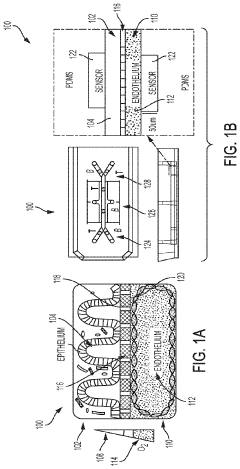
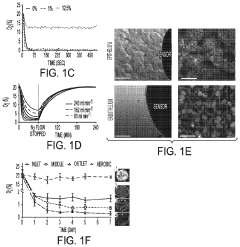
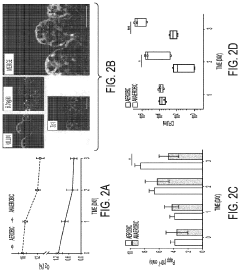
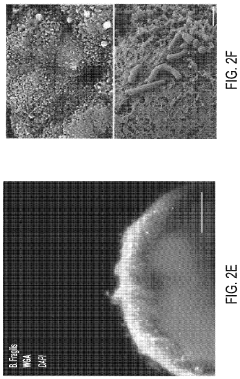
Protocols for co-culturing microbiome communities with gut epithelial cells in gut-on-chip models
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microbiome-Epithelial Co-culture Background and Objectives
The gut microbiome has emerged as a critical factor in human health, influencing numerous physiological processes including digestion, immune function, and even neurological development. Traditional in vitro models have struggled to accurately replicate the complex interactions between gut microbiota and epithelial cells, limiting our understanding of host-microbe relationships. The development of gut-on-chip technology represents a significant advancement in this field, offering a dynamic microenvironment that more closely mimics the in vivo conditions of the human intestine.
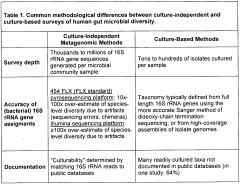
The evolution of microbiome research has progressed from simple culture techniques to sophisticated multi-omics approaches. Initially, researchers focused on identifying individual bacterial species through culture-dependent methods. This transitioned to culture-independent techniques like 16S rRNA sequencing, which revealed the vast diversity of the gut microbiome. Recent technological advances have enabled more comprehensive studies through metagenomic, metatranscriptomic, and metabolomic analyses, providing deeper insights into microbial community functions.
Concurrently, epithelial cell culture techniques have evolved from basic two-dimensional monolayers to complex three-dimensional organoids. The integration of these advances with microfluidic technology has given rise to gut-on-chip models, which incorporate dynamic flow conditions and mechanical forces that better represent the intestinal environment.
The primary objective of developing protocols for co-culturing microbiome communities with gut epithelial cells in gut-on-chip models is to establish reproducible methods that maintain both microbial diversity and epithelial cell viability. This presents significant technical challenges, including oxygen gradient management, nutrient balance, and prevention of microbial overgrowth while ensuring epithelial cell survival.
Additional goals include standardizing protocols to enable cross-laboratory comparisons, developing methods to introduce specific microbial consortia, and creating systems that can sustain long-term co-cultures for chronic disease modeling. These objectives align with the broader aim of creating physiologically relevant models for studying host-microbiome interactions in health and disease.
The technological trajectory points toward increasingly sophisticated systems that incorporate additional cell types, such as immune cells and enteric neurons, to better recapitulate the intestinal microenvironment. Future developments will likely focus on personalized models using patient-derived cells and microbiota, enabling precision medicine approaches for gastrointestinal disorders.
Achieving these objectives would significantly advance our understanding of microbiome-host interactions and potentially lead to novel therapeutic strategies for conditions ranging from inflammatory bowel disease to metabolic disorders and even neurological conditions linked to gut dysbiosis.
The evolution of microbiome research has progressed from simple culture techniques to sophisticated multi-omics approaches. Initially, researchers focused on identifying individual bacterial species through culture-dependent methods. This transitioned to culture-independent techniques like 16S rRNA sequencing, which revealed the vast diversity of the gut microbiome. Recent technological advances have enabled more comprehensive studies through metagenomic, metatranscriptomic, and metabolomic analyses, providing deeper insights into microbial community functions.
Concurrently, epithelial cell culture techniques have evolved from basic two-dimensional monolayers to complex three-dimensional organoids. The integration of these advances with microfluidic technology has given rise to gut-on-chip models, which incorporate dynamic flow conditions and mechanical forces that better represent the intestinal environment.
The primary objective of developing protocols for co-culturing microbiome communities with gut epithelial cells in gut-on-chip models is to establish reproducible methods that maintain both microbial diversity and epithelial cell viability. This presents significant technical challenges, including oxygen gradient management, nutrient balance, and prevention of microbial overgrowth while ensuring epithelial cell survival.
Additional goals include standardizing protocols to enable cross-laboratory comparisons, developing methods to introduce specific microbial consortia, and creating systems that can sustain long-term co-cultures for chronic disease modeling. These objectives align with the broader aim of creating physiologically relevant models for studying host-microbiome interactions in health and disease.
The technological trajectory points toward increasingly sophisticated systems that incorporate additional cell types, such as immune cells and enteric neurons, to better recapitulate the intestinal microenvironment. Future developments will likely focus on personalized models using patient-derived cells and microbiota, enabling precision medicine approaches for gastrointestinal disorders.
Achieving these objectives would significantly advance our understanding of microbiome-host interactions and potentially lead to novel therapeutic strategies for conditions ranging from inflammatory bowel disease to metabolic disorders and even neurological conditions linked to gut dysbiosis.
Market Analysis for Gut-on-Chip Microbiome Models
The global market for gut-on-chip microbiome models is experiencing significant growth, driven by increasing recognition of the gut microbiome's role in human health and disease. Current market valuations place this segment at approximately $120 million in 2023, with projections indicating a compound annual growth rate of 22% through 2030, potentially reaching $550 million by the end of the decade.
Pharmaceutical companies represent the largest market segment, accounting for roughly 45% of the current demand. These companies are increasingly incorporating gut-on-chip models into their drug development pipelines to better predict drug efficacy, toxicity, and interactions with the gut microbiome. This trend is particularly evident in therapeutic areas such as inflammatory bowel disease, metabolic disorders, and immuno-oncology.
Academic research institutions constitute the second-largest market segment at 30%, focusing primarily on fundamental microbiome research and method development. Biotechnology companies follow at 20%, with the remaining 5% distributed among contract research organizations and government laboratories.
Regionally, North America dominates the market with approximately 40% share, followed by Europe (35%), Asia-Pacific (20%), and rest of the world (5%). However, the Asia-Pacific region is expected to show the fastest growth rate in the coming years, particularly in China, Japan, and South Korea, where significant investments in microbiome research are being made.
Key market drivers include the rising prevalence of gastrointestinal disorders, increasing R&D investments in microbiome research, and growing recognition of limitations in traditional cell culture and animal models. The COVID-19 pandemic has further accelerated interest in host-microbiome interactions and their impact on immune response.
Market challenges include the high cost of gut-on-chip systems, technical complexities in maintaining stable co-cultures, and the need for standardized protocols. The average cost per gut-on-chip device ranges from $5,000 to $15,000, with additional expenses for specialized media, analytical tools, and trained personnel.
Customer demand is increasingly focused on systems that can maintain anaerobic conditions, support diverse microbial communities, and provide real-time monitoring capabilities. There is also growing interest in models that incorporate immune cells and neural components to better recapitulate the gut-brain-microbiome axis.
The market is expected to evolve toward more integrated systems that combine gut-on-chip technology with other organ models to create multi-organ platforms capable of studying systemic effects of microbiome-host interactions, representing a significant opportunity for technology developers and service providers in this space.
Pharmaceutical companies represent the largest market segment, accounting for roughly 45% of the current demand. These companies are increasingly incorporating gut-on-chip models into their drug development pipelines to better predict drug efficacy, toxicity, and interactions with the gut microbiome. This trend is particularly evident in therapeutic areas such as inflammatory bowel disease, metabolic disorders, and immuno-oncology.
Academic research institutions constitute the second-largest market segment at 30%, focusing primarily on fundamental microbiome research and method development. Biotechnology companies follow at 20%, with the remaining 5% distributed among contract research organizations and government laboratories.
Regionally, North America dominates the market with approximately 40% share, followed by Europe (35%), Asia-Pacific (20%), and rest of the world (5%). However, the Asia-Pacific region is expected to show the fastest growth rate in the coming years, particularly in China, Japan, and South Korea, where significant investments in microbiome research are being made.
Key market drivers include the rising prevalence of gastrointestinal disorders, increasing R&D investments in microbiome research, and growing recognition of limitations in traditional cell culture and animal models. The COVID-19 pandemic has further accelerated interest in host-microbiome interactions and their impact on immune response.
Market challenges include the high cost of gut-on-chip systems, technical complexities in maintaining stable co-cultures, and the need for standardized protocols. The average cost per gut-on-chip device ranges from $5,000 to $15,000, with additional expenses for specialized media, analytical tools, and trained personnel.
Customer demand is increasingly focused on systems that can maintain anaerobic conditions, support diverse microbial communities, and provide real-time monitoring capabilities. There is also growing interest in models that incorporate immune cells and neural components to better recapitulate the gut-brain-microbiome axis.
The market is expected to evolve toward more integrated systems that combine gut-on-chip technology with other organ models to create multi-organ platforms capable of studying systemic effects of microbiome-host interactions, representing a significant opportunity for technology developers and service providers in this space.
Current Challenges in Microbiome-Epithelial Co-culture Systems
Despite significant advancements in gut-on-chip technology, several critical challenges persist in establishing effective microbiome-epithelial co-culture systems. The primary obstacle remains maintaining viable microbial communities in aerobic cell culture environments, as most gut microbes are strict or facultative anaerobes. Current systems struggle to create appropriate oxygen gradients that simultaneously support both epithelial cell viability and diverse microbial populations.
Nutrient competition presents another significant hurdle, as microbes typically grow more rapidly than human cells and can quickly deplete available nutrients, potentially compromising epithelial cell function. This imbalance often leads to microbial overgrowth that disrupts the intended co-culture equilibrium, making long-term studies particularly challenging.
The establishment of physiologically relevant microbial diversity poses a substantial technical barrier. While single-strain or simplified consortia models offer experimental control, they fail to capture the complexity of the human gut microbiome. Conversely, attempts to incorporate complex microbial communities frequently result in selective enrichment of certain species, leading to reduced diversity over time and limiting the biological relevance of these models.
Medium formulation remains problematic, as traditional cell culture media contain components that can selectively promote or inhibit specific microbial populations. Antibiotics, commonly used in epithelial cell culture, are obviously incompatible with microbiome studies, necessitating alternative approaches to prevent contamination while maintaining experimental integrity.
Technical limitations in monitoring co-culture systems present additional challenges. Real-time assessment of microbial composition and metabolic activity without disrupting the culture environment requires sophisticated analytical approaches that are not yet standardized. Current methods often necessitate endpoint analyses that fail to capture dynamic interactions between microbes and epithelial cells.
The lack of standardized protocols represents perhaps the most significant barrier to progress in this field. Variations in chip design, flow rates, seeding densities, and analytical methods make cross-laboratory comparisons difficult. This heterogeneity in methodological approaches has hindered the establishment of reproducible models that could serve as platforms for mechanistic studies or pharmaceutical testing.
Addressing these challenges requires interdisciplinary collaboration between microbiologists, cell biologists, engineers, and computational scientists to develop innovative solutions that better recapitulate the complex microbiome-epithelial interface of the human gut.
Nutrient competition presents another significant hurdle, as microbes typically grow more rapidly than human cells and can quickly deplete available nutrients, potentially compromising epithelial cell function. This imbalance often leads to microbial overgrowth that disrupts the intended co-culture equilibrium, making long-term studies particularly challenging.
The establishment of physiologically relevant microbial diversity poses a substantial technical barrier. While single-strain or simplified consortia models offer experimental control, they fail to capture the complexity of the human gut microbiome. Conversely, attempts to incorporate complex microbial communities frequently result in selective enrichment of certain species, leading to reduced diversity over time and limiting the biological relevance of these models.
Medium formulation remains problematic, as traditional cell culture media contain components that can selectively promote or inhibit specific microbial populations. Antibiotics, commonly used in epithelial cell culture, are obviously incompatible with microbiome studies, necessitating alternative approaches to prevent contamination while maintaining experimental integrity.
Technical limitations in monitoring co-culture systems present additional challenges. Real-time assessment of microbial composition and metabolic activity without disrupting the culture environment requires sophisticated analytical approaches that are not yet standardized. Current methods often necessitate endpoint analyses that fail to capture dynamic interactions between microbes and epithelial cells.
The lack of standardized protocols represents perhaps the most significant barrier to progress in this field. Variations in chip design, flow rates, seeding densities, and analytical methods make cross-laboratory comparisons difficult. This heterogeneity in methodological approaches has hindered the establishment of reproducible models that could serve as platforms for mechanistic studies or pharmaceutical testing.
Addressing these challenges requires interdisciplinary collaboration between microbiologists, cell biologists, engineers, and computational scientists to develop innovative solutions that better recapitulate the complex microbiome-epithelial interface of the human gut.
Established Protocols for Microbiome-Epithelial Cell Co-culture
01 Microfluidic systems for co-culturing gut microbiome and epithelial cells
Microfluidic devices provide controlled environments for co-culturing gut microbiome communities with epithelial cells. These systems allow for precise control of flow rates, nutrient delivery, and oxygen gradients that mimic the intestinal environment. The devices typically include separate chambers for epithelial cell growth and microbial communities, connected by permeable membranes that allow for molecular exchange while preventing direct contact between microbes and host cells, simulating the intestinal barrier function.- In vitro co-culture systems for gut microbiome and epithelial cells: Various in vitro systems have been developed to co-culture gut microbiome communities with epithelial cells. These systems typically involve growing intestinal epithelial cell lines (such as Caco-2 or HT-29) in specialized chambers or devices that allow for the controlled introduction of microbial communities. These systems often incorporate features that mimic the physiological conditions of the gut, including oxygen gradients, fluid flow, and appropriate growth media to support both mammalian cells and microorganisms.
- Anaerobic culture techniques for maintaining microbiome viability: Specialized anaerobic culture techniques are essential for maintaining the viability of obligate anaerobic bacteria that comprise a significant portion of the gut microbiome. These protocols typically involve the use of anaerobic chambers, specialized gas mixtures, and oxygen-scavenging compounds to create and maintain oxygen-depleted environments. The co-culture systems often incorporate oxygen gradients to simultaneously support the growth of both aerobic epithelial cells and anaerobic microorganisms, mimicking the natural environment of the gut epithelium and its associated microbiota.
- Microfluidic and organ-on-chip technologies for co-culture: Advanced microfluidic and organ-on-chip technologies have been developed to create more physiologically relevant co-culture systems. These platforms typically consist of multiple chambers connected by channels that allow for the controlled flow of media and the establishment of distinct microenvironments. The systems often incorporate porous membranes that separate the epithelial cell layer from the microbial communities while allowing for molecular communication between them. These technologies enable the study of host-microbiome interactions under dynamic conditions that better mimic the in vivo environment.
- Media formulations and supplements for co-culture systems: Specialized media formulations and supplements have been developed to support the co-culture of gut epithelial cells and microbiome communities. These media typically contain a balance of nutrients that support both mammalian cell and microbial growth, including specific carbon sources, amino acids, vitamins, and minerals. Some protocols incorporate prebiotics, short-chain fatty acids, or other compounds that promote the growth of beneficial bacteria. The media may also contain selective agents that help maintain the desired composition of the microbial community while preventing overgrowth of fast-growing species.
- Analysis methods for co-culture experiments: Various analytical methods have been developed to assess the outcomes of microbiome-epithelial cell co-culture experiments. These include techniques for monitoring changes in epithelial cell morphology, barrier function, and gene expression, as well as methods for analyzing changes in microbial community composition and metabolic activity. Common approaches include transcriptomics, proteomics, and metabolomics analyses, as well as imaging techniques such as confocal microscopy and electron microscopy. These methods allow researchers to comprehensively evaluate the complex interactions between gut epithelial cells and their associated microbiota.
02 Anaerobic co-culture methods for oxygen-sensitive gut microbiota
Specialized protocols for maintaining anaerobic conditions are essential for co-culturing oxygen-sensitive gut microbiota with epithelial cells. These methods include the use of anaerobic chambers, oxygen-scavenging systems, and specialized media formulations that maintain viability of both strict anaerobes and oxygen-requiring epithelial cells. The protocols often involve establishing oxygen gradients that mimic the intestinal lumen environment, allowing for the cultivation of complex microbial communities that would otherwise be difficult to maintain in standard laboratory conditions.Expand Specific Solutions03 3D organoid models for microbiome-epithelial cell interactions
Three-dimensional organoid culture systems provide advanced models for studying microbiome-epithelial cell interactions. These organoids are derived from stem cells and self-organize into structures that mimic the architecture and function of the intestinal epithelium, including crypt-villus organization and cellular differentiation. Protocols for co-culturing microbiome communities with these organoids typically involve microinjection of bacterial communities into the organoid lumen or embedding organoids in matrices containing microbiota, allowing for more physiologically relevant studies of host-microbe interactions.Expand Specific Solutions04 Media formulations and supplements for co-culture stability
Specialized media formulations and supplements are critical for maintaining stable co-cultures of gut microbiome communities and epithelial cells. These formulations typically include specific growth factors, prebiotics, and nutrients that support both mammalian cell and microbial growth. The protocols often involve sequential adaptation steps, where media compositions are gradually adjusted to accommodate the metabolic requirements of both cell types. Some approaches include the use of conditioned media or specific metabolites to enhance epithelial barrier function while supporting diverse microbial communities.Expand Specific Solutions05 Host-microbiome interaction analysis techniques
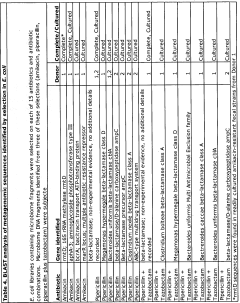
Advanced analytical techniques are employed to study the interactions between gut microbiome communities and epithelial cells in co-culture systems. These include transcriptomic, proteomic, and metabolomic analyses to assess changes in gene expression, protein production, and metabolite profiles. Imaging techniques such as confocal microscopy and live-cell imaging allow for visualization of bacterial adhesion, biofilm formation, and epithelial responses. Additionally, barrier function assays, cytokine measurements, and microbial community analysis through 16S rRNA sequencing provide comprehensive data on the dynamics of host-microbe interactions in these co-culture systems.Expand Specific Solutions
Leading Research Groups and Companies in Gut Microbiome Models
The field of gut-on-chip microbiome co-culture protocols is currently in an early growth phase, with significant research momentum but limited commercial maturity. The global market for organ-on-chip technology is expanding rapidly, projected to reach $220 million by 2025, with gut models representing a key segment. Emulate, Inc. leads commercial development with their Organs-on-Chips platform, while academic institutions like Harvard, University of Luxembourg, and Tsinghua University drive fundamental research innovations. Technical challenges in maintaining stable co-cultures and physiological relevance remain, with companies like BGI Research and NuBiyota pursuing specialized microbiome applications. The field is characterized by strong academic-industry partnerships, with research institutes like Dalian Institute of Chemical Physics developing enabling technologies for this emerging application area.
Emulate, Inc.
Technical Solution: Emulate has developed the Intestine-Chip, a sophisticated organ-on-chip platform specifically designed for co-culturing gut microbiome with human intestinal epithelial cells. Their technology utilizes a two-channel microfluidic device with a porous membrane separating the intestinal epithelial cells (grown on the top channel) from the microbiome communities (introduced in the bottom channel). The system incorporates mechanical forces that mimic peristalsis through cyclic stretching, creating physiologically relevant conditions. Emulate's protocols include specialized media formulations that support both human cells and diverse microbial communities simultaneously, with oxygen gradients carefully controlled to maintain anaerobic conditions for obligate anaerobes while supporting epithelial cell viability[1][3]. Their proprietary chip design includes specialized coatings that promote epithelial cell attachment and differentiation while preventing excessive bacterial adhesion to device surfaces.
Strengths: Industry-leading commercial platform with validated protocols, excellent reproducibility, and compatibility with various analytical techniques. Weaknesses: Relatively high cost of implementation, proprietary nature limits customization, and challenges in maintaining stable co-cultures beyond 72 hours without specialized expertise.
President & Fellows of Harvard College
Technical Solution: Harvard's Wyss Institute pioneered the Human Intestine Chip technology that enables long-term co-culture of human intestinal epithelium with complex microbiome communities. Their protocol utilizes a microfluidic device with two parallel microchannels separated by a porous extracellular matrix-coated membrane. The top channel hosts intestinal epithelial cells that form villi-like structures, while the bottom channel accommodates microbiome samples. The system incorporates mechanical stretching (10% strain, 0.15 Hz) to mimic peristaltic motions and employs specialized media formulations with carefully balanced nutrient compositions to support both human cells and diverse microbial populations[2][4]. Harvard researchers have developed methods to establish oxygen gradients across the membrane, allowing for the co-existence of obligate anaerobes with oxygen-requiring epithelial cells. Their protocols include specialized sampling techniques for real-time monitoring of microbial metabolites, cytokines, and barrier function without disrupting the co-culture system.
Strengths: Pioneering research with extensive validation, highly physiologically relevant model with demonstrated ability to maintain complex microbial communities for extended periods (7+ days). Weaknesses: Complex implementation requiring specialized expertise, limited throughput compared to simpler models, and challenges in standardizing microbiome inputs across experiments.
Key Innovations in Anaerobic-Aerobic Interface Maintenance
Complex Human Gut Microbiome Cultured In An Anaerobic Human Gut-On-A-Chip
PatentPendingUS20240002808A1
Innovation
- A microfluidic device with a membrane allowing oxygen flow between two channels, one containing human intestinal epithelium and the other vascular endothelium, creates a physiologically relevant oxygen gradient, enabling stable co-culture of complex microbial communities with real-time oxygen monitoring using embedded microscale oxygen sensors.
Cultured collection of gut microbial community
PatentWO2012122522A2
Innovation
- Development of in vitro and in vivo cultures of gut microbial communities that maintain a phylotypic composition similar to the original community, allowing for the analysis of perturbations and the use of clonally arrayed culture collections to represent the original community's composition and functions.
Standardization and Validation Methodologies
The standardization and validation of gut-on-chip models incorporating microbiome communities and epithelial cells represent critical challenges in advancing this technology toward widespread adoption. Current protocols exhibit significant variability across research institutions, hampering reproducibility and cross-study comparisons. Establishing standardized methodologies requires addressing multiple dimensions of these complex co-culture systems.
Validation frameworks must include comprehensive characterization of both microbial and epithelial components before, during, and after co-culture experiments. Quantitative metrics for epithelial barrier integrity, including transepithelial electrical resistance (TEER) measurements and permeability assays using fluorescent markers, should be consistently applied across studies. Similarly, standardized methods for assessing microbial viability, community composition stability, and metabolic activity are essential for meaningful interpretation of results.
Reference materials and control communities represent another crucial aspect of standardization efforts. The development of defined synthetic microbial consortia with known composition and behavior could serve as benchmarks against which experimental variations can be measured. These reference communities should ideally represent different functional groups found in the human gut microbiome while maintaining reproducible growth characteristics in microfluidic environments.
Quality control parameters must be established for chip fabrication, surface functionalization, and flow conditions. Variations in these physical parameters can significantly impact cellular adhesion, microbial biofilm formation, and overall system performance. Documentation standards should include detailed reporting of chip dimensions, material properties, surface treatments, and flow rates to enable replication across laboratories.
Validation protocols should incorporate multi-omics approaches to characterize system outputs comprehensively. Standardized sampling procedures for transcriptomic, proteomic, and metabolomic analyses would facilitate integration of data across different experimental platforms. Additionally, imaging protocols for visualizing spatial organization of microbial communities and their interactions with epithelial surfaces require standardization regarding microscopy settings, staining procedures, and image analysis algorithms.
Regulatory considerations must also be addressed in standardization efforts, particularly for applications in drug development and toxicology testing. Establishing clear guidelines for demonstrating physiological relevance and predictive capacity of gut-on-chip models will accelerate their acceptance as alternatives to traditional cell culture and animal testing approaches. This includes developing validation protocols that compare chip-based results with established in vivo and clinical observations.
Validation frameworks must include comprehensive characterization of both microbial and epithelial components before, during, and after co-culture experiments. Quantitative metrics for epithelial barrier integrity, including transepithelial electrical resistance (TEER) measurements and permeability assays using fluorescent markers, should be consistently applied across studies. Similarly, standardized methods for assessing microbial viability, community composition stability, and metabolic activity are essential for meaningful interpretation of results.
Reference materials and control communities represent another crucial aspect of standardization efforts. The development of defined synthetic microbial consortia with known composition and behavior could serve as benchmarks against which experimental variations can be measured. These reference communities should ideally represent different functional groups found in the human gut microbiome while maintaining reproducible growth characteristics in microfluidic environments.
Quality control parameters must be established for chip fabrication, surface functionalization, and flow conditions. Variations in these physical parameters can significantly impact cellular adhesion, microbial biofilm formation, and overall system performance. Documentation standards should include detailed reporting of chip dimensions, material properties, surface treatments, and flow rates to enable replication across laboratories.
Validation protocols should incorporate multi-omics approaches to characterize system outputs comprehensively. Standardized sampling procedures for transcriptomic, proteomic, and metabolomic analyses would facilitate integration of data across different experimental platforms. Additionally, imaging protocols for visualizing spatial organization of microbial communities and their interactions with epithelial surfaces require standardization regarding microscopy settings, staining procedures, and image analysis algorithms.
Regulatory considerations must also be addressed in standardization efforts, particularly for applications in drug development and toxicology testing. Establishing clear guidelines for demonstrating physiological relevance and predictive capacity of gut-on-chip models will accelerate their acceptance as alternatives to traditional cell culture and animal testing approaches. This includes developing validation protocols that compare chip-based results with established in vivo and clinical observations.
Regulatory Considerations for Microbiome-Based Drug Development
The regulatory landscape for microbiome-based therapeutics and gut-on-chip models presents unique challenges due to their novel nature and complex biological interactions. Currently, both the FDA and EMA are developing frameworks to address these innovative approaches, with the FDA's Center for Biologics Evaluation and Research (CBER) taking the lead on microbiome-based products classification and regulation.
Microbiome-based therapeutics often fall into a regulatory gray area between drugs, biologics, and medical devices. For co-culture protocols in gut-on-chip models, researchers must navigate regulations for both the microbiome components and the chip technology itself. The FDA has issued guidance documents addressing aspects of microbiome therapeutics, particularly focusing on fecal microbiota transplantation (FMT) and live biotherapeutic products (LBPs).
Key regulatory considerations for co-culture protocols include standardization of microbiome community composition, quality control measures for maintaining microbial viability, and validation of epithelial cell-microbiome interactions. Researchers must demonstrate that their protocols can consistently produce reliable and reproducible results across different laboratories and manufacturing settings.
Safety assessment frameworks require special attention when developing these models. Potential contamination, unintended microbial shifts during culture, and host-microbiome interactions that might trigger adverse responses must be thoroughly evaluated. The FDA recommends comprehensive characterization of microbial strains, including whole genome sequencing and functional analysis.
Manufacturing considerations present another regulatory hurdle. Good Manufacturing Practice (GMP) compliance for microbiome cultivation alongside human cells requires specialized facilities and protocols. Documentation must address how the co-culture environment maintains the intended microbial community structure while preventing opportunistic overgrowth of certain species.
Clinical translation pathways for findings from gut-on-chip models remain under development. The FDA's Drug Development Tool (DDT) Qualification Programs may provide avenues for validating these models as biomarkers or drug development tools. However, the correlation between in vitro observations and in vivo outcomes requires substantial validation.
International harmonization efforts are underway through initiatives like the International Council for Harmonisation (ICH) to develop consistent regulatory approaches for microbiome-based therapeutics. This is particularly important for multi-center studies utilizing gut-on-chip technologies across different regulatory jurisdictions.
Researchers developing co-culture protocols should engage early with regulatory agencies through programs like the FDA's Pre-IND consultation process. This proactive approach can help identify potential regulatory concerns before significant resources are invested in protocol development and validation.
Microbiome-based therapeutics often fall into a regulatory gray area between drugs, biologics, and medical devices. For co-culture protocols in gut-on-chip models, researchers must navigate regulations for both the microbiome components and the chip technology itself. The FDA has issued guidance documents addressing aspects of microbiome therapeutics, particularly focusing on fecal microbiota transplantation (FMT) and live biotherapeutic products (LBPs).
Key regulatory considerations for co-culture protocols include standardization of microbiome community composition, quality control measures for maintaining microbial viability, and validation of epithelial cell-microbiome interactions. Researchers must demonstrate that their protocols can consistently produce reliable and reproducible results across different laboratories and manufacturing settings.
Safety assessment frameworks require special attention when developing these models. Potential contamination, unintended microbial shifts during culture, and host-microbiome interactions that might trigger adverse responses must be thoroughly evaluated. The FDA recommends comprehensive characterization of microbial strains, including whole genome sequencing and functional analysis.
Manufacturing considerations present another regulatory hurdle. Good Manufacturing Practice (GMP) compliance for microbiome cultivation alongside human cells requires specialized facilities and protocols. Documentation must address how the co-culture environment maintains the intended microbial community structure while preventing opportunistic overgrowth of certain species.
Clinical translation pathways for findings from gut-on-chip models remain under development. The FDA's Drug Development Tool (DDT) Qualification Programs may provide avenues for validating these models as biomarkers or drug development tools. However, the correlation between in vitro observations and in vivo outcomes requires substantial validation.
International harmonization efforts are underway through initiatives like the International Council for Harmonisation (ICH) to develop consistent regulatory approaches for microbiome-based therapeutics. This is particularly important for multi-center studies utilizing gut-on-chip technologies across different regulatory jurisdictions.
Researchers developing co-culture protocols should engage early with regulatory agencies through programs like the FDA's Pre-IND consultation process. This proactive approach can help identify potential regulatory concerns before significant resources are invested in protocol development and validation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!