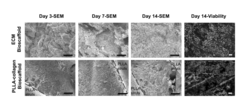
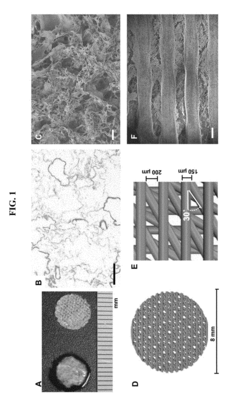
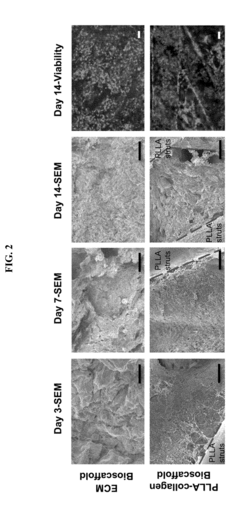
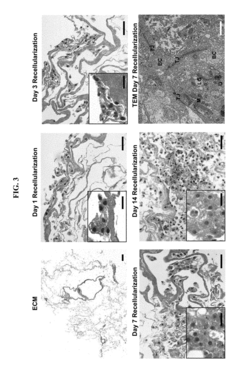
Functional characterization of iPSC-derived hepatocyte zonation under microfluidic oxygen gradients
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
iPSC-Derived Hepatocyte Zonation Background and Objectives
The field of hepatocyte zonation research has evolved significantly over the past decades, transitioning from basic observations of liver metabolic heterogeneity to sophisticated understanding of zone-specific functions. Initially identified in the 1970s through histochemical studies, liver zonation describes the spatial organization of hepatocytes with distinct metabolic functions along the porto-central axis. This phenomenon is critical for proper liver function, including detoxification, protein synthesis, and metabolic homeostasis.
Recent technological advances have enabled more precise characterization of hepatic zonation, particularly through single-cell RNA sequencing and spatial transcriptomics. These approaches have revealed comprehensive gene expression patterns that define the functional specialization of hepatocytes across different zones. The integration of these molecular insights with tissue engineering represents a frontier in liver biology research.
Induced pluripotent stem cell (iPSC) technology has emerged as a revolutionary platform for generating patient-specific hepatocytes, offering unprecedented opportunities for personalized medicine and disease modeling. However, conventional iPSC-derived hepatocyte cultures typically lack the zonation patterns observed in native liver tissue, limiting their physiological relevance and applicability in drug development and toxicology studies.
Microfluidic systems have recently gained attention for their ability to recreate physiological microenvironments, including oxygen gradients that naturally occur in the liver lobule. These gradients are now recognized as primary drivers of hepatic zonation, influencing gene expression patterns and metabolic functions. The combination of iPSC technology with microfluidic platforms presents a promising approach to recapitulate zonation in vitro.
The primary objective of this research is to develop and characterize a microfluidic system capable of establishing controlled oxygen gradients that induce zonation in iPSC-derived hepatocytes. This system aims to mimic the physiological oxygen distribution observed in liver sinusoids, ranging from oxygen-rich periportal regions to relatively hypoxic pericentral areas.
Secondary objectives include the comprehensive functional characterization of zone-specific hepatocyte phenotypes under these conditions, validation of zonation markers through transcriptomic and proteomic analyses, and assessment of zone-dependent drug metabolism and toxicity responses. The ultimate goal is to establish a physiologically relevant in vitro model that accurately reflects the functional heterogeneity of the human liver.
This research addresses a critical gap in current liver models and has significant implications for drug development, toxicology testing, and understanding liver pathophysiology. By recreating the complex microenvironment of the liver lobule, this technology could substantially improve the predictive value of preclinical studies and reduce reliance on animal testing in pharmaceutical research.
Recent technological advances have enabled more precise characterization of hepatic zonation, particularly through single-cell RNA sequencing and spatial transcriptomics. These approaches have revealed comprehensive gene expression patterns that define the functional specialization of hepatocytes across different zones. The integration of these molecular insights with tissue engineering represents a frontier in liver biology research.
Induced pluripotent stem cell (iPSC) technology has emerged as a revolutionary platform for generating patient-specific hepatocytes, offering unprecedented opportunities for personalized medicine and disease modeling. However, conventional iPSC-derived hepatocyte cultures typically lack the zonation patterns observed in native liver tissue, limiting their physiological relevance and applicability in drug development and toxicology studies.
Microfluidic systems have recently gained attention for their ability to recreate physiological microenvironments, including oxygen gradients that naturally occur in the liver lobule. These gradients are now recognized as primary drivers of hepatic zonation, influencing gene expression patterns and metabolic functions. The combination of iPSC technology with microfluidic platforms presents a promising approach to recapitulate zonation in vitro.
The primary objective of this research is to develop and characterize a microfluidic system capable of establishing controlled oxygen gradients that induce zonation in iPSC-derived hepatocytes. This system aims to mimic the physiological oxygen distribution observed in liver sinusoids, ranging from oxygen-rich periportal regions to relatively hypoxic pericentral areas.
Secondary objectives include the comprehensive functional characterization of zone-specific hepatocyte phenotypes under these conditions, validation of zonation markers through transcriptomic and proteomic analyses, and assessment of zone-dependent drug metabolism and toxicity responses. The ultimate goal is to establish a physiologically relevant in vitro model that accurately reflects the functional heterogeneity of the human liver.
This research addresses a critical gap in current liver models and has significant implications for drug development, toxicology testing, and understanding liver pathophysiology. By recreating the complex microenvironment of the liver lobule, this technology could substantially improve the predictive value of preclinical studies and reduce reliance on animal testing in pharmaceutical research.
Market Analysis for Liver-on-Chip Technologies
The liver-on-chip technology market is experiencing significant growth, driven by increasing demand for more physiologically relevant in vitro models for drug development and toxicity testing. The global market for organ-on-chip technologies was valued at approximately $21 million in 2019 and is projected to reach $220 million by 2025, with liver models representing one of the largest segments at roughly 25% of the total market share.
The primary market drivers include the pharmaceutical industry's need to reduce drug development costs and timelines, with an estimated $2.6 billion spent per successful drug development cycle. Liver toxicity remains a leading cause of drug failures and market withdrawals, creating urgent demand for better predictive models. Additionally, regulatory pressures to reduce animal testing, particularly in Europe following Directive 2010/63/EU, have accelerated adoption of alternative testing methods.
Market segmentation reveals three primary customer groups: pharmaceutical companies (65% of market), academic research institutions (25%), and contract research organizations (10%). Geographically, North America leads with 45% market share, followed by Europe (30%) and Asia-Pacific (20%), with the latter showing the fastest growth rate at approximately 25% annually.
The technology addressing iPSC-derived hepatocyte zonation under microfluidic oxygen gradients specifically targets a high-value niche within this market. This approach addresses the critical need to replicate the oxygen gradient-dependent zonation of liver functions, which is essential for accurate drug metabolism and toxicity prediction. Current estimates suggest this specialized segment could capture 15-20% of the liver-on-chip market within five years.
Key market challenges include high unit costs ($5,000-15,000 per device), technical complexity requiring specialized expertise, and regulatory uncertainty regarding validation and standardization. Despite these challenges, the compound annual growth rate for microfluidic liver models is projected at 35%, significantly outpacing traditional cell culture systems.
Investment in this sector has been robust, with venture capital funding exceeding $400 million between 2018-2021 for organ-on-chip companies. Strategic partnerships between technology developers and pharmaceutical companies have become increasingly common, with over 30 major collaborations announced in the past three years, indicating strong industry confidence in the technology's commercial potential.
The primary market drivers include the pharmaceutical industry's need to reduce drug development costs and timelines, with an estimated $2.6 billion spent per successful drug development cycle. Liver toxicity remains a leading cause of drug failures and market withdrawals, creating urgent demand for better predictive models. Additionally, regulatory pressures to reduce animal testing, particularly in Europe following Directive 2010/63/EU, have accelerated adoption of alternative testing methods.
Market segmentation reveals three primary customer groups: pharmaceutical companies (65% of market), academic research institutions (25%), and contract research organizations (10%). Geographically, North America leads with 45% market share, followed by Europe (30%) and Asia-Pacific (20%), with the latter showing the fastest growth rate at approximately 25% annually.
The technology addressing iPSC-derived hepatocyte zonation under microfluidic oxygen gradients specifically targets a high-value niche within this market. This approach addresses the critical need to replicate the oxygen gradient-dependent zonation of liver functions, which is essential for accurate drug metabolism and toxicity prediction. Current estimates suggest this specialized segment could capture 15-20% of the liver-on-chip market within five years.
Key market challenges include high unit costs ($5,000-15,000 per device), technical complexity requiring specialized expertise, and regulatory uncertainty regarding validation and standardization. Despite these challenges, the compound annual growth rate for microfluidic liver models is projected at 35%, significantly outpacing traditional cell culture systems.
Investment in this sector has been robust, with venture capital funding exceeding $400 million between 2018-2021 for organ-on-chip companies. Strategic partnerships between technology developers and pharmaceutical companies have become increasingly common, with over 30 major collaborations announced in the past three years, indicating strong industry confidence in the technology's commercial potential.
Current Challenges in Hepatic Zonation Modeling
Despite significant advancements in liver tissue engineering, creating accurate in vitro models of hepatic zonation remains a formidable challenge. Current hepatic zonation models struggle to recapitulate the complex oxygen gradient that exists along the liver sinusoid, which is crucial for proper metabolic zonation. Traditional static culture systems fail to maintain the dynamic microenvironment necessary for zone-specific hepatocyte function, resulting in homogeneous cell populations that do not reflect in vivo heterogeneity.
The use of iPSC-derived hepatocytes introduces additional complexities, as these cells often exhibit immature phenotypes compared to primary hepatocytes. While iPSCs offer advantages in terms of availability and reproducibility, their functional maturation into zone-specific hepatocytes has proven difficult to achieve consistently. Current differentiation protocols produce hepatocyte-like cells with mixed phenotypes rather than distinct zonation characteristics.
Microfluidic systems, while promising for creating oxygen gradients, face technical hurdles in maintaining stable gradients over extended periods required for hepatocyte maturation. The integration of oxygen sensors within these systems remains challenging, limiting real-time monitoring capabilities essential for experimental validation. Additionally, current microfluidic designs often prioritize either gradient generation or cell culture compatibility, rarely optimizing both simultaneously.
Analytical methods for assessing zone-specific functions present another significant obstacle. Current techniques lack the spatial resolution to detect subtle changes in gene expression and metabolic activities across the artificial sinusoid. This hampers efforts to validate whether engineered gradients truly induce physiologically relevant zonation patterns in iPSC-derived hepatocytes.
The translation of research findings to standardized protocols represents a persistent challenge. Variability in iPSC lines, differentiation efficiencies, and microfluidic system designs has resulted in poor reproducibility across laboratories. This inconsistency impedes progress toward establishing reliable models for drug metabolism studies and toxicity screening.
Interdisciplinary barriers further complicate advancement in this field. Effective hepatic zonation modeling requires expertise spanning stem cell biology, microfluidics engineering, hepatology, and systems biology. The limited cross-disciplinary collaboration has slowed innovation and implementation of comprehensive solutions.
Regulatory considerations and clinical translation pathways remain underdeveloped for these advanced in vitro models. Without clear frameworks for validation against in vivo data, the adoption of zonated hepatocyte models in drug development pipelines faces significant hurdles, despite their potential to provide more physiologically relevant data than current preclinical models.
The use of iPSC-derived hepatocytes introduces additional complexities, as these cells often exhibit immature phenotypes compared to primary hepatocytes. While iPSCs offer advantages in terms of availability and reproducibility, their functional maturation into zone-specific hepatocytes has proven difficult to achieve consistently. Current differentiation protocols produce hepatocyte-like cells with mixed phenotypes rather than distinct zonation characteristics.
Microfluidic systems, while promising for creating oxygen gradients, face technical hurdles in maintaining stable gradients over extended periods required for hepatocyte maturation. The integration of oxygen sensors within these systems remains challenging, limiting real-time monitoring capabilities essential for experimental validation. Additionally, current microfluidic designs often prioritize either gradient generation or cell culture compatibility, rarely optimizing both simultaneously.
Analytical methods for assessing zone-specific functions present another significant obstacle. Current techniques lack the spatial resolution to detect subtle changes in gene expression and metabolic activities across the artificial sinusoid. This hampers efforts to validate whether engineered gradients truly induce physiologically relevant zonation patterns in iPSC-derived hepatocytes.
The translation of research findings to standardized protocols represents a persistent challenge. Variability in iPSC lines, differentiation efficiencies, and microfluidic system designs has resulted in poor reproducibility across laboratories. This inconsistency impedes progress toward establishing reliable models for drug metabolism studies and toxicity screening.
Interdisciplinary barriers further complicate advancement in this field. Effective hepatic zonation modeling requires expertise spanning stem cell biology, microfluidics engineering, hepatology, and systems biology. The limited cross-disciplinary collaboration has slowed innovation and implementation of comprehensive solutions.
Regulatory considerations and clinical translation pathways remain underdeveloped for these advanced in vitro models. Without clear frameworks for validation against in vivo data, the adoption of zonated hepatocyte models in drug development pipelines faces significant hurdles, despite their potential to provide more physiologically relevant data than current preclinical models.
Established Methodologies for Hepatocyte Functional Characterization
01 Methods for generating iPSC-derived hepatocytes with zonation patterns
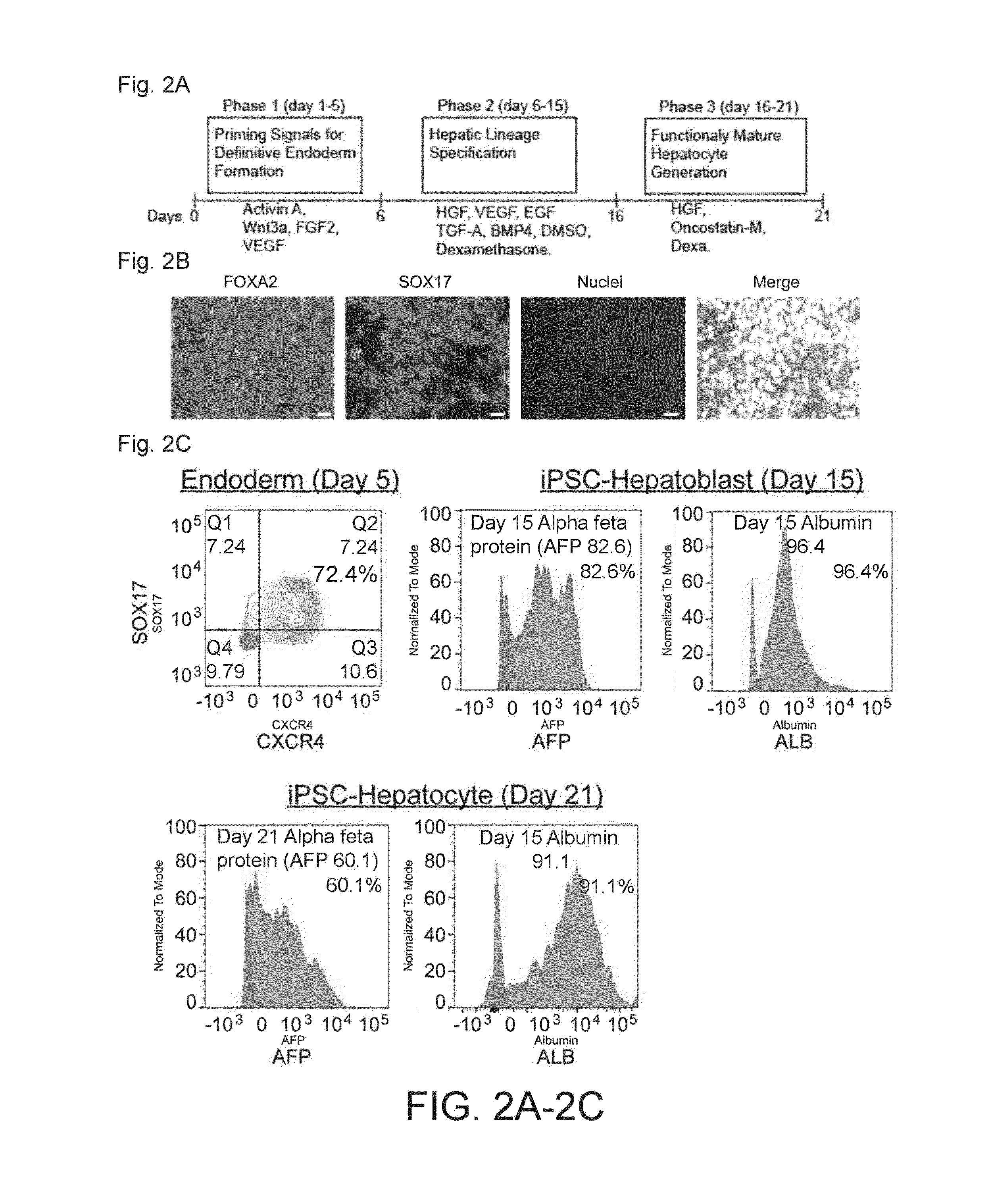
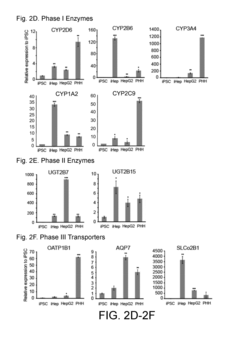
Various techniques have been developed to generate induced pluripotent stem cell (iPSC)-derived hepatocytes that exhibit zonation patterns similar to those found in the liver. These methods involve specific culture conditions, growth factors, and signaling molecules to induce differentiation of iPSCs into hepatocytes with zone-specific characteristics. The resulting hepatocytes display metabolic and functional properties that correspond to different zones of the liver lobule, which is crucial for accurate disease modeling and drug testing.- Methods for generating iPSC-derived hepatocytes with zonation patterns: Various methods have been developed to generate induced pluripotent stem cell (iPSC)-derived hepatocytes that exhibit zonation patterns similar to those found in the native liver. These methods typically involve specific culture conditions, growth factors, and signaling molecules that guide the differentiation of iPSCs into hepatocytes with zone-specific characteristics. The resulting hepatocytes display differential gene expression and metabolic functions that mimic the zonation observed in liver lobules, which is crucial for modeling liver physiology and disease.
- Microfluidic systems for establishing hepatic zonation: Microfluidic devices and bioreactors have been designed to create oxygen and nutrient gradients that induce zonation in iPSC-derived hepatocytes. These systems mimic the physiological microenvironment of the liver lobule, where hepatocytes are exposed to varying concentrations of oxygen, hormones, and nutrients along the portal-central vein axis. By controlling these gradients, researchers can generate hepatocytes with zone-specific functions and gene expression profiles, enabling more accurate modeling of liver metabolism and toxicity responses.
- Genetic and molecular markers for hepatic zonation characterization: Specific genetic and molecular markers are used to characterize and validate the zonation patterns in iPSC-derived hepatocytes. These markers include zone-specific enzymes, transporters, and transcription factors that are differentially expressed across the liver lobule. By analyzing the expression of these markers, researchers can confirm the presence of zonation in their hepatocyte cultures and assess the functionality of different hepatic zones. This approach is essential for developing accurate liver models for drug metabolism studies and disease modeling.
- Applications of zonated iPSC-derived hepatocytes in drug testing and disease modeling: Zonated iPSC-derived hepatocytes have significant applications in pharmaceutical research, toxicology testing, and disease modeling. These hepatocytes can recapitulate zone-specific drug metabolism and toxicity responses, providing more predictive models for drug development. They are also valuable for studying zone-specific liver diseases, such as non-alcoholic fatty liver disease, which predominantly affects certain zones of the liver lobule. The ability to model zonation in vitro allows for more accurate prediction of drug-induced liver injury and better understanding of liver pathophysiology.
- 3D culture systems for enhancing hepatic zonation: Three-dimensional culture systems have been developed to enhance the formation of zonation patterns in iPSC-derived hepatocytes. These systems include organoids, spheroids, and bioprinted constructs that better mimic the spatial organization of the liver lobule. The 3D architecture allows for the establishment of physiological cell-cell interactions and extracellular matrix composition, which are critical for proper hepatocyte differentiation and zonation. These advanced culture systems provide more accurate models of liver function and zonation compared to traditional 2D cultures.
02 Microfluidic systems for establishing hepatic zonation
Microfluidic devices and bioreactors have been designed to create oxygen and nutrient gradients that mimic the zonation patterns observed in the liver. These systems allow for the culture of iPSC-derived hepatocytes under conditions that recreate the microenvironment of different hepatic zones. By controlling factors such as oxygen tension, nutrient availability, and fluid flow, these platforms enable the development of hepatocytes with zone-specific functions and gene expression profiles.Expand Specific Solutions03 Genetic modification approaches for hepatic zonation
Genetic engineering techniques are employed to induce or enhance zonation patterns in iPSC-derived hepatocytes. These approaches involve the manipulation of key transcription factors and signaling pathways that regulate hepatic zonation, such as Wnt/β-catenin, Hnf4α, and hypoxia-inducible factors. By modulating the expression of these factors, researchers can generate hepatocytes that exhibit zone-specific gene expression patterns and metabolic functions, providing valuable tools for studying liver biology and disease.Expand Specific Solutions04 3D culture systems for enhancing hepatocyte zonation
Three-dimensional culture systems, including organoids and spheroids, have been developed to better recapitulate the spatial organization and zonation patterns of the liver. These systems provide a more physiologically relevant environment for iPSC-derived hepatocytes, allowing them to form complex structures with zone-specific characteristics. The 3D architecture facilitates cell-cell interactions and the establishment of gradients that drive zonation, resulting in hepatocytes that more closely resemble their in vivo counterparts.Expand Specific Solutions05 Applications of zonated iPSC-derived hepatocytes
Zonated iPSC-derived hepatocytes have numerous applications in drug development, toxicology testing, and disease modeling. These specialized cells enable more accurate prediction of zone-specific drug metabolism and toxicity, as well as improved modeling of liver diseases that affect particular zones. Additionally, zonated hepatocytes derived from patient-specific iPSCs can be used for personalized medicine approaches, including drug screening and the development of tailored therapeutic strategies.Expand Specific Solutions
Leading Organizations in iPSC-Derived Liver Models
The field of iPSC-derived hepatocyte zonation under microfluidic oxygen gradients is in an early growth phase, with market size estimated at $1.2-1.5 billion and expanding at 15-20% annually. The competitive landscape features academic institutions (University of North Carolina, Zhejiang University, Sichuan University) leading fundamental research, while specialized biotechnology companies (Emulate, FUJIFILM Cellular Dynamics, TreeFrog Therapeutics) are commercializing microfluidic platforms and cell technologies. Pharmaceutical companies are increasingly adopting these technologies for drug development and toxicity testing. Technical maturity remains moderate, with Emulate's organ-on-chip technology representing the most advanced commercial application, while companies like Vesta Therapeutics and Fate Therapeutics are developing therapeutic applications. Research institutions like Agency for Science, Technology & Research and Draper Laboratory are advancing the underlying microfluidic technologies.
TreeFrog Therapeutics SAS
Technical Solution: TreeFrog Therapeutics has developed C-Stem™ technology, a unique approach for large-scale production of iPSC-derived hepatocytes that can be applied to zonation studies in microfluidic systems. Their technology encapsulates iPSCs in biomimetic shells that promote 3D cell growth and differentiation while protecting cells from shear stress in microfluidic environments. For hepatocyte zonation studies, TreeFrog has adapted their platform to incorporate oxygen gradient controllers that establish physiologically relevant zones within their 3D hepatocyte cultures. The company's approach allows for the simultaneous culture of millions of hepatocytes in a single microfluidic device while maintaining zonation patterns. Their system has demonstrated the ability to recapitulate zone-specific drug metabolism and toxicity profiles, making it particularly valuable for pharmaceutical applications. TreeFrog's technology also enables the study of dynamic changes in zonation patterns in response to varying oxygen conditions or drug treatments.
Strengths: Unique 3D encapsulation technology that protects cells from shear stress; capability for large-scale production of zonated hepatocytes; excellent cell viability and functionality in long-term culture. Weaknesses: Relatively new technology with fewer published validation studies; complex implementation requiring specialized equipment; higher initial investment compared to conventional methods.
The Charles Stark Draper Laboratory, Inc.
Technical Solution: Draper Laboratory has developed advanced microfluidic liver platforms specifically designed for studying hepatocyte zonation under controlled oxygen gradients. Their Human Organ Systems (HOS) technology incorporates precision-engineered microchannels with integrated oxygen sensors that allow for real-time monitoring and adjustment of oxygen levels across the culture chamber. For iPSC-derived hepatocyte studies, Draper has created specialized culture surfaces that promote cell adhesion and maintenance of differentiated functions. Their system features multiple parallel channels that enable the simultaneous testing of different conditions or compounds while maintaining consistent oxygen gradients. Draper's platform has been validated for pharmaceutical applications, demonstrating zone-specific drug metabolism and toxicity responses that correlate with in vivo observations. The technology also incorporates computational modeling capabilities that predict oxygen distribution and cellular responses, allowing researchers to optimize experimental conditions for specific applications.
Strengths: Highly sophisticated engineering with precise control over microfluidic parameters; integrated sensing capabilities for real-time monitoring; strong computational modeling support. Weaknesses: Complex system requiring specialized technical expertise; higher cost compared to simpler microfluidic platforms; limited commercial availability outside of partnerships.
Key Technical Innovations in Microfluidic Liver Zonation
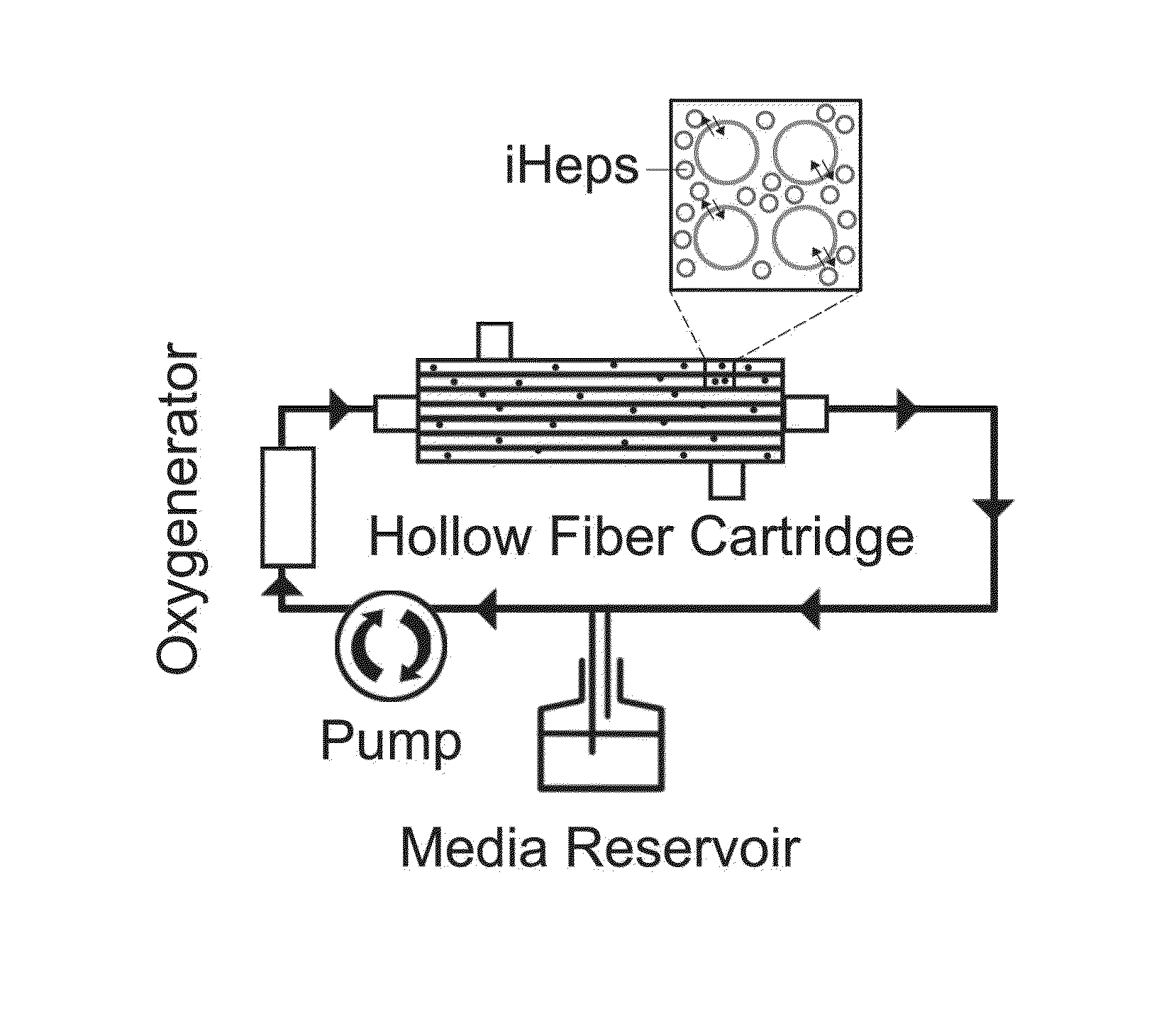
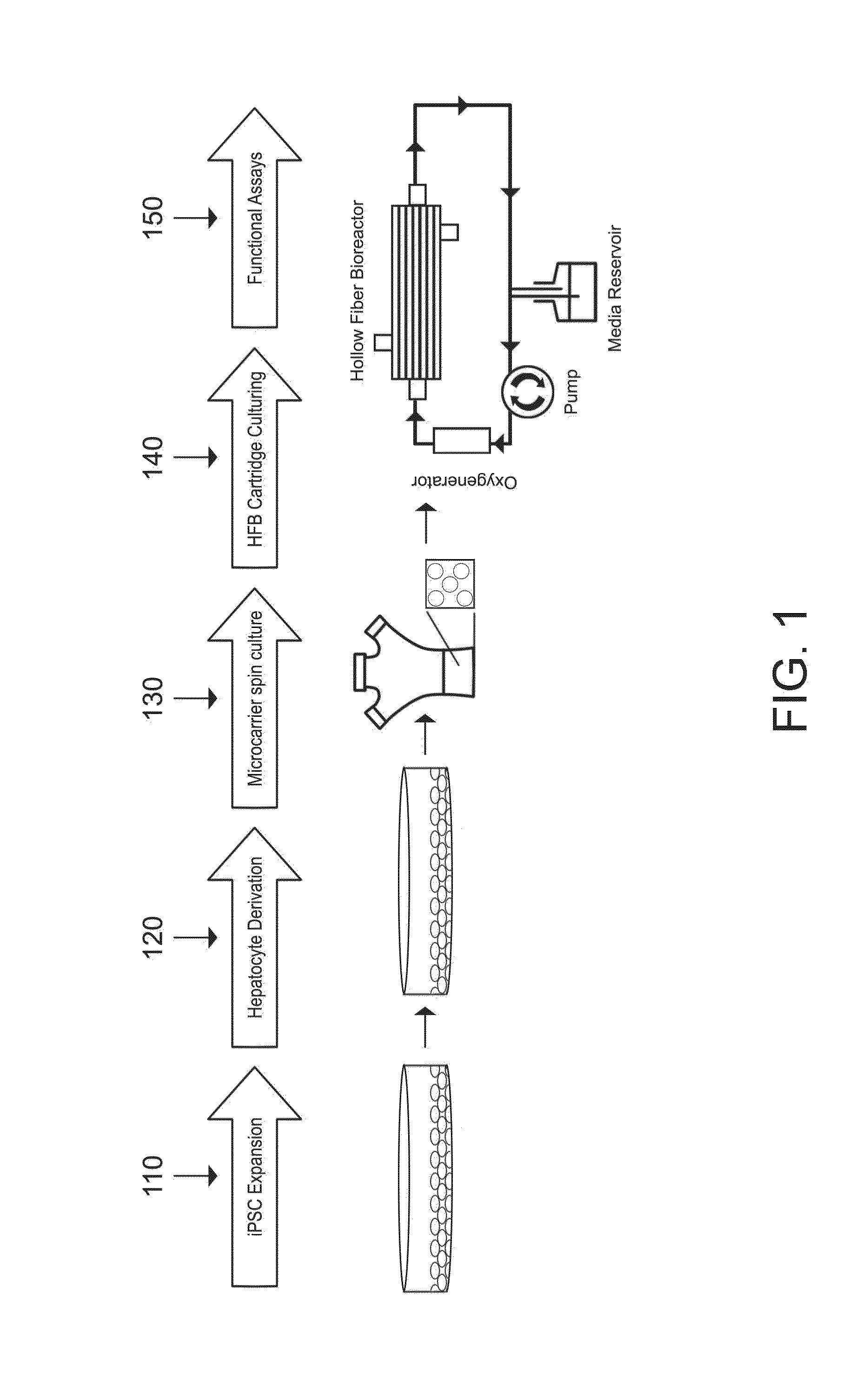
Induced pluripotent stem cell-derived hepatocyte based bioartificial liver device
PatentInactiveUS20160256672A1
Innovation
- Development of a bioartificial liver device using metabolically active human hepatocytes derived from induced pluripotent stem cells (iPSCs), which are differentiated and matured in a hollow fiber bioreactor, providing a nearly unlimited supply and reducing the risk of disease transmission, and mimicking the liver's microarchitecture for efficient detoxification and protein secretion.
Acellular scaffolds for maturation of ipsc-hepatocytes
PatentInactiveUS20180016548A1
Innovation
- The use of decellularized extracellular matrix (ECM) as a bioscaffold to mature iPSC-derived hepatocytes, enhancing their hepatocytic biomarkers and functionality, thereby improving liver-specific enzyme expression and activity.
Regulatory Considerations for Organ-on-Chip Technologies
The regulatory landscape for organ-on-chip (OOC) technologies, particularly those involving iPSC-derived hepatocyte zonation under microfluidic oxygen gradients, presents complex challenges that require careful navigation. These microphysiological systems occupy an intersection between medical devices, cell therapies, and in vitro diagnostic tools, creating regulatory ambiguity.
The FDA has begun addressing these technologies through its regulatory framework, primarily through the Center for Devices and Radiological Health (CDRH) and the Center for Biologics Evaluation and Research (CBER). For microfluidic systems modeling hepatic zonation, qualification processes focus on demonstrating reproducibility of oxygen gradients and consistent cellular responses across different batches of iPSC-derived hepatocytes.
European regulatory bodies approach these technologies under the Medical Device Regulation (MDR) framework, with specific attention to risk classification based on intended use. Systems designed for toxicology screening face different requirements than those intended for personalized medicine applications, with the latter requiring more stringent validation protocols.
Validation requirements present significant hurdles, as regulatory agencies increasingly demand correlation between in vitro microfluidic models and in vivo outcomes. For hepatocyte zonation models, this necessitates demonstration that zone-specific metabolic functions under oxygen gradients accurately reflect physiological conditions, supported by comparative clinical data.
Quality control standards for iPSC-derived hepatocytes introduce additional complexity, requiring standardized protocols for cell characterization, differentiation efficiency, and functional stability under varying oxygen tensions. The International Council for Harmonisation (ICH) guidelines provide some direction, but specific standards for zonated liver models remain underdeveloped.
Data integrity and reproducibility standards are particularly stringent for these technologies. Regulatory bodies require comprehensive documentation of microfluidic design parameters, oxygen gradient stability, and cellular response metrics across multiple experimental runs and manufacturing lots.
Looking forward, regulatory frameworks are evolving toward adaptive licensing approaches that accommodate the iterative development nature of OOC technologies. This includes provisional approval pathways that allow market entry with continued post-market surveillance and data collection, particularly valuable for novel applications like zonated hepatocyte models.
International harmonization efforts through organizations like the OECD and ISO are working to establish standardized validation protocols specifically for microphysiological systems, which will facilitate global acceptance of data generated using these platforms and streamline regulatory approval processes across different jurisdictions.
The FDA has begun addressing these technologies through its regulatory framework, primarily through the Center for Devices and Radiological Health (CDRH) and the Center for Biologics Evaluation and Research (CBER). For microfluidic systems modeling hepatic zonation, qualification processes focus on demonstrating reproducibility of oxygen gradients and consistent cellular responses across different batches of iPSC-derived hepatocytes.
European regulatory bodies approach these technologies under the Medical Device Regulation (MDR) framework, with specific attention to risk classification based on intended use. Systems designed for toxicology screening face different requirements than those intended for personalized medicine applications, with the latter requiring more stringent validation protocols.
Validation requirements present significant hurdles, as regulatory agencies increasingly demand correlation between in vitro microfluidic models and in vivo outcomes. For hepatocyte zonation models, this necessitates demonstration that zone-specific metabolic functions under oxygen gradients accurately reflect physiological conditions, supported by comparative clinical data.
Quality control standards for iPSC-derived hepatocytes introduce additional complexity, requiring standardized protocols for cell characterization, differentiation efficiency, and functional stability under varying oxygen tensions. The International Council for Harmonisation (ICH) guidelines provide some direction, but specific standards for zonated liver models remain underdeveloped.
Data integrity and reproducibility standards are particularly stringent for these technologies. Regulatory bodies require comprehensive documentation of microfluidic design parameters, oxygen gradient stability, and cellular response metrics across multiple experimental runs and manufacturing lots.
Looking forward, regulatory frameworks are evolving toward adaptive licensing approaches that accommodate the iterative development nature of OOC technologies. This includes provisional approval pathways that allow market entry with continued post-market surveillance and data collection, particularly valuable for novel applications like zonated hepatocyte models.
International harmonization efforts through organizations like the OECD and ISO are working to establish standardized validation protocols specifically for microphysiological systems, which will facilitate global acceptance of data generated using these platforms and streamline regulatory approval processes across different jurisdictions.
Translational Applications in Drug Development and Toxicology
The integration of iPSC-derived hepatocyte zonation models with microfluidic oxygen gradients represents a significant advancement in translational medicine, particularly for drug development and toxicology applications. These biomimetic systems offer unprecedented opportunities to predict human-specific drug responses and toxicity profiles with greater accuracy than traditional testing methods.
Pharmaceutical companies can leverage these advanced liver models to evaluate drug metabolism and toxicity in pre-clinical stages, potentially reducing the high attrition rates currently plaguing drug development pipelines. The ability to recapitulate zone-specific metabolic functions allows for more precise assessment of zone-dependent drug metabolism, which is crucial for compounds primarily processed in specific hepatic regions.
The technology enables improved detection of idiosyncratic drug-induced liver injury (DILI), a leading cause of drug withdrawal from markets. By incorporating patient-specific iPSCs, these systems can identify population subgroups at higher risk for adverse reactions, supporting the development of personalized medicine approaches and potentially saving billions in development costs and post-market complications.
For toxicological screening, these microfluidic liver models provide more physiologically relevant alternatives to animal testing, addressing both ethical concerns and the limitations of interspecies differences. The controlled oxygen gradients allow for the study of zone-specific toxicity mechanisms and the evaluation of chronic low-dose exposures that may affect particular metabolic zones differently.
Regulatory agencies are increasingly recognizing the value of such advanced in vitro models. The technology aligns with global initiatives to reduce animal testing while improving predictive capabilities, potentially accelerating the path toward regulatory acceptance of alternative testing methods for safety assessment.
The pharmaceutical industry can also benefit from these systems for mechanistic investigations of drug-drug interactions, particularly those involving zone-specific enzymes. This application is especially valuable for polypharmacy scenarios common in aging populations and patients with multiple comorbidities.
Furthermore, the technology offers opportunities for developing liver-on-chip platforms for high-throughput screening, enabling more efficient early-stage drug candidate evaluation and toxicity assessment. Integration with other organ-on-chip systems could eventually lead to multi-organ platforms capable of modeling complex systemic drug effects and metabolite-mediated toxicity.
Pharmaceutical companies can leverage these advanced liver models to evaluate drug metabolism and toxicity in pre-clinical stages, potentially reducing the high attrition rates currently plaguing drug development pipelines. The ability to recapitulate zone-specific metabolic functions allows for more precise assessment of zone-dependent drug metabolism, which is crucial for compounds primarily processed in specific hepatic regions.
The technology enables improved detection of idiosyncratic drug-induced liver injury (DILI), a leading cause of drug withdrawal from markets. By incorporating patient-specific iPSCs, these systems can identify population subgroups at higher risk for adverse reactions, supporting the development of personalized medicine approaches and potentially saving billions in development costs and post-market complications.
For toxicological screening, these microfluidic liver models provide more physiologically relevant alternatives to animal testing, addressing both ethical concerns and the limitations of interspecies differences. The controlled oxygen gradients allow for the study of zone-specific toxicity mechanisms and the evaluation of chronic low-dose exposures that may affect particular metabolic zones differently.
Regulatory agencies are increasingly recognizing the value of such advanced in vitro models. The technology aligns with global initiatives to reduce animal testing while improving predictive capabilities, potentially accelerating the path toward regulatory acceptance of alternative testing methods for safety assessment.
The pharmaceutical industry can also benefit from these systems for mechanistic investigations of drug-drug interactions, particularly those involving zone-specific enzymes. This application is especially valuable for polypharmacy scenarios common in aging populations and patients with multiple comorbidities.
Furthermore, the technology offers opportunities for developing liver-on-chip platforms for high-throughput screening, enabling more efficient early-stage drug candidate evaluation and toxicity assessment. Integration with other organ-on-chip systems could eventually lead to multi-organ platforms capable of modeling complex systemic drug effects and metabolite-mediated toxicity.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!