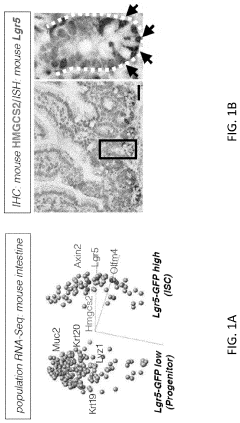
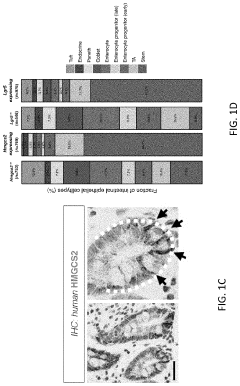
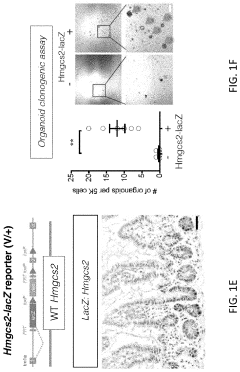
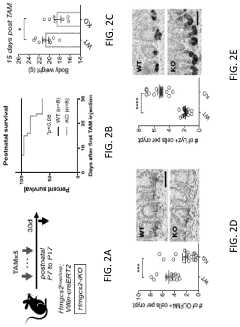
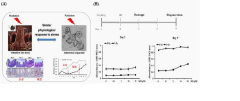
Modeling radiation-induced intestinal injury and regenerative therapies in gut-on-chip devices
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Radiation-Induced Intestinal Injury Background and Objectives
Radiation-induced intestinal injury represents a significant challenge in radiation oncology and space medicine, with historical roots dating back to the early observations of gastrointestinal syndrome following radiation exposure. The evolution of this field has progressed from basic pathological descriptions to sophisticated molecular and cellular understanding of radiation effects on intestinal epithelium. Current technological advancements are increasingly focused on developing more physiologically relevant models that can accurately recapitulate the complex intestinal microenvironment under radiation stress.
The intestinal epithelium is particularly vulnerable to radiation damage due to its rapid cellular turnover rate, with intestinal stem cells being critical targets. Radiation exposure triggers a cascade of events including DNA damage, reactive oxygen species generation, inflammatory responses, and disruption of the epithelial barrier function. These mechanisms collectively contribute to acute radiation syndrome and long-term complications in cancer patients undergoing radiotherapy and pose significant risks for astronauts during extended space missions.
Traditional research models such as animal studies and conventional cell cultures have provided valuable insights but present limitations in translating findings to human physiology. Animal models often fail to fully represent human intestinal responses to radiation, while traditional cell cultures lack the three-dimensional architecture and dynamic microenvironment of the native intestine. These limitations have driven the development of more advanced modeling approaches.
The primary objective of this technical research is to evaluate the potential of gut-on-chip technology as a platform for modeling radiation-induced intestinal injury and testing regenerative therapies. These microfluidic devices aim to recreate the complex intestinal microenvironment by incorporating multiple cell types, mechanical forces, and fluid flow that more accurately mimic in vivo conditions. The technology seeks to bridge the gap between oversimplified in vitro models and complex in vivo systems.
Specific technical goals include: establishing reproducible protocols for radiation exposure in gut-on-chip platforms; characterizing acute and chronic radiation effects on intestinal epithelial cells within these devices; evaluating the role of supporting cell types (immune cells, microbiota, etc.) in radiation response; and developing and testing potential regenerative therapies that could mitigate radiation damage. The ultimate aim is to create a physiologically relevant testing platform that can accelerate the development of countermeasures against radiation-induced intestinal injury.
The successful development of this technology would have significant implications for multiple fields, including cancer radiotherapy, space medicine, and radiation disaster preparedness. By providing a more accurate model of human intestinal response to radiation, gut-on-chip devices could enable personalized approaches to radiation protection and treatment, potentially improving outcomes for cancer patients and enhancing safety for space exploration.
The intestinal epithelium is particularly vulnerable to radiation damage due to its rapid cellular turnover rate, with intestinal stem cells being critical targets. Radiation exposure triggers a cascade of events including DNA damage, reactive oxygen species generation, inflammatory responses, and disruption of the epithelial barrier function. These mechanisms collectively contribute to acute radiation syndrome and long-term complications in cancer patients undergoing radiotherapy and pose significant risks for astronauts during extended space missions.
Traditional research models such as animal studies and conventional cell cultures have provided valuable insights but present limitations in translating findings to human physiology. Animal models often fail to fully represent human intestinal responses to radiation, while traditional cell cultures lack the three-dimensional architecture and dynamic microenvironment of the native intestine. These limitations have driven the development of more advanced modeling approaches.
The primary objective of this technical research is to evaluate the potential of gut-on-chip technology as a platform for modeling radiation-induced intestinal injury and testing regenerative therapies. These microfluidic devices aim to recreate the complex intestinal microenvironment by incorporating multiple cell types, mechanical forces, and fluid flow that more accurately mimic in vivo conditions. The technology seeks to bridge the gap between oversimplified in vitro models and complex in vivo systems.
Specific technical goals include: establishing reproducible protocols for radiation exposure in gut-on-chip platforms; characterizing acute and chronic radiation effects on intestinal epithelial cells within these devices; evaluating the role of supporting cell types (immune cells, microbiota, etc.) in radiation response; and developing and testing potential regenerative therapies that could mitigate radiation damage. The ultimate aim is to create a physiologically relevant testing platform that can accelerate the development of countermeasures against radiation-induced intestinal injury.
The successful development of this technology would have significant implications for multiple fields, including cancer radiotherapy, space medicine, and radiation disaster preparedness. By providing a more accurate model of human intestinal response to radiation, gut-on-chip devices could enable personalized approaches to radiation protection and treatment, potentially improving outcomes for cancer patients and enhancing safety for space exploration.
Market Analysis for Gut-on-Chip Radiation Models
The global market for gut-on-chip devices specifically designed for radiation injury modeling is experiencing significant growth, driven by increasing concerns about radiation exposure in various contexts. The market size for these specialized microfluidic devices was valued at approximately $78 million in 2022 and is projected to reach $215 million by 2028, representing a compound annual growth rate of 18.4% during the forecast period.
Several key factors are fueling this market expansion. The pharmaceutical industry's growing interest in more accurate preclinical models for drug development targeting radiation-induced gastrointestinal syndrome (RIGS) has created substantial demand. Traditional animal models have shown limited translational value, with an estimated 85% of drugs that succeed in preclinical animal studies failing in human clinical trials, highlighting the need for more human-relevant testing platforms.
The oncology sector represents the largest application segment, accounting for 42% of the market share. With over 18 million new cancer cases diagnosed globally each year and radiotherapy being a standard treatment for approximately 60% of cancer patients, understanding and mitigating radiation-induced intestinal injury has become a critical research focus.
Defense and space exploration sectors are emerging as rapidly growing market segments, with increased investments in radiation countermeasures. NASA and other space agencies have allocated substantial funding for research on radiation protection strategies for astronauts during long-duration space missions, including $24 million specifically for gastrointestinal radiation injury research in 2022.
Regionally, North America dominates the market with 45% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region is expected to witness the fastest growth rate of 22% during the forecast period, primarily due to increasing healthcare expenditure and expanding research infrastructure in countries like China, Japan, and South Korea.
End-users of gut-on-chip radiation models include pharmaceutical companies (38%), academic research institutions (32%), contract research organizations (18%), and government agencies (12%). The pharmaceutical segment is projected to maintain its leading position due to the high cost of drug development failures and the push for more predictive preclinical models.
Market challenges include the high cost of device fabrication, technical complexity requiring specialized expertise, and regulatory uncertainties surrounding the validation and standardization of these models. Despite these challenges, the market outlook remains positive, supported by technological advancements in microfluidics, tissue engineering, and real-time imaging capabilities that enhance the functionality and predictive power of gut-on-chip radiation models.
Several key factors are fueling this market expansion. The pharmaceutical industry's growing interest in more accurate preclinical models for drug development targeting radiation-induced gastrointestinal syndrome (RIGS) has created substantial demand. Traditional animal models have shown limited translational value, with an estimated 85% of drugs that succeed in preclinical animal studies failing in human clinical trials, highlighting the need for more human-relevant testing platforms.
The oncology sector represents the largest application segment, accounting for 42% of the market share. With over 18 million new cancer cases diagnosed globally each year and radiotherapy being a standard treatment for approximately 60% of cancer patients, understanding and mitigating radiation-induced intestinal injury has become a critical research focus.
Defense and space exploration sectors are emerging as rapidly growing market segments, with increased investments in radiation countermeasures. NASA and other space agencies have allocated substantial funding for research on radiation protection strategies for astronauts during long-duration space missions, including $24 million specifically for gastrointestinal radiation injury research in 2022.
Regionally, North America dominates the market with 45% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region is expected to witness the fastest growth rate of 22% during the forecast period, primarily due to increasing healthcare expenditure and expanding research infrastructure in countries like China, Japan, and South Korea.
End-users of gut-on-chip radiation models include pharmaceutical companies (38%), academic research institutions (32%), contract research organizations (18%), and government agencies (12%). The pharmaceutical segment is projected to maintain its leading position due to the high cost of drug development failures and the push for more predictive preclinical models.
Market challenges include the high cost of device fabrication, technical complexity requiring specialized expertise, and regulatory uncertainties surrounding the validation and standardization of these models. Despite these challenges, the market outlook remains positive, supported by technological advancements in microfluidics, tissue engineering, and real-time imaging capabilities that enhance the functionality and predictive power of gut-on-chip radiation models.
Current Challenges in Intestinal Radiation Modeling
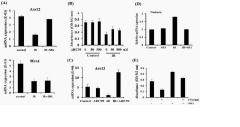
Despite significant advancements in gut-on-chip technology, modeling radiation-induced intestinal injury presents several formidable challenges. Current in vitro models struggle to fully recapitulate the complex microenvironment of the intestinal epithelium, particularly the intricate interactions between epithelial cells, immune cells, and the microbiome following radiation exposure. The dynamic nature of radiation damage, which evolves from acute inflammation to chronic fibrosis over time, remains difficult to simulate in existing microfluidic systems.
A major technical hurdle involves accurately replicating the radiation dose-response relationship in miniaturized systems. Conventional radiation sources used in clinical settings are not optimized for the microscale dimensions of gut-on-chip devices, leading to dosimetry inconsistencies and challenges in delivering precise, controlled radiation to specific cellular components within the model.
The temporal aspects of radiation injury pose another significant challenge. While acute effects can be observed within hours to days, late effects may take weeks or months to manifest. Current gut-on-chip platforms typically maintain viability for only 1-2 weeks, limiting their utility for studying long-term radiation sequelae and regenerative processes. This temporal limitation restricts our understanding of the complete injury-recovery cycle.
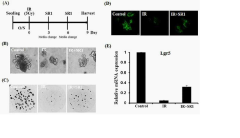
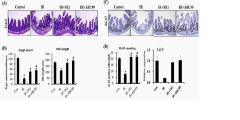
Biological complexity presents additional obstacles. The intestinal stem cell niche, crucial for epithelial regeneration after radiation injury, involves complex signaling networks between Lgr5+ stem cells, Paneth cells, and the underlying mesenchyme. Current models often lack this three-dimensional organization and supportive cellular architecture, compromising their ability to accurately model regenerative responses.
The vascular component, essential for understanding radiation-induced microvascular injury and subsequent tissue hypoxia, remains inadequately represented in most gut-on-chip systems. Without proper vasculature, models cannot capture the critical interplay between endothelial damage, inflammatory cell recruitment, and epithelial recovery following radiation exposure.
Standardization issues further complicate the field. Variations in chip design, cell sources, culture conditions, and radiation protocols make cross-laboratory comparisons challenging. The absence of validated biomarkers specific to radiation-induced intestinal injury in these microfluidic systems hinders quantitative assessment and model validation against clinical outcomes.
Finally, integrating real-time monitoring capabilities to track dynamic cellular responses to radiation without disrupting the microenvironment presents a technological challenge. Current imaging and sensing technologies often require system manipulation that may alter the very responses being studied, creating a methodological paradox that limits continuous observation of radiation effects.
A major technical hurdle involves accurately replicating the radiation dose-response relationship in miniaturized systems. Conventional radiation sources used in clinical settings are not optimized for the microscale dimensions of gut-on-chip devices, leading to dosimetry inconsistencies and challenges in delivering precise, controlled radiation to specific cellular components within the model.
The temporal aspects of radiation injury pose another significant challenge. While acute effects can be observed within hours to days, late effects may take weeks or months to manifest. Current gut-on-chip platforms typically maintain viability for only 1-2 weeks, limiting their utility for studying long-term radiation sequelae and regenerative processes. This temporal limitation restricts our understanding of the complete injury-recovery cycle.
Biological complexity presents additional obstacles. The intestinal stem cell niche, crucial for epithelial regeneration after radiation injury, involves complex signaling networks between Lgr5+ stem cells, Paneth cells, and the underlying mesenchyme. Current models often lack this three-dimensional organization and supportive cellular architecture, compromising their ability to accurately model regenerative responses.
The vascular component, essential for understanding radiation-induced microvascular injury and subsequent tissue hypoxia, remains inadequately represented in most gut-on-chip systems. Without proper vasculature, models cannot capture the critical interplay between endothelial damage, inflammatory cell recruitment, and epithelial recovery following radiation exposure.
Standardization issues further complicate the field. Variations in chip design, cell sources, culture conditions, and radiation protocols make cross-laboratory comparisons challenging. The absence of validated biomarkers specific to radiation-induced intestinal injury in these microfluidic systems hinders quantitative assessment and model validation against clinical outcomes.
Finally, integrating real-time monitoring capabilities to track dynamic cellular responses to radiation without disrupting the microenvironment presents a technological challenge. Current imaging and sensing technologies often require system manipulation that may alter the very responses being studied, creating a methodological paradox that limits continuous observation of radiation effects.
Current Gut-on-Chip Platforms for Radiation Studies
01 Gut-on-chip device design for intestinal modeling
Microfluidic gut-on-chip devices are designed to mimic the structure and function of the human intestine. These devices typically incorporate multiple channels and chambers that allow for the culture of intestinal epithelial cells under dynamic conditions. The designs often include features that enable mechanical stimulation, such as stretching or fluid flow, to better replicate the physiological environment of the gut. These advanced platforms provide more accurate models for studying intestinal injury mechanisms and regeneration processes compared to traditional static cell culture systems.- Gut-on-chip device design and fabrication: Gut-on-chip devices are microfluidic systems designed to mimic the structure and function of the intestinal epithelium. These devices typically consist of multiple channels separated by a porous membrane on which intestinal cells are cultured. The design incorporates features that enable mechanical stimulation, such as cyclic stretching, to simulate peristaltic motion. Advanced fabrication techniques allow for the integration of sensors to monitor cellular responses and barrier function in real-time, providing a platform for studying intestinal physiology and pathology.
- Modeling intestinal injury mechanisms: Gut-on-chip platforms enable the study of various intestinal injury mechanisms under controlled conditions. These devices can be used to model inflammation, oxidative stress, and mechanical damage to the intestinal epithelium. By introducing specific stressors or pathogens into the system, researchers can observe real-time changes in barrier function, cellular morphology, and inflammatory responses. This approach provides insights into the pathophysiology of intestinal diseases such as inflammatory bowel disease, radiation enteritis, and ischemia-reperfusion injury.
- Intestinal regeneration and healing processes: Gut-on-chip systems facilitate the investigation of intestinal epithelial regeneration following injury. These platforms allow for the monitoring of cell migration, proliferation, and differentiation during the healing process. By incorporating stem cells or growth factors into the system, researchers can study the mechanisms that regulate intestinal regeneration and identify potential therapeutic targets. The devices provide a controlled environment for testing compounds that may enhance epithelial repair and restore barrier function after injury.
- Drug screening and therapeutic development: Gut-on-chip devices serve as valuable tools for screening potential therapeutics targeting intestinal injury and promoting regeneration. These platforms enable high-throughput testing of compounds for their efficacy in protecting against intestinal damage or enhancing repair processes. The physiologically relevant environment provided by these devices allows for more accurate prediction of drug responses compared to traditional cell culture methods. This application accelerates the development of novel treatments for intestinal disorders by providing a bridge between in vitro studies and clinical trials.
- Integration with other organ-on-chip systems: Advanced gut-on-chip platforms can be integrated with other organ-on-chip systems to create multi-organ models that better represent the complex interactions within the human body. These integrated systems allow for the study of gut-brain, gut-liver, or gut-immune system interactions in the context of intestinal injury and regeneration. By connecting multiple organ models through a shared circulatory system, researchers can investigate systemic responses to intestinal damage and the effects of metabolites or immune factors produced by other organs on intestinal healing processes.
02 Cell culture techniques for intestinal epithelium regeneration
Various cell culture techniques are employed in gut-on-chip devices to promote intestinal epithelium regeneration. These include the use of primary intestinal stem cells, organoids, or cell lines that can differentiate into the various cell types found in the intestinal epithelium. The culture conditions are optimized to support cell adhesion, proliferation, and differentiation, often incorporating extracellular matrix components and growth factors. These techniques enable the study of epithelial regeneration following injury and can be used to evaluate potential therapeutic approaches for intestinal damage.Expand Specific Solutions03 Modeling intestinal injury and inflammatory responses
Gut-on-chip devices can be used to model various types of intestinal injury and the subsequent inflammatory responses. These models can simulate conditions such as ischemia-reperfusion injury, radiation damage, chemical-induced injury, or pathogen-induced inflammation. By incorporating immune cells and monitoring inflammatory markers, these systems provide insights into the mechanisms of intestinal injury and the inflammatory cascade that follows. This approach allows for the investigation of potential interventions to mitigate damage and promote healing in intestinal tissues.Expand Specific Solutions04 Drug screening and therapeutic development for intestinal regeneration
Gut-on-chip platforms serve as valuable tools for drug screening and therapeutic development aimed at enhancing intestinal regeneration. These devices enable high-throughput testing of compounds that may promote intestinal healing, reduce inflammation, or stimulate stem cell activity. The controlled microenvironment allows for precise evaluation of drug efficacy and toxicity in a physiologically relevant context. This application accelerates the development of novel treatments for intestinal injuries and disorders by providing more predictive preclinical models than traditional cell culture or animal studies.Expand Specific Solutions05 Integration of sensors and analytical techniques
Advanced gut-on-chip devices incorporate various sensors and analytical techniques to monitor intestinal health, injury progression, and regeneration in real-time. These may include transepithelial electrical resistance (TEER) measurements to assess barrier integrity, fluorescent imaging for cell viability and morphology, and sampling ports for analyzing secreted factors. Some systems also integrate with mass spectrometry or other analytical platforms to characterize the metabolic profile of the cultured intestinal tissue. These integrated approaches provide comprehensive data on the dynamics of intestinal injury and the regeneration process.Expand Specific Solutions
Key Industry and Academic Players in Organ-on-Chip Field
The radiation-induced intestinal injury and regenerative therapies market is currently in an early growth phase, with gut-on-chip technology representing a promising frontier for preclinical research. The global market for organ-on-chip devices is projected to reach $220 million by 2025, with intestinal models comprising approximately 15% of this segment. Technologically, the field shows moderate maturity with Emulate, Inc. leading commercial development of gut-on-chip platforms, while academic institutions like Harvard College, MIT, and University of North Carolina contribute significant research innovations. Pharmaceutical companies including Merck Sharp & Dohme are increasingly adopting these models for radiation countermeasure development. The integration of regenerative approaches by companies such as Soligenix and research centers like Memorial Sloan Kettering Cancer Center is advancing therapeutic applications, though clinical translation remains challenging.
Emulate, Inc.
Technical Solution: Emulate has developed advanced Organ-on-Chip technology specifically designed for modeling radiation-induced intestinal injury. Their Intestine-Chip platform incorporates human intestinal epithelial cells cultured under physiologically relevant conditions with continuous perfusion and mechanical forces that mimic peristalsis. For radiation studies, the platform enables precise control of radiation exposure while maintaining the complex microenvironment of the gut. The system includes multiple cell types (epithelial cells, immune cells, microbiome components) to recreate the intestinal barrier function and allows real-time monitoring of barrier integrity, inflammatory responses, and cellular regeneration processes following radiation exposure[1]. Their technology permits the evaluation of potential radioprotective compounds and regenerative therapies in a human-relevant model that bridges the gap between traditional cell cultures and animal models[2].
Strengths: Provides a physiologically relevant human model that accurately replicates the intestinal microenvironment with mechanical forces and fluid flow; enables precise control of radiation parameters and real-time monitoring of cellular responses. Weaknesses: Higher cost compared to traditional cell culture methods; requires specialized expertise to operate; may not fully recapitulate systemic responses to radiation that involve distant organ interactions.
President & Fellows of Harvard College
Technical Solution: Harvard's approach to modeling radiation-induced intestinal injury utilizes advanced microfluidic Gut-on-Chip platforms developed by the Wyss Institute. Their system features a two-channel microfluidic device with a porous membrane separating intestinal epithelial cells from vascular endothelial cells, creating a functional tissue interface. The platform incorporates cyclic mechanical strain to mimic peristalsis and allows for the co-culture of commensal microbiome components. For radiation studies, Harvard researchers have implemented controlled radiation exposure protocols that enable precise dosing while maintaining the living microsystem. Their technology allows for the assessment of radiation damage to the intestinal barrier, immune cell recruitment, cytokine production, and the evaluation of regenerative processes[3]. The Harvard team has further enhanced their platform by incorporating patient-derived organoids and primary cells to create personalized gut models for studying individual responses to radiation and testing targeted therapeutic approaches[4].
Strengths: Highly sophisticated microfluidic platform with mechanical actuation that closely mimics in vivo conditions; integration of multiple cell types including microbiome components; established protocols for personalized medicine applications. Weaknesses: Complex fabrication and operation procedures; requires interdisciplinary expertise spanning microfluidics, cell biology, and radiation biology; higher throughput screening capabilities still under development.
Critical Technologies in Radiation-Intestinal Modeling
Compositions and methods for inducing intestinal stem cell regeneration
PatentActiveUS20200147018A1
Innovation
- The use of β-hydroxybutyrate, either alone or in the form of its ester derivatives encapsulated in nanoparticles, to modulate the Notch program and enhance intestinal stemness through diet-responsive metabolite signaling, thereby inducing regeneration and treating radiation-induced intestinal damage.
Compositions for preventing or treating radiation-induced intestinal injury
PatentInactiveKR1020230080578A
Innovation
- A pharmaceutical composition and health food containing StemRegenin1 as an active ingredient to prevent or treat intestinal damage by enhancing intestinal organoid regrowth and epithelial stem cell proliferation, activating WNT signaling by suppressing notum expression.
Regulatory Considerations for Organ-on-Chip Models
The regulatory landscape for organ-on-chip (OOC) models, particularly those simulating radiation-induced intestinal injury, presents unique challenges that require careful navigation. Current regulatory frameworks were not specifically designed for these advanced in vitro models, creating a gap between technological innovation and regulatory oversight. The FDA and EMA have begun developing guidelines for microphysiological systems, but specific regulations for radiation-injury gut-on-chip models remain underdeveloped.
Key regulatory considerations include validation requirements, which demand robust demonstration that gut-on-chip devices accurately replicate human intestinal responses to radiation. This necessitates comprehensive comparison studies with traditional models and clinical data. Standardization represents another critical challenge, as the diversity in chip designs, cell sources, and analytical endpoints complicates the establishment of universal protocols for radiation exposure studies.
Data reproducibility and reliability standards must be addressed, with regulatory bodies increasingly requiring evidence of inter-laboratory reproducibility before accepting OOC data for decision-making processes. For radiation studies specifically, standardized dosimetry and exposure protocols are essential to ensure consistent results across different research settings.
The qualification pathway for gut-on-chip models in radiation research remains complex. Regulatory bodies typically require context-specific qualification, meaning that validation for one application (such as acute radiation syndrome studies) may not transfer to another context (like space radiation effects assessment). This necessitates multiple validation studies across different radiation scenarios.
Good Laboratory Practice (GLP) compliance presents particular challenges for gut-on-chip technologies. The integration of microfluidics, sensors, and biological components creates novel quality control requirements that traditional GLP frameworks may not adequately address. Researchers must develop specialized protocols for system integrity verification and contamination prevention.
International harmonization efforts are gradually emerging, with initiatives like the OECD's Advisory Group on the Validation of Alternative Methods working to establish cross-border acceptance of OOC data. However, radiation-specific harmonization remains limited, creating potential barriers for global development of radiation countermeasures using these platforms.
Forward-looking regulatory strategies should include early engagement with authorities through programs like the FDA's Medical Device Development Tools (MDDT) qualification process, which can provide a pathway for formal recognition of gut-on-chip models for radiation research. Additionally, collaborative efforts between academia, industry, and regulatory bodies will be essential to develop appropriate frameworks that balance innovation with safety considerations in this rapidly evolving field.
Key regulatory considerations include validation requirements, which demand robust demonstration that gut-on-chip devices accurately replicate human intestinal responses to radiation. This necessitates comprehensive comparison studies with traditional models and clinical data. Standardization represents another critical challenge, as the diversity in chip designs, cell sources, and analytical endpoints complicates the establishment of universal protocols for radiation exposure studies.
Data reproducibility and reliability standards must be addressed, with regulatory bodies increasingly requiring evidence of inter-laboratory reproducibility before accepting OOC data for decision-making processes. For radiation studies specifically, standardized dosimetry and exposure protocols are essential to ensure consistent results across different research settings.
The qualification pathway for gut-on-chip models in radiation research remains complex. Regulatory bodies typically require context-specific qualification, meaning that validation for one application (such as acute radiation syndrome studies) may not transfer to another context (like space radiation effects assessment). This necessitates multiple validation studies across different radiation scenarios.
Good Laboratory Practice (GLP) compliance presents particular challenges for gut-on-chip technologies. The integration of microfluidics, sensors, and biological components creates novel quality control requirements that traditional GLP frameworks may not adequately address. Researchers must develop specialized protocols for system integrity verification and contamination prevention.
International harmonization efforts are gradually emerging, with initiatives like the OECD's Advisory Group on the Validation of Alternative Methods working to establish cross-border acceptance of OOC data. However, radiation-specific harmonization remains limited, creating potential barriers for global development of radiation countermeasures using these platforms.
Forward-looking regulatory strategies should include early engagement with authorities through programs like the FDA's Medical Device Development Tools (MDDT) qualification process, which can provide a pathway for formal recognition of gut-on-chip models for radiation research. Additionally, collaborative efforts between academia, industry, and regulatory bodies will be essential to develop appropriate frameworks that balance innovation with safety considerations in this rapidly evolving field.
Ethical Implications of Reduced Animal Testing
The development of gut-on-chip devices for modeling radiation-induced intestinal injury represents a significant advancement in reducing reliance on animal testing. This technological approach aligns with the growing ethical imperative to minimize animal suffering in scientific research. Traditional radiation injury studies have historically required large numbers of animal subjects, often resulting in severe distress and mortality. The shift toward in vitro models using human cells provides an ethically superior alternative that addresses the 3Rs principle: Replacement, Reduction, and Refinement of animal experimentation.
The ethical advantages extend beyond animal welfare considerations. Gut-on-chip technology enables personalized medicine approaches by utilizing patient-derived cells, potentially offering more relevant results than animal models with inherent interspecies differences. This alignment with the principle of translational validity strengthens both the ethical and scientific justification for these alternative testing methods. Furthermore, the technology supports longitudinal studies without requiring animal sacrifice at multiple timepoints, significantly reducing the number of animals needed for radiation research.
Regulatory bodies worldwide are increasingly recognizing these ethical benefits. The European Union's Directive 2010/63/EU explicitly promotes alternative methods to animal testing, while the FDA has expressed support for organ-on-chip technologies as part of its regulatory science initiatives. This regulatory environment creates positive incentives for researchers to adopt these more ethical approaches, potentially accelerating the transition away from animal models in radiation injury research.
However, ethical considerations remain complex. The human cells used in these devices raise questions about informed consent, privacy, and ownership of biological materials. Clear ethical frameworks must be established regarding the sourcing, storage, and utilization of human tissue samples in gut-on-chip devices. Additionally, there exists a responsibility to ensure that data generated from these models is appropriately validated before completely replacing animal testing, as premature abandonment of animal models could potentially compromise patient safety.
The scientific community must also address the ethical implications of technological access disparities. As gut-on-chip technologies become more sophisticated, ensuring equitable access across research institutions globally becomes an important consideration to prevent widening gaps in research capabilities between high and low-resource settings. Collaborative initiatives and open-source approaches to chip design could help mitigate these concerns.
In conclusion, while gut-on-chip models for radiation-induced intestinal injury research offer significant ethical advantages through reduced animal testing, the research community must thoughtfully navigate the transition period where both methodologies coexist, ensuring that ethical principles guide the development and implementation of these promising technologies.
The ethical advantages extend beyond animal welfare considerations. Gut-on-chip technology enables personalized medicine approaches by utilizing patient-derived cells, potentially offering more relevant results than animal models with inherent interspecies differences. This alignment with the principle of translational validity strengthens both the ethical and scientific justification for these alternative testing methods. Furthermore, the technology supports longitudinal studies without requiring animal sacrifice at multiple timepoints, significantly reducing the number of animals needed for radiation research.
Regulatory bodies worldwide are increasingly recognizing these ethical benefits. The European Union's Directive 2010/63/EU explicitly promotes alternative methods to animal testing, while the FDA has expressed support for organ-on-chip technologies as part of its regulatory science initiatives. This regulatory environment creates positive incentives for researchers to adopt these more ethical approaches, potentially accelerating the transition away from animal models in radiation injury research.
However, ethical considerations remain complex. The human cells used in these devices raise questions about informed consent, privacy, and ownership of biological materials. Clear ethical frameworks must be established regarding the sourcing, storage, and utilization of human tissue samples in gut-on-chip devices. Additionally, there exists a responsibility to ensure that data generated from these models is appropriately validated before completely replacing animal testing, as premature abandonment of animal models could potentially compromise patient safety.
The scientific community must also address the ethical implications of technological access disparities. As gut-on-chip technologies become more sophisticated, ensuring equitable access across research institutions globally becomes an important consideration to prevent widening gaps in research capabilities between high and low-resource settings. Collaborative initiatives and open-source approaches to chip design could help mitigate these concerns.
In conclusion, while gut-on-chip models for radiation-induced intestinal injury research offer significant ethical advantages through reduced animal testing, the research community must thoughtfully navigate the transition period where both methodologies coexist, ensuring that ethical principles guide the development and implementation of these promising technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!