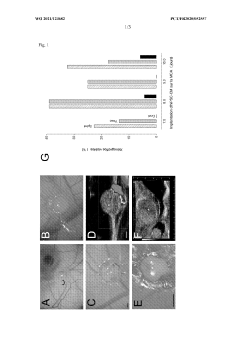
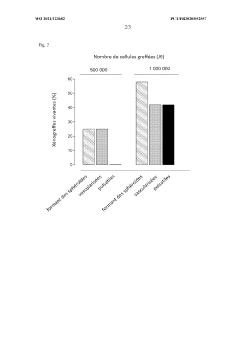
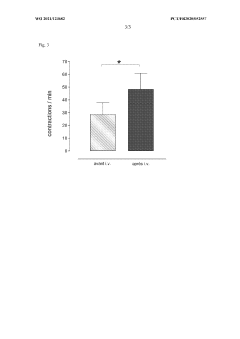
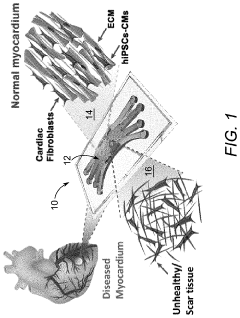
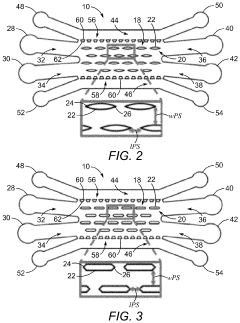
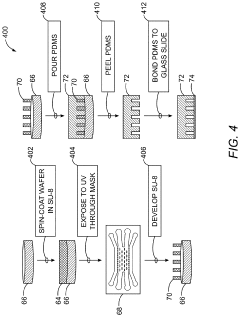
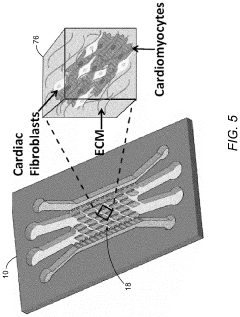
Generating vascularized cardiac microtissues from human adipose-derived stem cells on chip
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cardiac Microtissue Engineering Background and Objectives
Cardiac microtissue engineering has evolved significantly over the past two decades, transitioning from simple cell culture techniques to sophisticated three-dimensional constructs that better mimic native cardiac tissue. The field emerged from the broader tissue engineering discipline in the early 2000s, with pioneering work focusing on creating functional cardiac tissue equivalents for regenerative medicine applications and drug testing platforms.
The development trajectory has been characterized by progressive improvements in biomaterial scaffolds, cell sourcing strategies, and fabrication technologies. Early approaches relied primarily on synthetic polymers and cardiomyocytes derived from animal sources, which presented limitations in translational relevance. The discovery of human induced pluripotent stem cells (hiPSCs) in 2007 revolutionized the field by providing a potentially unlimited source of human cardiomyocytes.
A critical limitation in cardiac tissue engineering has been the lack of functional vasculature, which restricts the size and functionality of engineered constructs due to diffusion limitations of oxygen and nutrients. This challenge has driven research toward developing vascularized cardiac tissues that can better recapitulate the complex architecture and physiology of native myocardium.
Human adipose-derived stem cells (hADSCs) have emerged as a promising cell source due to their abundance, accessibility, and multilineage differentiation potential. These cells can be readily harvested through minimally invasive procedures and expanded in vitro, offering significant advantages over other stem cell sources. Furthermore, hADSCs have demonstrated the capacity to differentiate into both cardiomyocytes and vascular cell types, making them particularly suitable for generating vascularized cardiac tissues.
Microfluidic technologies and organ-on-chip platforms represent the latest frontier in this field, enabling precise control over the cellular microenvironment and facilitating the formation of functional vascular networks within engineered tissues. These technologies allow for continuous perfusion, which addresses the oxygen and nutrient diffusion limitations inherent in traditional static culture systems.
The primary objectives of current research in vascularized cardiac microtissue engineering include: developing physiologically relevant human cardiac tissue models for drug screening and toxicity testing; creating disease models for studying cardiac pathophysiology; advancing regenerative medicine approaches for treating heart disease; and establishing standardized protocols for reproducible tissue fabrication. The ultimate goal is to generate functional, vascularized cardiac tissues that accurately recapitulate the structural, mechanical, and electrophysiological properties of native myocardium.
The development trajectory has been characterized by progressive improvements in biomaterial scaffolds, cell sourcing strategies, and fabrication technologies. Early approaches relied primarily on synthetic polymers and cardiomyocytes derived from animal sources, which presented limitations in translational relevance. The discovery of human induced pluripotent stem cells (hiPSCs) in 2007 revolutionized the field by providing a potentially unlimited source of human cardiomyocytes.
A critical limitation in cardiac tissue engineering has been the lack of functional vasculature, which restricts the size and functionality of engineered constructs due to diffusion limitations of oxygen and nutrients. This challenge has driven research toward developing vascularized cardiac tissues that can better recapitulate the complex architecture and physiology of native myocardium.
Human adipose-derived stem cells (hADSCs) have emerged as a promising cell source due to their abundance, accessibility, and multilineage differentiation potential. These cells can be readily harvested through minimally invasive procedures and expanded in vitro, offering significant advantages over other stem cell sources. Furthermore, hADSCs have demonstrated the capacity to differentiate into both cardiomyocytes and vascular cell types, making them particularly suitable for generating vascularized cardiac tissues.
Microfluidic technologies and organ-on-chip platforms represent the latest frontier in this field, enabling precise control over the cellular microenvironment and facilitating the formation of functional vascular networks within engineered tissues. These technologies allow for continuous perfusion, which addresses the oxygen and nutrient diffusion limitations inherent in traditional static culture systems.
The primary objectives of current research in vascularized cardiac microtissue engineering include: developing physiologically relevant human cardiac tissue models for drug screening and toxicity testing; creating disease models for studying cardiac pathophysiology; advancing regenerative medicine approaches for treating heart disease; and establishing standardized protocols for reproducible tissue fabrication. The ultimate goal is to generate functional, vascularized cardiac tissues that accurately recapitulate the structural, mechanical, and electrophysiological properties of native myocardium.
Market Analysis for Vascularized Cardiac Tissue Models
The global market for vascularized cardiac tissue models is experiencing significant growth, driven by increasing prevalence of cardiovascular diseases and the pressing need for more effective drug discovery platforms. Currently valued at approximately $1.2 billion, this market segment is projected to grow at a compound annual growth rate of 15-18% over the next five years, potentially reaching $2.8 billion by 2028.
Pharmaceutical companies represent the largest market segment, accounting for roughly 45% of the total market share. These companies are increasingly adopting organ-on-chip technologies to reduce drug development costs, which currently average $2.6 billion per approved drug, and to address the high failure rates of cardiac drugs in clinical trials (estimated at 90%).
Academic research institutions constitute the second-largest market segment (30%), followed by contract research organizations (15%) and biotechnology companies (10%). Geographically, North America leads with 40% market share, followed by Europe (30%), Asia-Pacific (20%), and rest of the world (10%). The Asia-Pacific region, particularly China and Japan, is expected to witness the fastest growth due to increasing research investments and favorable regulatory environments.
Key market drivers include the rising demand for personalized medicine, stringent regulatory requirements for drug safety testing, and technological advancements in 3D bioprinting and microfluidics. The cost-effectiveness of these models compared to animal testing (potentially reducing preclinical testing costs by 25-30%) further accelerates market growth.
Vascularized cardiac microtissues derived from human adipose-derived stem cells offer distinct market advantages, including abundant and accessible cell source, reduced ethical concerns compared to embryonic stem cells, and potential for autologous applications. This specific technology addresses a critical market need for physiologically relevant cardiac models that accurately recapitulate human heart tissue vasculature.
Market challenges include high initial investment costs for organ-on-chip platforms, technical complexities in maintaining long-term tissue viability, and regulatory uncertainties regarding validation and standardization. Additionally, the market faces competition from alternative technologies such as traditional 2D cell cultures and animal models, which still dominate due to established protocols and regulatory acceptance.
Customer segments show varying needs: pharmaceutical companies prioritize predictive accuracy and throughput, academic researchers value versatility and cost-effectiveness, while clinical applications demand personalization capabilities and reproducibility. The pricing models range from $5,000-$15,000 for basic systems to $50,000-$100,000 for advanced integrated platforms with comprehensive analytical capabilities.
Pharmaceutical companies represent the largest market segment, accounting for roughly 45% of the total market share. These companies are increasingly adopting organ-on-chip technologies to reduce drug development costs, which currently average $2.6 billion per approved drug, and to address the high failure rates of cardiac drugs in clinical trials (estimated at 90%).
Academic research institutions constitute the second-largest market segment (30%), followed by contract research organizations (15%) and biotechnology companies (10%). Geographically, North America leads with 40% market share, followed by Europe (30%), Asia-Pacific (20%), and rest of the world (10%). The Asia-Pacific region, particularly China and Japan, is expected to witness the fastest growth due to increasing research investments and favorable regulatory environments.
Key market drivers include the rising demand for personalized medicine, stringent regulatory requirements for drug safety testing, and technological advancements in 3D bioprinting and microfluidics. The cost-effectiveness of these models compared to animal testing (potentially reducing preclinical testing costs by 25-30%) further accelerates market growth.
Vascularized cardiac microtissues derived from human adipose-derived stem cells offer distinct market advantages, including abundant and accessible cell source, reduced ethical concerns compared to embryonic stem cells, and potential for autologous applications. This specific technology addresses a critical market need for physiologically relevant cardiac models that accurately recapitulate human heart tissue vasculature.
Market challenges include high initial investment costs for organ-on-chip platforms, technical complexities in maintaining long-term tissue viability, and regulatory uncertainties regarding validation and standardization. Additionally, the market faces competition from alternative technologies such as traditional 2D cell cultures and animal models, which still dominate due to established protocols and regulatory acceptance.
Customer segments show varying needs: pharmaceutical companies prioritize predictive accuracy and throughput, academic researchers value versatility and cost-effectiveness, while clinical applications demand personalization capabilities and reproducibility. The pricing models range from $5,000-$15,000 for basic systems to $50,000-$100,000 for advanced integrated platforms with comprehensive analytical capabilities.
Current Challenges in Cardiac Tissue Vascularization
Despite significant advancements in cardiac tissue engineering, vascularization remains one of the most critical challenges in developing functional cardiac microtissues. The absence of adequate vascular networks severely limits the size and functionality of engineered cardiac tissues, as cells located more than 100-200 μm from a capillary suffer from hypoxia and nutrient deficiency. This diffusion limitation represents a fundamental obstacle in scaling up cardiac tissue constructs for both research and therapeutic applications.
Current vascularization approaches for cardiac tissues face several technical hurdles. The complex architecture of native cardiac vasculature, with its hierarchical organization of vessels and capillaries, proves extremely difficult to replicate artificially. Conventional methods often fail to create the dense capillary networks necessary for proper oxygen and nutrient distribution throughout the tissue construct, resulting in necrotic cores in larger tissue samples.
When specifically considering vascularized cardiac microtissues derived from human adipose-derived stem cells (hADSCs) on microfluidic chips, additional challenges emerge. The differentiation of hADSCs into both cardiomyocytes and vascular cells (endothelial cells and pericytes) with appropriate spatial organization remains inconsistent. Current protocols struggle to achieve the synchronized differentiation and self-organization required for functional vascularized cardiac tissues.
Microfluidic platforms, while promising for controlled tissue development, present their own set of challenges. The confined geometries of microfluidic channels can restrict cellular remodeling and matrix reorganization necessary for proper vessel formation. Additionally, achieving appropriate flow conditions that promote vascular development without causing cellular damage requires precise engineering that current systems have not fully mastered.
The integration of vascular networks with functional cardiac tissue presents another significant hurdle. Ensuring proper electromechanical coupling between cardiomyocytes while maintaining vascular integrity remains difficult. The contractile forces generated by cardiomyocytes can disrupt forming vessels, while the presence of vascular structures can interfere with the electrical synchronization of cardiac cells.
Long-term stability of engineered vascular networks constitutes another major challenge. Many current approaches achieve initial vessel formation, but these structures often regress over time due to insufficient pericyte coverage, inadequate basement membrane formation, or lack of appropriate biochemical and mechanical cues. This regression severely limits the clinical translation potential of these engineered tissues.
Finally, analytical methods for assessing vascular function within cardiac microtissues remain limited. Current imaging techniques struggle to provide real-time, non-destructive evaluation of vascular perfusion, permeability, and integration with cardiac tissue. This hampers iterative improvement of vascularization strategies and slows progress in the field.
Current vascularization approaches for cardiac tissues face several technical hurdles. The complex architecture of native cardiac vasculature, with its hierarchical organization of vessels and capillaries, proves extremely difficult to replicate artificially. Conventional methods often fail to create the dense capillary networks necessary for proper oxygen and nutrient distribution throughout the tissue construct, resulting in necrotic cores in larger tissue samples.
When specifically considering vascularized cardiac microtissues derived from human adipose-derived stem cells (hADSCs) on microfluidic chips, additional challenges emerge. The differentiation of hADSCs into both cardiomyocytes and vascular cells (endothelial cells and pericytes) with appropriate spatial organization remains inconsistent. Current protocols struggle to achieve the synchronized differentiation and self-organization required for functional vascularized cardiac tissues.
Microfluidic platforms, while promising for controlled tissue development, present their own set of challenges. The confined geometries of microfluidic channels can restrict cellular remodeling and matrix reorganization necessary for proper vessel formation. Additionally, achieving appropriate flow conditions that promote vascular development without causing cellular damage requires precise engineering that current systems have not fully mastered.
The integration of vascular networks with functional cardiac tissue presents another significant hurdle. Ensuring proper electromechanical coupling between cardiomyocytes while maintaining vascular integrity remains difficult. The contractile forces generated by cardiomyocytes can disrupt forming vessels, while the presence of vascular structures can interfere with the electrical synchronization of cardiac cells.
Long-term stability of engineered vascular networks constitutes another major challenge. Many current approaches achieve initial vessel formation, but these structures often regress over time due to insufficient pericyte coverage, inadequate basement membrane formation, or lack of appropriate biochemical and mechanical cues. This regression severely limits the clinical translation potential of these engineered tissues.
Finally, analytical methods for assessing vascular function within cardiac microtissues remain limited. Current imaging techniques struggle to provide real-time, non-destructive evaluation of vascular perfusion, permeability, and integration with cardiac tissue. This hampers iterative improvement of vascularization strategies and slows progress in the field.
State-of-the-Art Approaches for On-Chip Vascularization
01 Methods for generating vascularized cardiac microtissues from adipose-derived stem cells
Various techniques have been developed to generate vascularized cardiac microtissues using human adipose-derived stem cells (hADSCs). These methods typically involve culturing hADSCs under specific conditions to induce differentiation into cardiac lineages while promoting vascularization. The approaches may include 3D culture systems, co-culture with endothelial cells, and the use of growth factors to stimulate both cardiomyocyte development and blood vessel formation within the engineered tissues.- Methods for generating vascularized cardiac microtissues from adipose-derived stem cells: Various techniques have been developed to create vascularized cardiac microtissues using human adipose-derived stem cells (hADSCs). These methods typically involve culturing hADSCs under specific conditions that promote their differentiation into cardiac lineages while simultaneously encouraging vascularization. The processes often include three-dimensional culture systems, co-culture with endothelial cells, and the application of mechanical or electrical stimulation to enhance tissue formation and functionality.
- Growth factors and signaling molecules for promoting vascularization: Specific growth factors and signaling molecules play crucial roles in promoting vascularization of cardiac microtissues derived from adipose stem cells. These include vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), and angiopoietins. The controlled release or expression of these factors can significantly enhance the formation of blood vessel networks within the engineered cardiac tissues, improving their viability and functionality.
- Scaffold materials and biomaterials for supporting vascularized cardiac tissue: Various scaffold materials and biomaterials have been developed to support the growth and vascularization of cardiac microtissues. These include natural polymers (such as collagen, fibrin, and hyaluronic acid), synthetic polymers, and decellularized extracellular matrix. The physical and chemical properties of these scaffolds, including porosity, stiffness, and bioactive molecule presentation, can be tailored to enhance cell adhesion, migration, differentiation, and the formation of vascular networks within the cardiac constructs.
- Co-culture systems for enhancing vascularization: Co-culture systems involving adipose-derived stem cells and other cell types have been developed to enhance vascularization of cardiac microtissues. These systems typically combine adipose-derived stem cells with endothelial cells, smooth muscle cells, or cardiac cells. The interaction between these different cell types promotes the formation of more complex and functional vascular networks within the engineered tissues, mimicking the natural cardiac microenvironment and improving tissue viability and function.
- Bioreactor systems for optimizing vascularized cardiac tissue development: Specialized bioreactor systems have been designed to optimize the development of vascularized cardiac tissues from adipose-derived stem cells. These systems provide controlled environments with appropriate mechanical stimulation, electrical stimulation, perfusion, and oxygen tension to promote cell differentiation and vascular network formation. Dynamic culture conditions in these bioreactors can significantly enhance the maturation and functionality of the engineered cardiac tissues, resulting in more physiologically relevant constructs for research and potential therapeutic applications.
02 Scaffolds and biomaterials for vascularized cardiac tissue engineering
Specialized scaffolds and biomaterials play a crucial role in supporting the development of vascularized cardiac microtissues. These materials provide structural support while allowing for cell adhesion, migration, and the formation of vascular networks. Various natural and synthetic biomaterials have been employed, including hydrogels, decellularized extracellular matrix, and polymer-based scaffolds with specific properties to enhance vascularization and cardiac tissue development from adipose-derived stem cells.Expand Specific Solutions03 Growth factors and signaling molecules for promoting vascularization
Specific growth factors and signaling molecules are essential for inducing vascularization in cardiac microtissues derived from adipose stem cells. These bioactive factors include vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and platelet-derived growth factor (PDGF), which stimulate angiogenesis and vasculogenesis. The controlled release or supplementation of these factors in culture systems enhances the formation of functional blood vessels within the engineered cardiac tissues.Expand Specific Solutions04 Co-culture systems for enhanced vascularization of cardiac tissues
Co-culture systems involving adipose-derived stem cells and other cell types have been developed to enhance vascularization in cardiac microtissues. These approaches typically combine hADSCs with endothelial cells, pericytes, or other supporting cells to create a more physiologically relevant microenvironment. The interaction between different cell types promotes the formation of stable vascular networks and improves the functional properties of the engineered cardiac tissues.Expand Specific Solutions05 Clinical applications and therapeutic potential of vascularized cardiac microtissues
Vascularized cardiac microtissues derived from human adipose stem cells show significant potential for clinical applications in cardiac regenerative medicine. These engineered tissues can be used for drug screening, disease modeling, and potentially for treating heart conditions through transplantation. The presence of functional vasculature within these tissues enhances their survival after implantation and improves their integration with host tissue, making them promising candidates for treating myocardial infarction and other cardiac disorders.Expand Specific Solutions
Leading Research Groups and Companies in Cardiac Tissue Engineering
The field of vascularized cardiac microtissues from human adipose-derived stem cells is in an early growth phase, with market size estimated to expand significantly as regenerative medicine advances. The technology sits at the intersection of tissue engineering, microfluidics, and stem cell research, currently at the early-to-mid stage of technical maturity. Leading research institutions like Centre National de la Recherche Scientifique, Arizona State University, and Murdoch Childrens Research Institute are driving fundamental research, while specialized companies such as Heartseed, Inc., Jointechlabs, and iHeart Japan are commercializing applications. Academic-industry partnerships are emerging between universities (Harvard, Keio University) and pharmaceutical/biotech companies (Nissan Chemical), indicating growing commercial interest despite the technology still requiring significant validation for clinical applications.
Heartseed, Inc.
Technical Solution: Heartseed has developed a proprietary technology platform called "Heart-in-a-Dish" that enables the generation of highly purified, metabolically mature, and aligned cardiac tissues from human induced pluripotent stem cells (iPSCs). Their approach incorporates adipose-derived stem cells (ADSCs) as supporting cells to create vascularized cardiac microtissues in microfluidic devices. The platform utilizes a unique suspension culture method that promotes the formation of spherical cardiac tissue with enhanced cell-cell interactions and extracellular matrix development. Their chip-based system incorporates multiple microchannels that allow for controlled nutrient delivery and waste removal, mimicking natural vasculature. Heartseed's technology enables the creation of functional cardiac tissues with synchronized beating patterns and appropriate electrophysiological properties, making them suitable for drug screening and regenerative medicine applications.
Strengths: Highly specialized in cardiac tissue engineering with proprietary purification methods that ensure tissue functionality. Their platform achieves superior cell maturation and alignment compared to conventional methods. Weaknesses: The technology requires sophisticated equipment and expertise, potentially limiting widespread adoption. The scale-up process for clinical applications remains challenging.
President & Fellows of Harvard College
Technical Solution: Harvard's approach to generating vascularized cardiac microtissues utilizes a sophisticated organ-on-chip platform that integrates microfluidic technology with tissue engineering principles. Their system employs a multi-layer chip design with separate compartments for cardiac cells derived from human adipose stem cells and endothelial cells that form vascular networks. The platform incorporates micropatterned surfaces that guide tissue organization and alignment, critical for proper cardiac function. Harvard researchers have pioneered the use of sacrificial hydrogel templates to create perfusable vascular networks within the cardiac constructs, allowing for efficient nutrient and oxygen delivery. Their technology also incorporates electrical stimulation capabilities to promote cardiomyocyte maturation and contractile function. The Harvard platform enables real-time monitoring of tissue function through integrated sensors that measure contractile force, electrical activity, and metabolic parameters.
Strengths: World-class research infrastructure and multidisciplinary expertise in microfluidics, materials science, and stem cell biology. Their platforms achieve high physiological relevance with integrated sensing capabilities. Weaknesses: Complex fabrication processes may limit reproducibility and scalability. The technology requires significant expertise to implement effectively.
Key Technologies in Adipose-Derived Stem Cell Differentiation
Vascularised cardiac organoid model after incorporation of cardiomyocytes derived from human induced pluripotent stem cells
PatentWO2021123682A1
Innovation
- A vascularized cardiac organoid model is developed using human induced pluripotent stem cells (hiPSC-CM) grafted onto a fertilized egg's chorioallantoic membrane, allowing for spontaneous self-organization and vascularization without surgical intervention, enabling the creation of a flexible and personalized model for studying cardiomyocyte behavior and pharmaceutical effects.
Microfluidic devices and methods incorporating organized three-dimensional tissue constructs
PatentInactiveUS20210054321A1
Innovation
- A microfluidic device with a cell suspension region containing linear arrays of vertical posts that induce alignment of hydrogel-encapsulated tissues, allowing for the creation of a 3D biomimetic human cardiac tissue model that mimics the pathophysiological characteristics of diseased tissues, enabling therapeutic strategy development and functional outcome analysis.
Regulatory Considerations for Engineered Cardiac Tissues
The regulatory landscape for engineered cardiac tissues, particularly vascularized cardiac microtissues derived from human adipose-derived stem cells on chip, presents significant complexity that requires careful navigation. These innovative technologies fall under the jurisdiction of multiple regulatory frameworks globally, with the FDA in the United States and the EMA in Europe being primary authorities.
For clinical translation, these vascularized cardiac microtissues must meet stringent requirements for safety, efficacy, and quality control. The FDA typically classifies such engineered tissues as combination products, involving both biological and device components, necessitating review by multiple centers within the agency. The Center for Biologics Evaluation and Research (CBER) and the Center for Devices and Radiological Health (CDRH) would likely collaborate on the regulatory pathway.
Good Manufacturing Practice (GMP) compliance represents a critical regulatory hurdle. Developers must establish robust protocols for stem cell isolation, expansion, differentiation, and incorporation into microfluidic devices. Documentation of aseptic processing, quality control testing, and validation of the manufacturing process is essential for regulatory approval.
Preclinical testing requirements are particularly demanding for these technologies. Comprehensive biocompatibility testing, tumorigenicity assessments, and evaluation of potential immunogenicity are mandatory. Long-term stability studies and functional characterization of the vascularized cardiac microtissues must demonstrate consistent performance and safety profiles.
The regulatory pathway typically involves phased clinical trials, beginning with small safety studies and progressing to larger efficacy trials. For vascularized cardiac microtissues, endpoints may include measures of cardiac function, vascular integration, and tissue viability. Patient selection criteria and monitoring protocols require careful consideration and regulatory approval.
International harmonization efforts, such as those by the International Council for Harmonisation (ICH), are increasingly important for global development of these technologies. Developers should engage with multiple regulatory agencies early in the development process to align expectations and requirements across jurisdictions.
Ethical considerations also factor into the regulatory framework, particularly regarding informed consent for stem cell donation, genetic modification protocols, and potential therapeutic applications. Institutional Review Board (IRB) approval and compliance with national and international ethical guidelines are mandatory components of the regulatory process.
For clinical translation, these vascularized cardiac microtissues must meet stringent requirements for safety, efficacy, and quality control. The FDA typically classifies such engineered tissues as combination products, involving both biological and device components, necessitating review by multiple centers within the agency. The Center for Biologics Evaluation and Research (CBER) and the Center for Devices and Radiological Health (CDRH) would likely collaborate on the regulatory pathway.
Good Manufacturing Practice (GMP) compliance represents a critical regulatory hurdle. Developers must establish robust protocols for stem cell isolation, expansion, differentiation, and incorporation into microfluidic devices. Documentation of aseptic processing, quality control testing, and validation of the manufacturing process is essential for regulatory approval.
Preclinical testing requirements are particularly demanding for these technologies. Comprehensive biocompatibility testing, tumorigenicity assessments, and evaluation of potential immunogenicity are mandatory. Long-term stability studies and functional characterization of the vascularized cardiac microtissues must demonstrate consistent performance and safety profiles.
The regulatory pathway typically involves phased clinical trials, beginning with small safety studies and progressing to larger efficacy trials. For vascularized cardiac microtissues, endpoints may include measures of cardiac function, vascular integration, and tissue viability. Patient selection criteria and monitoring protocols require careful consideration and regulatory approval.
International harmonization efforts, such as those by the International Council for Harmonisation (ICH), are increasingly important for global development of these technologies. Developers should engage with multiple regulatory agencies early in the development process to align expectations and requirements across jurisdictions.
Ethical considerations also factor into the regulatory framework, particularly regarding informed consent for stem cell donation, genetic modification protocols, and potential therapeutic applications. Institutional Review Board (IRB) approval and compliance with national and international ethical guidelines are mandatory components of the regulatory process.
Translational Potential and Clinical Applications
The vascularized cardiac microtissues derived from human adipose-derived stem cells (hADSCs) on microfluidic chips represent a significant advancement with substantial translational potential for cardiovascular medicine. These engineered tissues address critical limitations in current cardiac disease modeling and therapeutic development by providing physiologically relevant human tissue models with functional vasculature.
In clinical diagnostics, these vascularized microtissues offer personalized medicine applications through patient-specific disease modeling. By generating tissues from a patient's own adipose-derived stem cells, clinicians can test drug responses and optimize treatment strategies before administration, potentially reducing adverse effects and improving therapeutic outcomes. This approach is particularly valuable for cardiovascular diseases where patient-specific responses to medications vary significantly.
For drug development and toxicology screening, these vascularized cardiac models provide more predictive platforms than traditional 2D cell cultures or animal models. Pharmaceutical companies can utilize these systems to evaluate cardiotoxicity profiles of candidate compounds earlier in the development pipeline, potentially reducing the high attrition rates and costs associated with late-stage drug failures. The presence of functional vasculature allows for more accurate assessment of drug distribution, metabolism, and side effects.
Regenerative medicine applications represent perhaps the most transformative potential of this technology. The ability to generate functional, vascularized cardiac tissues opens avenues for developing transplantable cardiac patches or even whole tissue constructs for patients with myocardial damage. The use of autologous hADSCs mitigates immunological rejection concerns while providing a relatively accessible and abundant stem cell source compared to other options.
Current clinical translation faces several challenges, including scalability of production, standardization of protocols, and regulatory considerations. However, ongoing collaborations between academic institutions, regulatory bodies, and industry partners are addressing these barriers. Early-stage clinical trials utilizing similar technologies for cardiac repair demonstrate promising safety profiles, suggesting a viable pathway toward clinical implementation.
The economic impact of successful translation would be substantial, potentially reducing healthcare costs associated with cardiovascular diseases through improved treatment efficacy and reduced hospitalization rates. Market analysis indicates growing interest from pharmaceutical companies and biotechnology firms in adopting these advanced tissue models, with projected market value exceeding $500 million by 2028 for organ-on-chip technologies in cardiovascular applications alone.
In clinical diagnostics, these vascularized microtissues offer personalized medicine applications through patient-specific disease modeling. By generating tissues from a patient's own adipose-derived stem cells, clinicians can test drug responses and optimize treatment strategies before administration, potentially reducing adverse effects and improving therapeutic outcomes. This approach is particularly valuable for cardiovascular diseases where patient-specific responses to medications vary significantly.
For drug development and toxicology screening, these vascularized cardiac models provide more predictive platforms than traditional 2D cell cultures or animal models. Pharmaceutical companies can utilize these systems to evaluate cardiotoxicity profiles of candidate compounds earlier in the development pipeline, potentially reducing the high attrition rates and costs associated with late-stage drug failures. The presence of functional vasculature allows for more accurate assessment of drug distribution, metabolism, and side effects.
Regenerative medicine applications represent perhaps the most transformative potential of this technology. The ability to generate functional, vascularized cardiac tissues opens avenues for developing transplantable cardiac patches or even whole tissue constructs for patients with myocardial damage. The use of autologous hADSCs mitigates immunological rejection concerns while providing a relatively accessible and abundant stem cell source compared to other options.
Current clinical translation faces several challenges, including scalability of production, standardization of protocols, and regulatory considerations. However, ongoing collaborations between academic institutions, regulatory bodies, and industry partners are addressing these barriers. Early-stage clinical trials utilizing similar technologies for cardiac repair demonstrate promising safety profiles, suggesting a viable pathway toward clinical implementation.
The economic impact of successful translation would be substantial, potentially reducing healthcare costs associated with cardiovascular diseases through improved treatment efficacy and reduced hospitalization rates. Market analysis indicates growing interest from pharmaceutical companies and biotechnology firms in adopting these advanced tissue models, with projected market value exceeding $500 million by 2028 for organ-on-chip technologies in cardiovascular applications alone.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!