advancements have enabled the integration of sensors, actuators, and automated control systems, allowing for real-time monitoring and adjustment of culture parameters.
Despite these advances, bubble formation remains a persistent challenge that significantly impacts the viability and functionality of long-term organ cultures. Bubbles can disrupt fluid flow, damage delicate cellular structures, and create localized regions of hypoxia or hyperoxia, ultimately compromising experimental outcomes and limiting the duration of viable culture periods.
The primary objective of this technical investigation is to comprehensively analyze the mechanisms of bubble formation in microfluidic organ culture systems and identify effective materials and engineering solutions to minimize this phenomenon. Specifically, we aim to evaluate the performance of various materials in terms of gas permeability, surface properties, and biocompatibility, while also exploring innovative engineering approaches such as bubble traps, degassing techniques, and flow control strategies.
Furthermore, this research seeks to establish standardized protocols and design principles that can be broadly applied across different microfluidic organ culture platforms, thereby enhancing reproducibility and reliability in long-term experiments. By addressing the bubble formation challenge, we anticipate extending viable culture durations from the current standard of several days to weeks or potentially months.
The successful development of bubble-minimizing strategies would significantly advance the field of organ-on-chip technology, enabling more accurate modeling of chronic diseases, long-term drug testing, and complex tissue engineering applications. This would bridge critical gaps between traditional cell culture methods and animal models, potentially reducing reliance on animal testing while providing more physiologically relevant data for translational research.
Additionally, this technical exploration aims to identify emerging trends in materials science and microfluidic engineering that could be leveraged for next-generation organ culture systems, positioning our organization at the forefront of this rapidly evolving field.
The trajectory of microfluidic organ culture development has been characterized by increasing complexity and functionality, moving from static culture systems to dynamic perfusion-based models that better replicate in vivo conditions. Recent technological advancements have enabled the integration of sensors, actuators, and automated control systems, allowing for real-time monitoring and adjustment of culture parameters.
Despite these advances, bubble formation remains a persistent challenge that significantly impacts the viability and functionality of long-term organ cultures. Bubbles can disrupt fluid flow, damage delicate cellular structures, and create localized regions of hypoxia or hyperoxia, ultimately compromising experimental outcomes and limiting the duration of viable culture periods.
The primary objective of this technical investigation is to comprehensively analyze the mechanisms of bubble formation in microfluidic organ culture systems and identify effective materials and engineering solutions to minimize this phenomenon. Specifically, we aim to evaluate the performance of various materials in terms of gas permeability, surface properties, and biocompatibility, while also exploring innovative engineering approaches such as bubble traps, degassing techniques, and flow control strategies.
Furthermore, this research seeks to establish standardized protocols and design principles that can be broadly applied across different microfluidic organ culture platforms, thereby enhancing reproducibility and reliability in long-term experiments. By addressing the bubble formation challenge, we anticipate extending viable culture durations from the current standard of several days to weeks or potentially months.
The successful development of bubble-minimizing strategies would significantly advance the field of organ-on-chip technology, enabling more accurate modeling of chronic diseases, long-term drug testing, and complex tissue engineering applications. This would bridge critical gaps between traditional cell culture methods and animal models, potentially reducing reliance on animal testing while providing more physiologically relevant data for translational research.
Additionally, this technical exploration aims to identify emerging trends in materials science and microfluidic engineering that could be leveraged for next-generation organ culture systems, positioning our organization at the forefront of this rapidly evolving field.
Market Analysis for Long-term Organ-on-Chip Systems
The global market for organ-on-chip (OOC) systems is experiencing rapid growth, driven by increasing demand for alternatives to animal testing and more physiologically relevant drug screening platforms. Currently valued at approximately $45 million in 2023, the market is projected to reach $297 million by 2028, representing a compound annual growth rate (CAGR) of 37.6%. This exceptional growth trajectory underscores the significant commercial potential for long-term microfluidic organ culture technologies.
The pharmaceutical industry remains the primary market segment, accounting for roughly 65% of the total market share. This dominance stems from the industry's urgent need to reduce drug development costs and failure rates in clinical trials. Long-term organ culture systems that can maintain functionality for weeks or months offer unprecedented value in drug toxicity screening and efficacy testing, potentially saving billions in development costs.
Academic research institutions constitute the second-largest market segment at approximately 20%, followed by cosmetics and chemical testing at 10%. The remaining 5% is distributed among various end-users including contract research organizations and government laboratories. Geographically, North America leads with 45% market share, followed by Europe (30%), Asia-Pacific (20%), and rest of the world (5%).
A critical market driver is the increasing regulatory pressure to reduce animal testing. The European Union's ban on animal testing for cosmetics and the FDA Modernization Act 2.0 in the United States have created strong regulatory tailwinds for alternative testing methods, including organ-on-chip technologies.
The bubble formation issue in long-term microfluidic cultures represents a significant market barrier, with industry surveys indicating that approximately 40% of users cite reliability and maintenance challenges as major obstacles to adoption. Solutions addressing this specific problem could unlock substantial market value, estimated at $30-50 million annually.
Customer segmentation reveals distinct needs: pharmaceutical companies prioritize reproducibility and validation against gold standards; academic researchers value flexibility and customization; while cosmetic companies emphasize throughput and cost-effectiveness. All segments, however, share the common requirement for systems that can maintain bubble-free operation during extended culture periods.
Market forecasts suggest that technologies effectively addressing bubble formation in long-term cultures could command premium pricing, with potential price points 15-25% higher than standard systems. Early movers in this solution space are positioned to capture significant market share and establish industry standards, particularly as the field moves toward more complex multi-organ systems and personalized medicine applications.
The pharmaceutical industry remains the primary market segment, accounting for roughly 65% of the total market share. This dominance stems from the industry's urgent need to reduce drug development costs and failure rates in clinical trials. Long-term organ culture systems that can maintain functionality for weeks or months offer unprecedented value in drug toxicity screening and efficacy testing, potentially saving billions in development costs.
Academic research institutions constitute the second-largest market segment at approximately 20%, followed by cosmetics and chemical testing at 10%. The remaining 5% is distributed among various end-users including contract research organizations and government laboratories. Geographically, North America leads with 45% market share, followed by Europe (30%), Asia-Pacific (20%), and rest of the world (5%).
A critical market driver is the increasing regulatory pressure to reduce animal testing. The European Union's ban on animal testing for cosmetics and the FDA Modernization Act 2.0 in the United States have created strong regulatory tailwinds for alternative testing methods, including organ-on-chip technologies.
The bubble formation issue in long-term microfluidic cultures represents a significant market barrier, with industry surveys indicating that approximately 40% of users cite reliability and maintenance challenges as major obstacles to adoption. Solutions addressing this specific problem could unlock substantial market value, estimated at $30-50 million annually.
Customer segmentation reveals distinct needs: pharmaceutical companies prioritize reproducibility and validation against gold standards; academic researchers value flexibility and customization; while cosmetic companies emphasize throughput and cost-effectiveness. All segments, however, share the common requirement for systems that can maintain bubble-free operation during extended culture periods.
Market forecasts suggest that technologies effectively addressing bubble formation in long-term cultures could command premium pricing, with potential price points 15-25% higher than standard systems. Early movers in this solution space are positioned to capture significant market share and establish industry standards, particularly as the field moves toward more complex multi-organ systems and personalized medicine applications.
Bubble Formation Challenges in Microfluidic Platforms
Bubble formation represents one of the most significant challenges in microfluidic organ culture systems, particularly for long-term applications. These air bubbles disrupt fluid flow patterns, create unpredictable shear stresses on cultured cells, and can physically damage delicate tissue structures. The problem becomes especially pronounced in extended culture periods where even minor bubble nucleation sites can develop into system-compromising obstructions over time.
The primary sources of bubble formation include gas permeability of device materials, temperature fluctuations during operation, and mechanical perturbations in the system. PDMS (polydimethylsiloxane), the most commonly used material in microfluidic fabrication, exhibits high gas permeability that facilitates oxygen exchange for cell cultures but simultaneously creates vulnerability to bubble formation. This fundamental material property presents a significant engineering dilemma.
Bubble nucleation typically occurs at surface imperfections, material interfaces, or regions with hydrophobic properties. Once formed, bubbles can grow through gas diffusion from surrounding media or coalesce with other bubbles, creating larger flow obstructions. In organ-on-chip applications, these bubbles can completely block perfusion channels, creating anoxic regions in tissue constructs and invalidating experimental results.
The dynamic nature of microfluidic systems exacerbates bubble challenges. Pressure fluctuations from pumping systems, temperature gradients across the device, and mechanical manipulations during media changes all contribute to bubble formation events. Even minor design flaws such as sharp corners or sudden channel expansions can serve as bubble nucleation sites or trapping points.
Long-term organ cultures present unique bubble-related challenges compared to short-term microfluidic applications. Extended culture periods allow sufficient time for even slow processes like gas diffusion through materials to manifest as problematic bubbles. Additionally, the biological components themselves can contribute to bubble formation through metabolic gas production or protein deposition that creates nucleation sites.
Current microfluidic organ culture systems employ various strategies to mitigate bubble formation, including bubble traps, degassing techniques, and surface treatments. However, these approaches often introduce additional complexity, require frequent intervention, or provide only temporary solutions. The need for truly robust, long-term bubble prevention strategies remains a critical barrier to widespread adoption of microfluidic organ culture technologies.
The economic impact of bubble-related failures in microfluidic organ cultures extends beyond lost experimental data. High-value cell populations, expensive growth factors, and significant researcher time can all be wasted when bubbles compromise system function. This creates substantial pressure to develop more reliable bubble prevention technologies for next-generation organ culture platforms.
The primary sources of bubble formation include gas permeability of device materials, temperature fluctuations during operation, and mechanical perturbations in the system. PDMS (polydimethylsiloxane), the most commonly used material in microfluidic fabrication, exhibits high gas permeability that facilitates oxygen exchange for cell cultures but simultaneously creates vulnerability to bubble formation. This fundamental material property presents a significant engineering dilemma.
Bubble nucleation typically occurs at surface imperfections, material interfaces, or regions with hydrophobic properties. Once formed, bubbles can grow through gas diffusion from surrounding media or coalesce with other bubbles, creating larger flow obstructions. In organ-on-chip applications, these bubbles can completely block perfusion channels, creating anoxic regions in tissue constructs and invalidating experimental results.
The dynamic nature of microfluidic systems exacerbates bubble challenges. Pressure fluctuations from pumping systems, temperature gradients across the device, and mechanical manipulations during media changes all contribute to bubble formation events. Even minor design flaws such as sharp corners or sudden channel expansions can serve as bubble nucleation sites or trapping points.
Long-term organ cultures present unique bubble-related challenges compared to short-term microfluidic applications. Extended culture periods allow sufficient time for even slow processes like gas diffusion through materials to manifest as problematic bubbles. Additionally, the biological components themselves can contribute to bubble formation through metabolic gas production or protein deposition that creates nucleation sites.
Current microfluidic organ culture systems employ various strategies to mitigate bubble formation, including bubble traps, degassing techniques, and surface treatments. However, these approaches often introduce additional complexity, require frequent intervention, or provide only temporary solutions. The need for truly robust, long-term bubble prevention strategies remains a critical barrier to widespread adoption of microfluidic organ culture technologies.
The economic impact of bubble-related failures in microfluidic organ cultures extends beyond lost experimental data. High-value cell populations, expensive growth factors, and significant researcher time can all be wasted when bubbles compromise system function. This creates substantial pressure to develop more reliable bubble prevention technologies for next-generation organ culture platforms.
Current Materials and Engineering Solutions
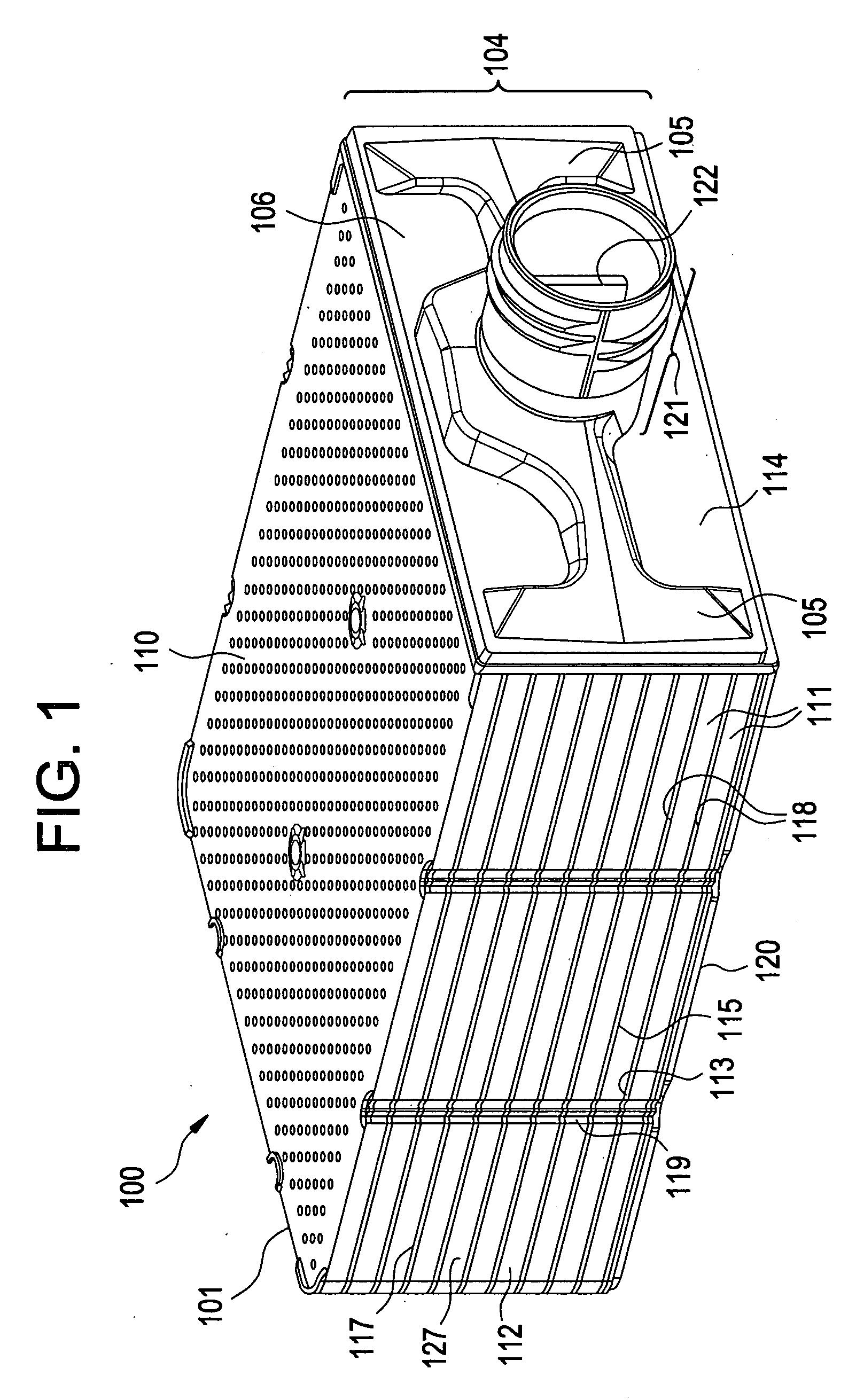
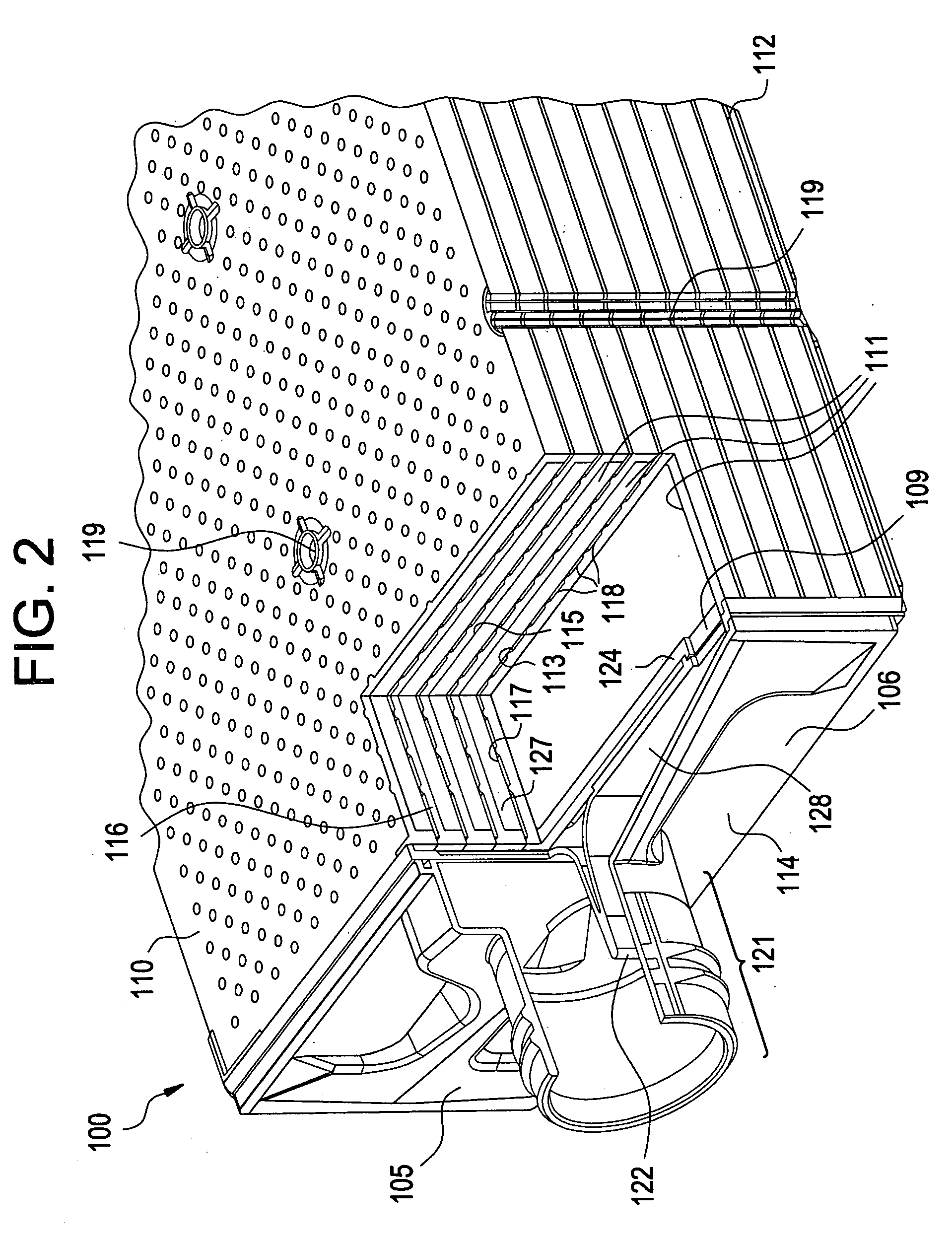
01 Microfluidic devices for organ culture with bubble prevention
Microfluidic devices designed specifically for organ culture applications incorporate features to prevent bubble formation, which can disrupt cell growth and damage delicate tissue structures. These devices often include specialized chambers, channels, and flow control mechanisms that minimize turbulence and prevent air entrapment. Some designs incorporate bubble traps or degassing components to remove bubbles before they reach the culture area, ensuring stable and consistent culture conditions for organ models.- Bubble prevention techniques in microfluidic organ cultures: Various techniques can be employed to prevent bubble formation in microfluidic organ culture systems. These include specialized channel designs, surface treatments to modify hydrophobicity/hydrophilicity, and pressure control mechanisms. Preventing bubbles is crucial as they can disrupt fluid flow, damage cultured tissues, and compromise experimental results in organ-on-chip platforms.
- Bubble trapping and removal systems: Microfluidic devices for organ cultures can incorporate dedicated bubble trapping chambers and removal systems. These structures are designed to capture bubbles before they reach sensitive culture areas, using principles such as buoyancy, surface tension differences, or specialized geometries. Some systems include active removal mechanisms like vacuum extraction ports or membrane-based gas exchange regions.
- Perfusion systems with bubble management for organ cultures: Advanced perfusion systems for microfluidic organ cultures incorporate specific features to manage bubble formation during continuous medium flow. These systems may include degassing components, bubble sensors, automated flow control, and specialized pumping mechanisms. Proper perfusion with bubble management is essential for maintaining physiologically relevant conditions in long-term organ cultures.
- Bubble-mediated cell and tissue manipulation: Controlled bubble formation can be harnessed for specific applications in microfluidic organ cultures. These include cell patterning, tissue stimulation, targeted drug delivery, and creating mechanical forces that mimic physiological conditions. By precisely controlling bubble generation and collapse, researchers can manipulate cellular environments in ways that enhance organ culture functionality.
- Monitoring and detection systems for bubbles in organ cultures: Specialized systems for detecting and monitoring bubbles in microfluidic organ cultures help maintain optimal conditions. These include optical detection methods, impedance-based sensors, acoustic monitoring, and integrated imaging systems. Real-time bubble detection allows for automated interventions to preserve culture integrity and experimental validity in organ-on-chip platforms.
02 Bubble removal techniques in organ-on-chip systems
Various techniques have been developed to remove unwanted bubbles in microfluidic organ culture systems. These include passive methods such as strategically placed expansion chambers that trap bubbles away from culture areas, and active methods like acoustic wave generators that dislodge bubbles from surfaces. Some systems employ hydrophilic surface treatments to reduce bubble adherence or incorporate microporous membranes that allow gas exchange while preventing bubble formation. These techniques are crucial for maintaining the integrity and functionality of organ cultures in microfluidic devices.Expand Specific Solutions03 Flow control strategies to minimize bubble formation
Controlling fluid flow dynamics is essential for preventing bubble formation in microfluidic organ cultures. Strategies include gradual flow rate transitions to avoid pressure fluctuations, specialized channel geometries that minimize dead zones where bubbles can form, and precise control of inlet/outlet configurations. Some systems incorporate pressure regulators or pulsation dampeners to maintain stable flow conditions. Advanced flow control algorithms can dynamically adjust flow parameters based on feedback from integrated sensors, further reducing the risk of bubble formation during extended culture periods.Expand Specific Solutions04 Materials and surface treatments for bubble prevention
The choice of materials and surface treatments plays a critical role in preventing bubble formation in microfluidic organ cultures. Hydrophilic surface modifications can reduce bubble nucleation and adherence to channel walls. Certain polymers and coatings can be applied to microfluidic channels to alter their surface properties and minimize gas-liquid interfaces where bubbles tend to form. Some approaches involve pre-treatment of culture media to reduce dissolved gas content or the addition of surfactants that lower surface tension, making bubble formation less energetically favorable while maintaining biocompatibility with cultured tissues.Expand Specific Solutions05 Integrated sensing and feedback systems for bubble detection
Advanced microfluidic organ culture systems incorporate integrated sensing technologies to detect bubble formation in real-time. These systems use optical, electrical, or acoustic sensors to monitor the presence of bubbles within the microfluidic channels. When bubbles are detected, automated feedback mechanisms can adjust flow parameters, activate bubble removal systems, or alert researchers. Some platforms include image processing algorithms that can distinguish between bubbles and cellular structures, enabling precise intervention only when necessary. These integrated approaches help maintain optimal culture conditions by addressing bubble formation issues before they impact organ culture viability.Expand Specific Solutions
Leading Companies and Research Institutions
The microfluidic organ culture technology market is currently in its growth phase, characterized by increasing research activities and commercial applications. The market size is expanding due to rising demand for advanced in vitro models that better mimic human physiology. Technologically, solutions for bubble formation in long-term cultures represent a critical challenge that is being addressed through various approaches. Leading players include Corning, Inc., which leverages its materials expertise to develop specialized microfluidic substrates, and Siemens AG, which contributes engineering solutions. Academic institutions like Harvard, MIT, and UC system are driving innovation through fundamental research, while companies like ibidi GmbH and QuantuMDx are developing specialized microfluidic platforms. The collaboration between industry leaders and research institutions indicates the technology is advancing toward maturity, though challenges in long-term culture stability remain.
Corning, Inc.
Technical Solution: Corning has developed an advanced microfluidic solution called "BubbleShield™" that specifically addresses bubble formation in long-term organ cultures. Their approach leverages Corning's extensive expertise in materials science and surface chemistry to create more stable culture environments. The company has engineered a specialized glass-polymer composite material for microfluidic channels that maintains exceptional hydrophilicity over extended periods, significantly reducing bubble nucleation sites. Their system incorporates proprietary surface treatments that create nanoscale surface patterns, disrupting the formation of bubble nucleation sites while promoting consistent fluid flow. Corning's microfluidic devices feature strategically designed expansion chambers that accommodate pressure fluctuations without allowing bubble formation or growth. A key innovation is their development of integrated degassing membranes with precisely controlled gas permeability, allowing continuous removal of dissolved gases before they can form disruptive bubbles. Their platform also includes specialized medium additives that modify surface tension properties, further reducing the likelihood of bubble formation while maintaining optimal conditions for cell growth and differentiation.
Strengths: Leverages Corning's extensive materials science expertise; comprehensive solution addresses multiple bubble formation mechanisms; commercially available system with established quality control. Weaknesses: May require specific Corning consumables for optimal performance; higher cost compared to generic microfluidic systems; some components may require regular replacement for extended culture periods.
President & Fellows of Harvard College
Technical Solution: Harvard's approach to minimizing bubble formation in microfluidic organ cultures focuses on advanced surface modification techniques and innovative device designs. Their researchers have developed a proprietary surface treatment that creates ultra-hydrophilic channel walls, significantly reducing the likelihood of bubble nucleation at the material interfaces. The technology incorporates specialized microstructures within channels that act as bubble traps, effectively capturing and removing bubbles before they can disrupt the culture environment. Harvard has also pioneered a pressure equilibration system that maintains consistent fluid dynamics throughout extended culture periods, preventing pressure differentials that commonly lead to bubble formation. Their organ-on-chip platforms feature integrated degassing membranes made of gas-permeable PDMS (polydimethylsiloxane) that allow continuous removal of dissolved gases before they can form disruptive bubbles. This comprehensive approach has demonstrated success in maintaining bubble-free cultures for periods exceeding 30 days in various organ models.
Strengths: Superior surface modification technology provides exceptional hydrophilicity; integrated bubble trap designs effectively capture bubbles before they affect cultures; comprehensive system approach addresses multiple bubble formation mechanisms simultaneously. Weaknesses: Complex fabrication processes may increase production costs; some solutions are organ-specific and may not translate across all tissue types; requires specialized expertise for implementation.
Key Patents and Technical Innovations
Microfluidic cartridge channel with reduced bubble formation
PatentActiveGB2462364B
Innovation
- A microfluidic cartridge with a polymeric coating on its inner surfaces, specifically using an amphiphilic polymer like Pluronics F127, which reduces surface tension and anchors to the substrate, preventing bubble formation and enhancing fluid flow patterns.
Device and method for reducing bubble formation in cell culture
PatentActiveUS20080206857A1
Innovation
- A device with a manifold that separates the inflow of liquid and outflow of gas through a cell culture vessel, using a necked opening and barrier plate with apertures to direct media flow and vent air, reducing the mixing of media with air and minimizing foam formation.
Biocompatibility and Cell Viability Considerations
Biocompatibility represents a critical consideration in the development of microfluidic organ culture systems designed for long-term operation. The materials selected for these platforms must not only minimize bubble formation but also maintain optimal cell viability throughout extended experimental periods. Traditional microfluidic materials such as polydimethylsiloxane (PDMS) exhibit excellent optical clarity and gas permeability but may absorb small hydrophobic molecules from culture media, potentially affecting cellular responses and experimental outcomes.
Advanced biocompatible materials including medical-grade silicones, polyethylene glycol (PEG)-modified surfaces, and thermoplastics like cyclic olefin copolymer (COC) demonstrate superior performance in maintaining cell health during prolonged culture periods. These materials significantly reduce protein adsorption and cellular adhesion to non-designated areas, which can compromise experimental results and cellular function. Recent studies indicate that surface modifications with hydrophilic coatings can simultaneously enhance biocompatibility and reduce bubble nucleation sites.
The interface between engineering solutions and biological requirements presents unique challenges in microfluidic organ cultures. Shear stress generated by fluid flow must be carefully controlled to prevent cellular damage while maintaining adequate nutrient delivery and waste removal. Research indicates that physiological shear stress ranges between 0.1-10 dyn/cm², with values exceeding this threshold potentially triggering mechanotransduction pathways that alter cellular phenotype and function.
Oxygen and nutrient gradients represent another critical consideration affecting cell viability in microfluidic systems. The materials and channel geometries must facilitate appropriate gas exchange while preventing bubble formation. Studies demonstrate that oxygen permeability coefficients of materials directly correlate with cellular metabolic activity and viability in long-term cultures. PDMS exhibits an oxygen permeability coefficient of approximately 3.4×10^-9 m²/s, whereas glass and hard plastics demonstrate significantly lower values, necessitating alternative oxygenation strategies.
Temperature stability and pH maintenance also significantly impact cell viability in microfluidic organ cultures. Materials with high thermal conductivity can help maintain consistent temperatures throughout the system, while buffer capacity within media formulations must be optimized to counteract pH shifts resulting from cellular metabolism. Engineering solutions incorporating integrated sensing elements for real-time monitoring of these parameters can substantially improve experimental outcomes and cellular health.
The integration of extracellular matrix components within microfluidic devices represents an emerging approach to enhance biocompatibility. Hydrogel-based matrices composed of collagen, fibronectin, or Matrigel provide physiologically relevant microenvironments that support cellular function while potentially reducing bubble formation through altered surface tension properties at material interfaces. These biomimetic approaches demonstrate promising results in maintaining cellular phenotype and function during extended culture periods.
Advanced biocompatible materials including medical-grade silicones, polyethylene glycol (PEG)-modified surfaces, and thermoplastics like cyclic olefin copolymer (COC) demonstrate superior performance in maintaining cell health during prolonged culture periods. These materials significantly reduce protein adsorption and cellular adhesion to non-designated areas, which can compromise experimental results and cellular function. Recent studies indicate that surface modifications with hydrophilic coatings can simultaneously enhance biocompatibility and reduce bubble nucleation sites.
The interface between engineering solutions and biological requirements presents unique challenges in microfluidic organ cultures. Shear stress generated by fluid flow must be carefully controlled to prevent cellular damage while maintaining adequate nutrient delivery and waste removal. Research indicates that physiological shear stress ranges between 0.1-10 dyn/cm², with values exceeding this threshold potentially triggering mechanotransduction pathways that alter cellular phenotype and function.
Oxygen and nutrient gradients represent another critical consideration affecting cell viability in microfluidic systems. The materials and channel geometries must facilitate appropriate gas exchange while preventing bubble formation. Studies demonstrate that oxygen permeability coefficients of materials directly correlate with cellular metabolic activity and viability in long-term cultures. PDMS exhibits an oxygen permeability coefficient of approximately 3.4×10^-9 m²/s, whereas glass and hard plastics demonstrate significantly lower values, necessitating alternative oxygenation strategies.
Temperature stability and pH maintenance also significantly impact cell viability in microfluidic organ cultures. Materials with high thermal conductivity can help maintain consistent temperatures throughout the system, while buffer capacity within media formulations must be optimized to counteract pH shifts resulting from cellular metabolism. Engineering solutions incorporating integrated sensing elements for real-time monitoring of these parameters can substantially improve experimental outcomes and cellular health.
The integration of extracellular matrix components within microfluidic devices represents an emerging approach to enhance biocompatibility. Hydrogel-based matrices composed of collagen, fibronectin, or Matrigel provide physiologically relevant microenvironments that support cellular function while potentially reducing bubble formation through altered surface tension properties at material interfaces. These biomimetic approaches demonstrate promising results in maintaining cellular phenotype and function during extended culture periods.
Scalability and Manufacturing Challenges
The transition from laboratory-scale microfluidic organ culture systems to commercial manufacturing presents significant challenges that must be addressed for widespread adoption. Current manufacturing processes for microfluidic devices typically involve cleanroom facilities and specialized equipment, resulting in high production costs that limit scalability. Traditional fabrication methods such as soft lithography with PDMS, while excellent for prototyping, face limitations in mass production scenarios due to time-consuming manual processes and batch-to-batch variability.
Material selection becomes increasingly critical when scaling production. Materials that perform well in laboratory settings may not be suitable for high-throughput manufacturing or may introduce inconsistencies when produced at scale. For instance, the gas permeability of PDMS—beneficial for cellular respiration but problematic for bubble formation—requires careful consideration when transitioning to industrial production. Alternative materials like thermoplastics offer better manufacturing scalability but may present different challenges regarding surface properties and biocompatibility.
Standardization represents another major hurdle in scaling microfluidic organ culture systems. The lack of industry-wide standards for components, connections, and testing protocols complicates quality control and regulatory compliance. Manufacturers must develop robust quality assurance processes to ensure consistent performance across thousands of devices, particularly regarding bubble prevention features which require precise dimensional control and surface characteristics.
The integration of bubble prevention technologies into mass-produced devices introduces additional complexity. Features such as bubble traps, hydrophilic surface treatments, and degassing components must be incorporated without significantly increasing production costs or compromising device functionality. Automated assembly processes must be developed to replace manual operations while maintaining precision in critical areas affecting fluid dynamics and bubble formation.
Supply chain considerations also impact scalability, as specialized materials and components may have limited suppliers or long lead times. Establishing reliable sourcing for materials with consistent properties is essential for maintaining quality in scaled production. Additionally, packaging and sterilization processes must be developed that preserve the integrity of bubble prevention features while meeting regulatory requirements for medical devices.
Cost-effectiveness ultimately determines commercial viability. The manufacturing process must balance production efficiency with performance reliability, particularly for long-term culture applications where bubble formation can cause catastrophic failure. Innovative approaches such as modular designs, simplified fabrication techniques, and automated quality control systems will be necessary to achieve economically viable manufacturing while maintaining the technical performance required for successful organ culture applications.
Material selection becomes increasingly critical when scaling production. Materials that perform well in laboratory settings may not be suitable for high-throughput manufacturing or may introduce inconsistencies when produced at scale. For instance, the gas permeability of PDMS—beneficial for cellular respiration but problematic for bubble formation—requires careful consideration when transitioning to industrial production. Alternative materials like thermoplastics offer better manufacturing scalability but may present different challenges regarding surface properties and biocompatibility.
Standardization represents another major hurdle in scaling microfluidic organ culture systems. The lack of industry-wide standards for components, connections, and testing protocols complicates quality control and regulatory compliance. Manufacturers must develop robust quality assurance processes to ensure consistent performance across thousands of devices, particularly regarding bubble prevention features which require precise dimensional control and surface characteristics.
The integration of bubble prevention technologies into mass-produced devices introduces additional complexity. Features such as bubble traps, hydrophilic surface treatments, and degassing components must be incorporated without significantly increasing production costs or compromising device functionality. Automated assembly processes must be developed to replace manual operations while maintaining precision in critical areas affecting fluid dynamics and bubble formation.
Supply chain considerations also impact scalability, as specialized materials and components may have limited suppliers or long lead times. Establishing reliable sourcing for materials with consistent properties is essential for maintaining quality in scaled production. Additionally, packaging and sterilization processes must be developed that preserve the integrity of bubble prevention features while meeting regulatory requirements for medical devices.
Cost-effectiveness ultimately determines commercial viability. The manufacturing process must balance production efficiency with performance reliability, particularly for long-term culture applications where bubble formation can cause catastrophic failure. Innovative approaches such as modular designs, simplified fabrication techniques, and automated quality control systems will be necessary to achieve economically viable manufacturing while maintaining the technical performance required for successful organ culture applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!