Modeling metastatic extravasation using vascularized tumor-on-chip platforms
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Metastatic Extravasation Modeling Background and Objectives
Metastatic cancer progression represents one of the most significant challenges in oncology, accounting for approximately 90% of cancer-related deaths worldwide. The process of extravasation—where circulating tumor cells exit the bloodstream to establish secondary tumors—remains poorly understood despite its critical role in metastasis. Historical approaches to studying this phenomenon have relied heavily on animal models, which, while valuable, often fail to capture the human-specific cellular interactions and microenvironmental factors that influence extravasation dynamics.
The evolution of tumor-on-chip technology over the past decade has created unprecedented opportunities to model complex biological processes in controlled microenvironments. These platforms integrate microfluidic engineering with tissue culture techniques to recreate physiologically relevant vascular structures that mimic the in vivo environment where extravasation occurs. The field has progressed from simple 2D co-culture systems to sophisticated 3D vascularized models that incorporate multiple cell types and extracellular matrix components.
Recent technological advances have enabled the development of vascularized tumor-on-chip platforms that specifically address the challenges of modeling extravasation. These systems allow for real-time visualization of tumor cell interactions with the endothelial barrier, providing insights into the mechanical and biochemical factors that facilitate transendothelial migration. The integration of patient-derived cells further enhances the clinical relevance of these models, potentially enabling personalized approaches to cancer treatment.
The primary objective of this research direction is to establish robust, reproducible vascularized tumor-on-chip platforms that accurately recapitulate the process of metastatic extravasation. These platforms aim to elucidate the cellular and molecular mechanisms underlying this critical step in metastasis, with particular focus on the role of endothelial junction integrity, tumor cell adhesion molecules, and microenvironmental factors such as inflammatory cytokines and shear stress.
Secondary objectives include the development of standardized protocols for platform fabrication and operation, validation against existing in vivo models, and demonstration of clinical relevance through correlation with patient outcomes. Additionally, these platforms are intended to serve as testing grounds for novel anti-metastatic therapeutics, potentially accelerating the drug development pipeline by providing more predictive preclinical models than traditional approaches.
The long-term vision for this technology encompasses integration with other emerging technologies such as single-cell sequencing, advanced imaging techniques, and computational modeling to create comprehensive systems for studying cancer metastasis. By combining experimental data with predictive algorithms, researchers aim to develop tools that can forecast metastatic potential and guide personalized treatment strategies, ultimately contributing to reduced mortality from metastatic disease.
The evolution of tumor-on-chip technology over the past decade has created unprecedented opportunities to model complex biological processes in controlled microenvironments. These platforms integrate microfluidic engineering with tissue culture techniques to recreate physiologically relevant vascular structures that mimic the in vivo environment where extravasation occurs. The field has progressed from simple 2D co-culture systems to sophisticated 3D vascularized models that incorporate multiple cell types and extracellular matrix components.
Recent technological advances have enabled the development of vascularized tumor-on-chip platforms that specifically address the challenges of modeling extravasation. These systems allow for real-time visualization of tumor cell interactions with the endothelial barrier, providing insights into the mechanical and biochemical factors that facilitate transendothelial migration. The integration of patient-derived cells further enhances the clinical relevance of these models, potentially enabling personalized approaches to cancer treatment.
The primary objective of this research direction is to establish robust, reproducible vascularized tumor-on-chip platforms that accurately recapitulate the process of metastatic extravasation. These platforms aim to elucidate the cellular and molecular mechanisms underlying this critical step in metastasis, with particular focus on the role of endothelial junction integrity, tumor cell adhesion molecules, and microenvironmental factors such as inflammatory cytokines and shear stress.
Secondary objectives include the development of standardized protocols for platform fabrication and operation, validation against existing in vivo models, and demonstration of clinical relevance through correlation with patient outcomes. Additionally, these platforms are intended to serve as testing grounds for novel anti-metastatic therapeutics, potentially accelerating the drug development pipeline by providing more predictive preclinical models than traditional approaches.
The long-term vision for this technology encompasses integration with other emerging technologies such as single-cell sequencing, advanced imaging techniques, and computational modeling to create comprehensive systems for studying cancer metastasis. By combining experimental data with predictive algorithms, researchers aim to develop tools that can forecast metastatic potential and guide personalized treatment strategies, ultimately contributing to reduced mortality from metastatic disease.
Market Analysis for Vascularized Tumor-on-Chip Technologies
The global market for vascularized tumor-on-chip technologies is experiencing significant growth, driven by increasing demand for more physiologically relevant cancer models in drug discovery and personalized medicine. Current market valuations estimate the overall organ-on-chip market at approximately $45 million in 2022, with projections to reach $600 million by 2030, representing a compound annual growth rate (CAGR) of 39.2%. Within this broader market, vascularized tumor models are emerging as a particularly valuable segment.
Key market drivers include the rising costs of traditional drug development, with estimates suggesting pharmaceutical companies spend over $2.6 billion to bring a single drug to market, and failure rates exceeding 90% in clinical trials. This economic pressure has created strong incentives for more predictive preclinical models that can better recapitulate human physiology and disease progression, particularly metastatic processes.
The pharmaceutical and biotechnology sectors represent the largest customer base, accounting for roughly 65% of the current market. Academic research institutions constitute approximately 25% of the market, while contract research organizations make up the remaining 10%. Geographically, North America dominates with 45% market share, followed by Europe (30%), Asia-Pacific (20%), and rest of world (5%).
Investor interest in this technology has been robust, with venture capital funding for organ-on-chip companies exceeding $400 million between 2018 and 2022. Notable funding rounds include CN Bio ($15 million), TissUse ($23 million), and Emulate ($82 million), highlighting strong commercial confidence in the technology's potential.
Market challenges include high production costs, with current vascularized tumor-on-chip platforms typically priced between $10,000-$50,000 per system, limiting widespread adoption. Additionally, regulatory uncertainties regarding validation requirements and standardization pose barriers to market penetration in clinical applications.
Customer demand is increasingly focused on platforms that can model specific aspects of the metastatic cascade, with extravasation models commanding premium pricing due to their critical role in understanding cancer progression. End-users report willingness to pay 30-40% more for systems that accurately model the extravasation process compared to standard tumor-on-chip platforms.
Future market growth is expected to be driven by integration with complementary technologies such as AI-powered image analysis, multi-omics capabilities, and automation systems that enhance throughput and reproducibility. The development of standardized validation protocols and regulatory acceptance will likely accelerate market adoption, particularly in pharmaceutical development pipelines.
Key market drivers include the rising costs of traditional drug development, with estimates suggesting pharmaceutical companies spend over $2.6 billion to bring a single drug to market, and failure rates exceeding 90% in clinical trials. This economic pressure has created strong incentives for more predictive preclinical models that can better recapitulate human physiology and disease progression, particularly metastatic processes.
The pharmaceutical and biotechnology sectors represent the largest customer base, accounting for roughly 65% of the current market. Academic research institutions constitute approximately 25% of the market, while contract research organizations make up the remaining 10%. Geographically, North America dominates with 45% market share, followed by Europe (30%), Asia-Pacific (20%), and rest of world (5%).
Investor interest in this technology has been robust, with venture capital funding for organ-on-chip companies exceeding $400 million between 2018 and 2022. Notable funding rounds include CN Bio ($15 million), TissUse ($23 million), and Emulate ($82 million), highlighting strong commercial confidence in the technology's potential.
Market challenges include high production costs, with current vascularized tumor-on-chip platforms typically priced between $10,000-$50,000 per system, limiting widespread adoption. Additionally, regulatory uncertainties regarding validation requirements and standardization pose barriers to market penetration in clinical applications.
Customer demand is increasingly focused on platforms that can model specific aspects of the metastatic cascade, with extravasation models commanding premium pricing due to their critical role in understanding cancer progression. End-users report willingness to pay 30-40% more for systems that accurately model the extravasation process compared to standard tumor-on-chip platforms.
Future market growth is expected to be driven by integration with complementary technologies such as AI-powered image analysis, multi-omics capabilities, and automation systems that enhance throughput and reproducibility. The development of standardized validation protocols and regulatory acceptance will likely accelerate market adoption, particularly in pharmaceutical development pipelines.
Current Challenges in Metastatic Extravasation Modeling
Despite significant advancements in tumor-on-chip technologies, modeling metastatic extravasation faces several critical challenges that limit the clinical relevance and predictive power of current platforms. The primary obstacle remains the accurate recapitulation of the complex vascular microenvironment where cancer cells interact with endothelial barriers. Conventional models often fail to reproduce the heterogeneous architecture of blood vessels found in different tissues, which significantly influences extravasation efficiency and patterns.
The dynamic nature of the extravasation process presents another substantial challenge. Current platforms struggle to capture the temporal aspects of cancer cell-endothelium interactions, including initial attachment, transmigration, and post-extravasation behavior. Most systems provide only static snapshots rather than continuous monitoring of these dynamic cellular events, limiting our understanding of the complete extravasation cascade.
Physiological flow conditions represent a significant hurdle in existing models. Recreating appropriate shear stress and flow patterns that cancer cells experience in vivo requires sophisticated microfluidic engineering. Many current platforms either lack flow altogether or implement simplified flow patterns that inadequately represent the complex hemodynamics of the circulatory system, particularly in microvascular networks where extravasation predominantly occurs.
The composition of the extracellular matrix (ECM) surrounding blood vessels significantly influences extravasation mechanics but remains poorly represented in most models. Current platforms typically utilize simplified ECM formulations that fail to mimic the tissue-specific matrix compositions and mechanical properties found at metastatic sites. This limitation hampers the study of how matrix stiffness, porosity, and biochemical composition affect cancer cell invasion post-extravasation.
Incorporating immune components presents another major challenge. The immune system plays a dual role in extravasation—both inhibiting and sometimes facilitating the process. Most current vascularized tumor-on-chip platforms lack immune cell components or offer only simplified immune cell interactions, failing to capture the complex immunomodulatory aspects of the metastatic cascade.
Scaling and throughput limitations restrict the application of these technologies for drug screening purposes. Many sophisticated vascularized models are labor-intensive, difficult to reproduce consistently, and not amenable to high-throughput applications, limiting their utility in pharmaceutical development pipelines.
Finally, validation against clinical data remains problematic. Correlating observations from tumor-on-chip platforms with actual patient outcomes represents a significant challenge, as the simplified nature of these models may not fully predict the complexity of metastatic behavior in human patients. This validation gap undermines confidence in using these platforms for personalized medicine applications.
The dynamic nature of the extravasation process presents another substantial challenge. Current platforms struggle to capture the temporal aspects of cancer cell-endothelium interactions, including initial attachment, transmigration, and post-extravasation behavior. Most systems provide only static snapshots rather than continuous monitoring of these dynamic cellular events, limiting our understanding of the complete extravasation cascade.
Physiological flow conditions represent a significant hurdle in existing models. Recreating appropriate shear stress and flow patterns that cancer cells experience in vivo requires sophisticated microfluidic engineering. Many current platforms either lack flow altogether or implement simplified flow patterns that inadequately represent the complex hemodynamics of the circulatory system, particularly in microvascular networks where extravasation predominantly occurs.
The composition of the extracellular matrix (ECM) surrounding blood vessels significantly influences extravasation mechanics but remains poorly represented in most models. Current platforms typically utilize simplified ECM formulations that fail to mimic the tissue-specific matrix compositions and mechanical properties found at metastatic sites. This limitation hampers the study of how matrix stiffness, porosity, and biochemical composition affect cancer cell invasion post-extravasation.
Incorporating immune components presents another major challenge. The immune system plays a dual role in extravasation—both inhibiting and sometimes facilitating the process. Most current vascularized tumor-on-chip platforms lack immune cell components or offer only simplified immune cell interactions, failing to capture the complex immunomodulatory aspects of the metastatic cascade.
Scaling and throughput limitations restrict the application of these technologies for drug screening purposes. Many sophisticated vascularized models are labor-intensive, difficult to reproduce consistently, and not amenable to high-throughput applications, limiting their utility in pharmaceutical development pipelines.
Finally, validation against clinical data remains problematic. Correlating observations from tumor-on-chip platforms with actual patient outcomes represents a significant challenge, as the simplified nature of these models may not fully predict the complexity of metastatic behavior in human patients. This validation gap undermines confidence in using these platforms for personalized medicine applications.
Current Vascularized Tumor-on-Chip Methodologies
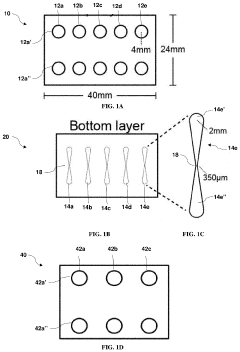
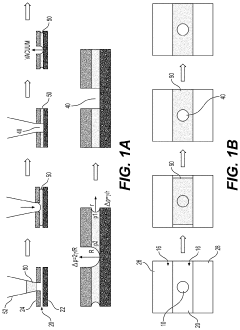
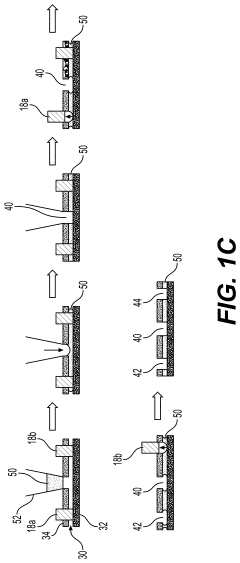
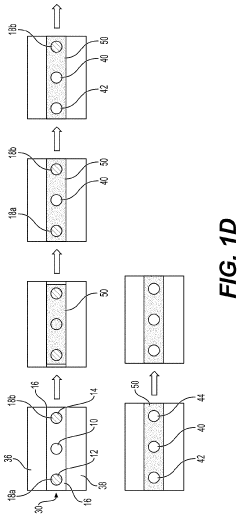
01 Microfluidic vascularized tumor-on-chip platforms
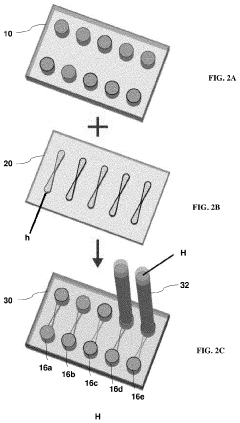
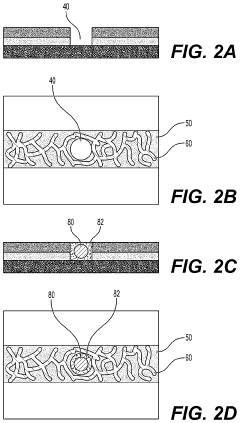
Microfluidic devices that incorporate vascularized tumor models allow for the study of cancer cell extravasation during metastasis. These platforms typically consist of multiple chambers connected by microchannels that mimic blood vessels. The devices enable real-time visualization and quantification of cancer cell interactions with the endothelial barrier, providing insights into the mechanisms of extravasation. These systems can be used to test potential anti-metastatic therapies by evaluating their effects on cancer cell migration and invasion.- Microfluidic tumor-on-chip platforms for studying metastasis: Microfluidic devices designed to mimic the tumor microenvironment and vascular networks for studying cancer cell extravasation during metastasis. These platforms incorporate multiple cell types and extracellular matrix components to recreate the complex interactions that occur during the metastatic cascade. The devices allow for real-time visualization and quantification of cancer cell behavior, including adhesion to endothelial cells, transmigration across the vascular barrier, and invasion into surrounding tissues.
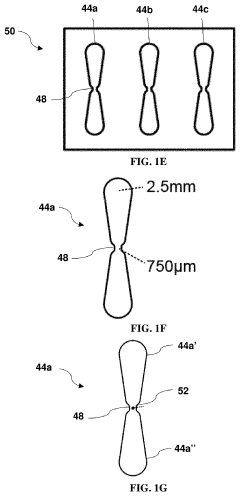
- Vascularized 3D tissue models for extravasation studies: Three-dimensional tissue constructs with integrated vascular networks that enable the study of tumor cell extravasation in a physiologically relevant environment. These models incorporate perfusable blood vessels lined with endothelial cells surrounded by supporting cells and extracellular matrix. The 3D architecture better recapitulates the in vivo conditions compared to traditional 2D models, allowing researchers to observe how cancer cells interact with the vascular wall, breach the endothelial barrier, and establish metastatic colonies in distant tissues.
- Biomaterials and fabrication techniques for vascularized tumor models: Advanced biomaterials and fabrication methods used to create vascularized tumor-on-chip platforms. These include hydrogels with tunable mechanical properties, bioprinting techniques, photolithography, and soft lithography approaches to generate complex microfluidic architectures. The materials and methods enable precise control over the geometry, stiffness, and biochemical composition of the tumor microenvironment, which are critical factors influencing cancer cell extravasation during metastasis.
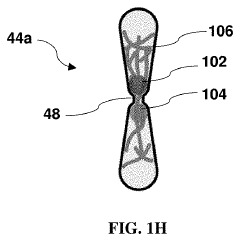
- Imaging and analysis methods for extravasation dynamics: Specialized imaging techniques and analytical approaches for monitoring and quantifying cancer cell extravasation in tumor-on-chip platforms. These include high-resolution confocal microscopy, time-lapse imaging, fluorescence labeling of cells, and computational image analysis. These methods enable researchers to track individual cancer cells as they interact with the vascular endothelium, undergo morphological changes, and migrate through the vessel wall, providing insights into the mechanisms of metastatic spread.
- Drug screening applications using vascularized tumor models: Utilization of vascularized tumor-on-chip platforms for screening and evaluating anti-metastatic drugs. These models provide a physiologically relevant system for testing compounds that target various steps of the metastatic cascade, particularly extravasation. The platforms enable assessment of drug efficacy, toxicity, and mechanisms of action in a controlled environment that recapitulates key aspects of human physiology, potentially reducing the need for animal testing and accelerating the development of new therapies for metastatic cancer.
02 3D biomimetic vasculature for metastasis modeling
Three-dimensional biomimetic vascular structures can be incorporated into tumor-on-chip platforms to better recapitulate the in vivo environment of metastatic extravasation. These structures typically use hydrogels embedded with endothelial cells that self-organize into vessel-like networks. The 3D architecture provides a more physiologically relevant model compared to 2D systems, allowing for the study of complex cell-cell and cell-matrix interactions during the extravasation process. These platforms can be used to investigate the role of mechanical forces, such as fluid shear stress, on cancer cell extravasation.Expand Specific Solutions03 Co-culture systems for tumor-endothelial interactions
Co-culture systems incorporating both tumor cells and endothelial cells enable the study of heterotypic cellular interactions during metastatic extravasation. These platforms allow researchers to investigate how cancer cells interact with the vascular endothelium, including adhesion, transmigration, and invasion processes. By incorporating additional cell types such as immune cells or fibroblasts, these systems can model the complex tumor microenvironment that influences extravasation. The co-culture approach provides insights into the bidirectional signaling between cancer cells and endothelial cells that facilitates metastatic spread.Expand Specific Solutions04 Real-time imaging and monitoring of extravasation
Advanced imaging techniques integrated with tumor-on-chip platforms allow for real-time visualization and quantification of the extravasation process. These systems typically incorporate transparent materials and compatible imaging modalities to track cancer cell movement across the endothelial barrier. Fluorescent labeling of different cell types and extracellular matrix components enables multi-parameter analysis of the extravasation process. These platforms can be used to identify key steps and molecular players in metastatic extravasation, providing potential targets for therapeutic intervention.Expand Specific Solutions05 Patient-derived models for personalized medicine
Tumor-on-chip platforms incorporating patient-derived cells or tissues provide personalized models for studying metastatic extravasation. These systems use primary tumor cells or circulating tumor cells from patients to create individualized models that reflect patient-specific characteristics. By testing different therapeutic agents on these personalized platforms, researchers can identify the most effective treatments for preventing extravasation in specific patients. This approach has potential applications in precision medicine, allowing for tailored treatment strategies based on individual tumor biology.Expand Specific Solutions
Leading Organizations in Vascularized Microfluidic Systems
The field of modeling metastatic extravasation using vascularized tumor-on-chip platforms is currently in an early growth phase, with significant research momentum but limited commercial maturity. The global market for organ-on-chip technologies is expanding rapidly, projected to reach $220 million by 2025, driven by pharmaceutical companies seeking alternatives to animal testing. Key players include academic institutions (MIT, Columbia University, Arizona State University) leading fundamental research, while specialized companies like Emulate, Inc., Aracari Biosciences, and D1 Medical Technology are commercializing these platforms. The technology remains primarily in research settings, with Emulate and LifeBridge Innovations advancing toward clinical applications, though widespread adoption faces regulatory and standardization challenges.
Massachusetts Institute of Technology
Technical Solution: MIT has developed advanced microfluidic tumor-on-chip platforms that accurately model the metastatic extravasation process. Their technology incorporates multi-layered microchannels with precisely controlled flow dynamics to mimic blood vessels, surrounded by extracellular matrix components. The platform features endothelial cell layers that form functional vasculature with tight junctions, allowing researchers to visualize and quantify cancer cell extravasation in real-time. MIT's system integrates oxygen gradient controllers to simulate hypoxic tumor microenvironments, which significantly influences extravasation behavior. Their platforms also incorporate tissue-specific cells (such as lung, brain, or bone marrow) to study organ-specific metastatic tropism. Recent innovations include integration of mechanical stimulation to mimic blood flow shear stress and incorporation of immune cells to study their role in extravasation dynamics. The platform allows for high-throughput screening of anti-metastatic compounds with automated image analysis capabilities.
Strengths: Superior microfluidic engineering with precise control over physical and biochemical parameters; integration of multiple cell types for physiologically relevant models; advanced imaging capabilities for real-time visualization. Weaknesses: Higher technical complexity requiring specialized expertise; relatively expensive setup compared to conventional models; challenges in scaling up for high-throughput pharmaceutical screening applications.
The Trustees of Columbia University in The City of New York
Technical Solution: Columbia University has developed a sophisticated vascularized tumor-on-chip platform that uniquely incorporates self-organizing blood vessel networks. Their approach utilizes a combination of endothelial cells, pericytes, and fibroblasts seeded in hydrogels that spontaneously form physiologically relevant vascular networks with proper lumen formation and barrier function. The platform features multiple compartments allowing for the co-culture of patient-derived tumor organoids alongside the vascular network, creating a more realistic tumor-vasculature interface. Columbia's system incorporates advanced biomaterials with tunable stiffness and composition to mimic different tissue microenvironments, which significantly impacts extravasation efficiency. Their technology enables the study of bidirectional communication between tumor cells and the vasculature through both soluble factors and direct cell-cell interactions. The platform supports long-term culture (up to 3 weeks) to observe the complete metastatic cascade from intravasation through extravasation to micrometastasis formation. Recent innovations include integration of optogenetic tools to precisely control cell signaling during extravasation events and incorporation of patient-specific immune components to study personalized immunotherapy responses.
Strengths: Self-organizing vascular networks that better recapitulate in vivo vasculature complexity; compatibility with patient-derived cells for personalized medicine applications; excellent optical properties for high-resolution imaging. Weaknesses: Higher biological variability between chips due to self-organization process; longer preparation time compared to pre-formed channel systems; more complex analysis required to quantify extravasation in 3D networks.
Key Technical Innovations in Extravasation Modeling
Fluidic platforms for perfusable vascularized tissues
PatentPendingUS20220338465A1
Innovation
- A microfluidic platform with gel-filled channels that mimics interstitial fluid flow to support the formation of perfusable microvascular networks by seeding endothelial cells and extracellular matrix components, using controlled fluid flow to manipulate tissue morphology and enhance vasculogenesis, with the interplay between interstitial flow and MMP-2 activity regulating key morphological parameters.
Fluidic platforms for perfusable vascularized tissues with infiltrates
PatentPendingUS20230146860A1
Innovation
- The development of microfluidic devices with a central gel channel and adjacent media channels, featuring phase guides and ports for introducing organoids/spheroids/tissues, which form perfusable vascular networks within a gel solution containing endothelial cells and extracellular matrix components, enabling interstitial and luminal flows that mimic tissue microenvironments and facilitate immune cell recruitment and drug transport.
Translational Potential for Drug Discovery Applications
Vascularized tumor-on-chip platforms represent a revolutionary approach for drug discovery applications, offering significant translational potential that bridges the gap between traditional in vitro models and clinical trials. These advanced microfluidic systems provide pharmaceutical researchers with physiologically relevant environments that accurately recapitulate the complex process of metastatic extravasation, a critical step in cancer progression.
The immediate translational value lies in drug screening capabilities, where these platforms enable high-throughput testing of anti-metastatic compounds under conditions that closely mimic human physiology. This approach significantly reduces the reliance on animal models, addressing both ethical concerns and the fundamental limitations of interspecies differences that have historically hampered drug development efforts.
Furthermore, these platforms facilitate personalized medicine approaches by incorporating patient-derived cells, allowing for tailored therapeutic strategies based on individual tumor characteristics. This patient-specific testing capability represents a paradigm shift in oncology drug development, potentially increasing success rates in clinical trials by pre-identifying responsive patient populations.
From an economic perspective, the implementation of vascularized tumor-on-chip platforms in early-stage drug discovery could substantially reduce development costs by enabling earlier elimination of ineffective compounds. Industry analysts estimate that identifying drug failures earlier in the development pipeline could save pharmaceutical companies millions per compound, significantly improving R&D efficiency.
Regulatory agencies have shown increasing interest in these technologies as complementary tools for safety and efficacy assessment. The FDA's Advancing Alternative Methods initiative specifically acknowledges the potential of organ-on-chip technologies to provide more predictive data than conventional preclinical models, potentially accelerating the regulatory approval process for promising cancer therapeutics.
Several pharmaceutical companies have already established partnerships with microfluidic technology developers to integrate these platforms into their drug discovery workflows. These collaborations demonstrate the growing recognition of tumor-on-chip systems as valuable tools for identifying novel therapeutic targets and validating drug candidates that specifically interrupt the metastatic cascade.
The future translational trajectory suggests potential expansion beyond traditional pharmaceutical applications into combination therapy optimization, where these platforms could help identify synergistic drug combinations that specifically target the extravasation process, potentially leading to more effective metastasis prevention strategies with minimal side effects.
The immediate translational value lies in drug screening capabilities, where these platforms enable high-throughput testing of anti-metastatic compounds under conditions that closely mimic human physiology. This approach significantly reduces the reliance on animal models, addressing both ethical concerns and the fundamental limitations of interspecies differences that have historically hampered drug development efforts.
Furthermore, these platforms facilitate personalized medicine approaches by incorporating patient-derived cells, allowing for tailored therapeutic strategies based on individual tumor characteristics. This patient-specific testing capability represents a paradigm shift in oncology drug development, potentially increasing success rates in clinical trials by pre-identifying responsive patient populations.
From an economic perspective, the implementation of vascularized tumor-on-chip platforms in early-stage drug discovery could substantially reduce development costs by enabling earlier elimination of ineffective compounds. Industry analysts estimate that identifying drug failures earlier in the development pipeline could save pharmaceutical companies millions per compound, significantly improving R&D efficiency.
Regulatory agencies have shown increasing interest in these technologies as complementary tools for safety and efficacy assessment. The FDA's Advancing Alternative Methods initiative specifically acknowledges the potential of organ-on-chip technologies to provide more predictive data than conventional preclinical models, potentially accelerating the regulatory approval process for promising cancer therapeutics.
Several pharmaceutical companies have already established partnerships with microfluidic technology developers to integrate these platforms into their drug discovery workflows. These collaborations demonstrate the growing recognition of tumor-on-chip systems as valuable tools for identifying novel therapeutic targets and validating drug candidates that specifically interrupt the metastatic cascade.
The future translational trajectory suggests potential expansion beyond traditional pharmaceutical applications into combination therapy optimization, where these platforms could help identify synergistic drug combinations that specifically target the extravasation process, potentially leading to more effective metastasis prevention strategies with minimal side effects.
Standardization and Validation Requirements
The standardization and validation of vascularized tumor-on-chip platforms for modeling metastatic extravasation represent critical challenges that must be addressed to ensure reproducibility, reliability, and clinical relevance of research findings. Currently, there exists significant heterogeneity in platform design, experimental protocols, and reporting standards across different research groups, hampering cross-study comparisons and slowing translational progress.
Establishing standardized fabrication protocols is essential for consistent device production. This includes specifications for materials selection, channel dimensions, membrane properties, and surface treatments. The microfluidic architecture must be precisely defined to ensure uniform flow dynamics and cellular interactions that accurately recapitulate the in vivo extravasation process. Additionally, standardized methods for endothelial barrier formation and characterization are needed to ensure consistent vascular integrity across experiments.
Cell sourcing and preparation protocols require rigorous standardization. Guidelines must specify acceptable passage numbers, culture conditions, and quality control metrics for both endothelial and tumor cells. Patient-derived cells introduce additional complexity, necessitating standardized isolation and characterization procedures to maintain reproducibility while preserving patient-specific characteristics.
Validation requirements must address both biological and engineering aspects of these platforms. Biological validation should include comparison with in vivo extravasation dynamics, verification of endothelial barrier function, and confirmation of physiologically relevant tumor cell behaviors. Engineering validation must ensure consistent fluid dynamics, stable temperature and gas exchange, and reliable imaging capabilities across different experimental setups.
Quantitative metrics for extravasation events need standardization to enable objective assessment and comparison. These metrics should include extravasation efficiency, transmigration time, endothelial junction disruption measurements, and post-extravasation tumor cell behavior characterization. Advanced imaging protocols and analysis algorithms should be standardized to minimize subjective interpretation of results.
Regulatory considerations must also be addressed, particularly for platforms intended for drug screening or personalized medicine applications. This includes establishing validation criteria that satisfy regulatory requirements for preclinical models and developing quality control procedures that ensure consistent performance over time.
Inter-laboratory validation studies represent a crucial step toward broader acceptance of these platforms. Collaborative efforts involving multiple research groups using identical protocols with different equipment and personnel would significantly strengthen confidence in the robustness and reproducibility of vascularized tumor-on-chip platforms for modeling metastatic extravasation.
Establishing standardized fabrication protocols is essential for consistent device production. This includes specifications for materials selection, channel dimensions, membrane properties, and surface treatments. The microfluidic architecture must be precisely defined to ensure uniform flow dynamics and cellular interactions that accurately recapitulate the in vivo extravasation process. Additionally, standardized methods for endothelial barrier formation and characterization are needed to ensure consistent vascular integrity across experiments.
Cell sourcing and preparation protocols require rigorous standardization. Guidelines must specify acceptable passage numbers, culture conditions, and quality control metrics for both endothelial and tumor cells. Patient-derived cells introduce additional complexity, necessitating standardized isolation and characterization procedures to maintain reproducibility while preserving patient-specific characteristics.
Validation requirements must address both biological and engineering aspects of these platforms. Biological validation should include comparison with in vivo extravasation dynamics, verification of endothelial barrier function, and confirmation of physiologically relevant tumor cell behaviors. Engineering validation must ensure consistent fluid dynamics, stable temperature and gas exchange, and reliable imaging capabilities across different experimental setups.
Quantitative metrics for extravasation events need standardization to enable objective assessment and comparison. These metrics should include extravasation efficiency, transmigration time, endothelial junction disruption measurements, and post-extravasation tumor cell behavior characterization. Advanced imaging protocols and analysis algorithms should be standardized to minimize subjective interpretation of results.
Regulatory considerations must also be addressed, particularly for platforms intended for drug screening or personalized medicine applications. This includes establishing validation criteria that satisfy regulatory requirements for preclinical models and developing quality control procedures that ensure consistent performance over time.
Inter-laboratory validation studies represent a crucial step toward broader acceptance of these platforms. Collaborative efforts involving multiple research groups using identical protocols with different equipment and personnel would significantly strengthen confidence in the robustness and reproducibility of vascularized tumor-on-chip platforms for modeling metastatic extravasation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!