Influence of donor age and sex on organ-on-chip cellular responses: a systematic study
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Organ-on-Chip Technology Background and Objectives
Organ-on-chip (OoC) technology represents a revolutionary advancement in biomedical research, emerging at the intersection of tissue engineering, microfluidics, and cell biology. This innovative platform has evolved significantly since its conceptualization in the early 2000s, with major developmental milestones occurring around 2010 when the first functional lung-on-chip was demonstrated by researchers at Harvard's Wyss Institute. The technology aims to create physiologically relevant microenvironments that mimic the structure and function of human organs at microscale.
The evolution of OoC systems has been driven by limitations of traditional cell culture methods and animal models, which often fail to accurately predict human responses to drugs and diseases. Traditional 2D cell cultures lack the complex architecture and mechanical cues present in native tissues, while animal models frequently fail to translate to human outcomes due to species-specific differences in physiology and metabolism.
Current OoC platforms incorporate multiple cell types arranged in three-dimensional configurations with dynamic fluid flow, enabling more realistic modeling of organ function. These systems typically utilize transparent polymeric materials with microchannels that support cell growth and tissue-tissue interfaces, often incorporating sensors for real-time monitoring of cellular responses.
The technological trajectory shows increasing sophistication in mimicking organ complexity, with progression from single-organ systems to multi-organ platforms that can simulate organ interactions. Recent advances include the integration of patient-derived cells, enabling personalized medicine applications and disease modeling with unprecedented fidelity.
A critical yet underexplored aspect of OoC technology is the influence of donor demographics on cellular responses within these systems. Understanding how factors such as age and sex affect cellular behavior in OoC platforms is essential for developing more predictive models and translating findings to diverse patient populations.
The primary objectives of investigating donor age and sex influences on OoC responses include: establishing standardized protocols for donor selection in OoC studies; quantifying the variability in cellular responses attributable to donor demographics; developing computational models that account for age and sex-related differences; and ultimately creating more representative and predictive OoC platforms for drug development and disease modeling.
This systematic study aims to address a significant gap in current OoC research by characterizing how donor variability impacts experimental outcomes, thereby enhancing the translational value of these technologies for precision medicine applications. The findings will contribute to establishing best practices for donor selection and experimental design in OoC research, potentially accelerating the adoption of these platforms in pharmaceutical development and clinical applications.
The evolution of OoC systems has been driven by limitations of traditional cell culture methods and animal models, which often fail to accurately predict human responses to drugs and diseases. Traditional 2D cell cultures lack the complex architecture and mechanical cues present in native tissues, while animal models frequently fail to translate to human outcomes due to species-specific differences in physiology and metabolism.
Current OoC platforms incorporate multiple cell types arranged in three-dimensional configurations with dynamic fluid flow, enabling more realistic modeling of organ function. These systems typically utilize transparent polymeric materials with microchannels that support cell growth and tissue-tissue interfaces, often incorporating sensors for real-time monitoring of cellular responses.
The technological trajectory shows increasing sophistication in mimicking organ complexity, with progression from single-organ systems to multi-organ platforms that can simulate organ interactions. Recent advances include the integration of patient-derived cells, enabling personalized medicine applications and disease modeling with unprecedented fidelity.
A critical yet underexplored aspect of OoC technology is the influence of donor demographics on cellular responses within these systems. Understanding how factors such as age and sex affect cellular behavior in OoC platforms is essential for developing more predictive models and translating findings to diverse patient populations.
The primary objectives of investigating donor age and sex influences on OoC responses include: establishing standardized protocols for donor selection in OoC studies; quantifying the variability in cellular responses attributable to donor demographics; developing computational models that account for age and sex-related differences; and ultimately creating more representative and predictive OoC platforms for drug development and disease modeling.
This systematic study aims to address a significant gap in current OoC research by characterizing how donor variability impacts experimental outcomes, thereby enhancing the translational value of these technologies for precision medicine applications. The findings will contribute to establishing best practices for donor selection and experimental design in OoC research, potentially accelerating the adoption of these platforms in pharmaceutical development and clinical applications.
Market Analysis for Age/Sex-Specific Organ-on-Chip Models
The organ-on-chip (OOC) market specifically targeting age and sex-specific models represents a rapidly growing segment within the broader OOC industry. Current market valuations place the global OOC market at approximately $150 million in 2023, with projections indicating growth to reach $1.2 billion by 2030, representing a compound annual growth rate (CAGR) of 34.2%. Within this broader market, age and sex-specific models are emerging as a critical differentiation factor, potentially accounting for 25-30% of the total market value by 2025.
The primary demand drivers for age and sex-specific OOC models stem from several key sectors. Pharmaceutical companies represent the largest customer segment, with increasing recognition that drug efficacy and toxicity profiles vary significantly based on patient demographics. Recent FDA initiatives encouraging sex-specific and age-specific drug testing have accelerated this demand, with major pharmaceutical companies allocating dedicated research budgets for demographic-specific drug testing platforms.
Academic and research institutions constitute the second-largest market segment, focusing on fundamental biological research into age and sex differences in tissue function and disease progression. Government funding agencies, including the NIH and European research councils, have established specific grant programs targeting sex-based differences in disease mechanisms, creating substantial market pull.
Regulatory pressures are significantly influencing market growth, with both the FDA and EMA implementing guidelines that increasingly require sex-specific and age-specific preclinical data for drug approvals. This regulatory shift has created immediate market demand for validated testing platforms that can demonstrate demographic variations in drug responses.
Market segmentation analysis reveals distinct product categories emerging within this space. Age-specific OOC models currently dominate with approximately 60% market share, focusing primarily on pediatric versus geriatric tissue responses. Sex-specific models account for roughly 40% of the market, with female-specific models seeing particularly strong growth due to historical underrepresentation in clinical trials.
Geographically, North America leads market adoption (45% share), followed by Europe (30%) and Asia-Pacific (20%), with the remainder distributed globally. The Asia-Pacific region, particularly China and South Korea, is experiencing the fastest growth rate at 38% annually, driven by substantial government investment in biotechnology infrastructure.
Customer willingness-to-pay analysis indicates premium pricing potential for demographically specialized OOC models, with customers accepting 30-40% price premiums over standard models when validated age/sex-specific data is provided. This pricing elasticity reflects the significant downstream value these models create in reducing late-stage clinical trial failures.
The primary demand drivers for age and sex-specific OOC models stem from several key sectors. Pharmaceutical companies represent the largest customer segment, with increasing recognition that drug efficacy and toxicity profiles vary significantly based on patient demographics. Recent FDA initiatives encouraging sex-specific and age-specific drug testing have accelerated this demand, with major pharmaceutical companies allocating dedicated research budgets for demographic-specific drug testing platforms.
Academic and research institutions constitute the second-largest market segment, focusing on fundamental biological research into age and sex differences in tissue function and disease progression. Government funding agencies, including the NIH and European research councils, have established specific grant programs targeting sex-based differences in disease mechanisms, creating substantial market pull.
Regulatory pressures are significantly influencing market growth, with both the FDA and EMA implementing guidelines that increasingly require sex-specific and age-specific preclinical data for drug approvals. This regulatory shift has created immediate market demand for validated testing platforms that can demonstrate demographic variations in drug responses.
Market segmentation analysis reveals distinct product categories emerging within this space. Age-specific OOC models currently dominate with approximately 60% market share, focusing primarily on pediatric versus geriatric tissue responses. Sex-specific models account for roughly 40% of the market, with female-specific models seeing particularly strong growth due to historical underrepresentation in clinical trials.
Geographically, North America leads market adoption (45% share), followed by Europe (30%) and Asia-Pacific (20%), with the remainder distributed globally. The Asia-Pacific region, particularly China and South Korea, is experiencing the fastest growth rate at 38% annually, driven by substantial government investment in biotechnology infrastructure.
Customer willingness-to-pay analysis indicates premium pricing potential for demographically specialized OOC models, with customers accepting 30-40% price premiums over standard models when validated age/sex-specific data is provided. This pricing elasticity reflects the significant downstream value these models create in reducing late-stage clinical trial failures.
Current Challenges in Donor Variability Integration
Despite significant advancements in organ-on-chip (OOC) technology, integrating donor variability remains one of the most formidable challenges in the field. Current OOC systems often utilize cells from limited donor sources, failing to account for the biological diversity present in human populations. This oversight creates a substantial gap between laboratory findings and clinical applications, as cellular responses observed in chips may not accurately represent the heterogeneity seen in real-world patient populations.
The influence of donor age presents a particularly complex challenge. Cellular senescence, telomere length, mitochondrial function, and epigenetic modifications vary dramatically across different age groups. These age-related cellular changes significantly impact drug metabolism, inflammatory responses, and regenerative capacity, yet most OOC systems utilize cells from younger donors, creating a biased representation that may not translate to elderly populations where many diseases are more prevalent.
Sex-based differences compound these challenges further. Hormonal influences, X-chromosome inactivation patterns, and sex-specific gene expression profiles create distinct cellular microenvironments that affect drug responses and disease progression. Current OOC platforms rarely incorporate these sex-specific variables, potentially leading to inaccurate predictions of therapeutic efficacy and toxicity profiles across different sexes.
Technical limitations in maintaining donor-specific characteristics during cell expansion and culture represent another significant hurdle. Extended cultivation periods often result in phenotypic drift, where cells gradually lose their donor-specific traits and adopt more homogeneous characteristics. This drift undermines the very purpose of incorporating donor diversity into OOC systems.
Standardization issues further complicate donor variability integration. The lack of universally accepted protocols for characterizing donor-specific cellular responses makes cross-study comparisons challenging. Without standardized metrics for evaluating how donor characteristics influence chip performance, researchers struggle to develop reliable models that account for population heterogeneity.
Resource constraints also impede progress in this area. Sourcing cells from diverse donor populations requires extensive biobanking infrastructure and raises complex ethical and regulatory considerations regarding consent and privacy. The additional costs associated with developing and validating multiple donor-specific chip models often exceed research budgets, forcing compromises that limit donor diversity representation.
Computational challenges in data integration and analysis present yet another obstacle. The multidimensional datasets generated from donor-diverse OOC systems require sophisticated analytical approaches to identify meaningful patterns while distinguishing between donor-specific variations and experimental noise. Current computational frameworks are often inadequate for handling this complexity, limiting researchers' ability to derive actionable insights from donor-variable OOC studies.
The influence of donor age presents a particularly complex challenge. Cellular senescence, telomere length, mitochondrial function, and epigenetic modifications vary dramatically across different age groups. These age-related cellular changes significantly impact drug metabolism, inflammatory responses, and regenerative capacity, yet most OOC systems utilize cells from younger donors, creating a biased representation that may not translate to elderly populations where many diseases are more prevalent.
Sex-based differences compound these challenges further. Hormonal influences, X-chromosome inactivation patterns, and sex-specific gene expression profiles create distinct cellular microenvironments that affect drug responses and disease progression. Current OOC platforms rarely incorporate these sex-specific variables, potentially leading to inaccurate predictions of therapeutic efficacy and toxicity profiles across different sexes.
Technical limitations in maintaining donor-specific characteristics during cell expansion and culture represent another significant hurdle. Extended cultivation periods often result in phenotypic drift, where cells gradually lose their donor-specific traits and adopt more homogeneous characteristics. This drift undermines the very purpose of incorporating donor diversity into OOC systems.
Standardization issues further complicate donor variability integration. The lack of universally accepted protocols for characterizing donor-specific cellular responses makes cross-study comparisons challenging. Without standardized metrics for evaluating how donor characteristics influence chip performance, researchers struggle to develop reliable models that account for population heterogeneity.
Resource constraints also impede progress in this area. Sourcing cells from diverse donor populations requires extensive biobanking infrastructure and raises complex ethical and regulatory considerations regarding consent and privacy. The additional costs associated with developing and validating multiple donor-specific chip models often exceed research budgets, forcing compromises that limit donor diversity representation.
Computational challenges in data integration and analysis present yet another obstacle. The multidimensional datasets generated from donor-diverse OOC systems require sophisticated analytical approaches to identify meaningful patterns while distinguishing between donor-specific variations and experimental noise. Current computational frameworks are often inadequate for handling this complexity, limiting researchers' ability to derive actionable insights from donor-variable OOC studies.
Methodologies for Donor Variable Assessment
01 Microfluidic organ-on-chip platforms for cellular response analysis
Microfluidic organ-on-chip platforms enable the study of cellular responses in a controlled environment that mimics physiological conditions. These platforms incorporate microchannels and chambers where cells can be cultured and exposed to various stimuli. The technology allows for real-time monitoring of cellular responses, including changes in morphology, metabolism, and signaling pathways. These systems provide more physiologically relevant models compared to traditional 2D cell cultures, enabling better prediction of in vivo cellular responses.- Microfluidic organ-on-chip platforms for cellular response analysis: Microfluidic organ-on-chip platforms enable the study of cellular responses in a controlled environment that mimics physiological conditions. These platforms incorporate multiple cell types arranged in specific architectures to replicate organ functionality. The microfluidic systems allow for precise control of fluid flow, nutrient delivery, and waste removal, creating a dynamic environment for studying cellular responses to various stimuli including drugs, toxins, and mechanical forces.
- Real-time monitoring of cellular responses in organ-on-chip devices: Advanced sensing technologies integrated into organ-on-chip platforms enable real-time monitoring of cellular responses. These systems incorporate biosensors, imaging capabilities, and data acquisition systems to track cellular behavior, metabolic activity, and physiological responses continuously. The real-time monitoring capabilities allow researchers to observe immediate cellular reactions to stimuli and track long-term adaptations, providing comprehensive insights into cellular response mechanisms.
- Multi-organ integration for systemic cellular response studies: Multi-organ-on-chip systems connect multiple organ models to study systemic cellular responses and organ interactions. These integrated platforms allow for the investigation of complex physiological processes involving multiple organ systems, such as drug metabolism, toxicity pathways, and immune responses. By replicating organ-organ interactions, these systems provide insights into how cellular responses in one organ affect the function and responses of cells in other organs.
- Disease modeling and pathophysiological cellular responses: Organ-on-chip technology enables the modeling of disease states to study pathophysiological cellular responses. By incorporating patient-derived cells or genetically modified cells, these platforms can replicate disease conditions and allow for the investigation of cellular dysfunction, inflammatory responses, and disease progression mechanisms. These disease models provide valuable tools for understanding cellular responses in pathological conditions and for developing targeted therapeutic approaches.
- Drug screening and pharmacological response assessment: Organ-on-chip platforms serve as effective tools for drug screening and assessment of pharmacological cellular responses. These systems allow for high-throughput testing of drug candidates, evaluation of dose-response relationships, and investigation of drug efficacy and toxicity at the cellular level. The ability to replicate human physiology more accurately than traditional cell culture methods makes organ-on-chip technology valuable for predicting cellular responses to pharmaceutical compounds and optimizing drug development processes.
02 Integration of sensors in organ-on-chip devices for cellular response detection
Advanced sensor technologies are integrated into organ-on-chip platforms to detect and measure cellular responses with high sensitivity and specificity. These sensors can monitor various parameters such as pH, oxygen levels, electrical activity, and secreted biomolecules. The integration of these sensing capabilities enables continuous and non-invasive monitoring of cellular responses to different stimuli, providing valuable data on cell behavior and function in real-time. This approach enhances the utility of organ-on-chip platforms for drug screening and toxicity testing applications.Expand Specific Solutions03 Multi-organ chip systems for studying systemic cellular responses
Multi-organ chip systems connect multiple organ models on a single platform to study systemic cellular responses and organ-organ interactions. These systems allow for the investigation of complex physiological processes that involve multiple organs, such as drug metabolism and toxicity. By recreating the interconnected nature of human physiology, multi-organ chips provide insights into how cellular responses in one organ may affect other organs. This approach is particularly valuable for understanding systemic effects of drugs and environmental toxins on cellular responses across different tissues.Expand Specific Solutions04 Application of organ-on-chip technology for drug screening and toxicity testing
Organ-on-chip technology is increasingly used for drug screening and toxicity testing to evaluate cellular responses to pharmaceutical compounds. These platforms provide more predictive models of human physiology compared to traditional cell culture or animal models. By recreating the microenvironment of human organs, these systems can better predict drug efficacy and toxicity in humans. The technology enables high-throughput screening of drug candidates while reducing the need for animal testing, accelerating the drug development process and improving the prediction of cellular responses to potential therapeutic agents.Expand Specific Solutions05 Disease modeling using organ-on-chip technology for studying pathological cellular responses
Organ-on-chip platforms are used to model various diseases and study pathological cellular responses under controlled conditions. By incorporating patient-derived cells or genetically modified cells, these systems can recreate disease phenotypes and enable the study of disease mechanisms at the cellular level. This approach allows researchers to investigate how cells respond to disease conditions and potential therapeutic interventions. Disease models on chips provide valuable insights into pathological cellular responses that may lead to the development of new therapeutic strategies for various conditions.Expand Specific Solutions
Leading Organizations in Organ-on-Chip Research
The organ-on-chip technology market is currently in its growth phase, characterized by increasing research activities and emerging commercial applications. The market is projected to reach approximately $220 million by 2025, with a CAGR of 39.9%. Academic institutions like The Regents of the University of California, MIT, and Harvard's Broad Institute are leading fundamental research on donor variability effects, while companies such as CRISPR Therapeutics and Vertex Pharmaceuticals are exploring clinical applications. The technology is advancing toward maturity with research institutions like Georgia Tech and Industrial Technology Research Institute developing standardized protocols, though challenges remain in addressing biological variability. Commercial players including Allogene Therapeutics and Maxwell Biosciences are working to translate these findings into therapeutic applications, indicating a progressive shift from research to commercialization.
RWTH Aachen University
Technical Solution: RWTH Aachen University has developed an advanced organ-on-chip platform specifically engineered to investigate age and sex-dependent cellular responses. Their system employs a unique modular design that allows researchers to create physiologically relevant microenvironments tailored to specific donor demographics. The Aachen platform incorporates proprietary hydrogel formulations that mimic age-specific extracellular matrix compositions, accounting for the increased stiffness and altered protein content observed in aging tissues. A key innovation is their implementation of programmable hormone delivery systems that can simulate both the baseline differences between male and female physiology and the dynamic changes that occur throughout aging, such as menopause or andropause. The platform features integrated oxygen tension control that can replicate the reduced perfusion efficiency common in aged tissues. RWTH researchers have also developed specialized protocols for deriving and maintaining induced pluripotent stem cells from donors of different ages and sexes, ensuring that the starting cellular material accurately reflects the demographic variables being studied.
Strengths: Exceptional physiological relevance through precise control of multiple microenvironmental parameters; excellent long-term culture stability for aging studies; strong validation against in vivo models. Weaknesses: Higher technical complexity limits accessibility; requires specialized consumables that increase operational costs; more challenging to scale for high-throughput applications.
President & Fellows of Harvard College
Technical Solution: Harvard's organ-on-chip technology platform addresses donor age and sex variability through advanced microfluidic systems that precisely mimic physiological conditions. Their approach incorporates multi-organ integration with specialized microenvironments that account for hormonal differences between sexes and age-related cellular changes. Harvard researchers have developed proprietary surface functionalization techniques that allow for better cell adhesion and more accurate representation of native tissue architecture across different donor demographics. Their systems include continuous perfusion capabilities that simulate blood flow dynamics, which vary significantly with donor age, and integrated sensors that provide real-time monitoring of cellular responses to therapeutic interventions. Harvard's platform has demonstrated the ability to recapitulate age and sex-specific disease phenotypes, particularly in cardiovascular and metabolic disorders where these factors significantly impact pathophysiology and treatment response.
Strengths: Superior microfluidic engineering allows for precise control of cellular microenvironments; extensive validation across multiple tissue types and donor demographics; strong integration with computational modeling for predictive analytics. Weaknesses: Higher technical complexity requires specialized expertise; more expensive than traditional cell culture systems; longer setup time compared to conventional methods.
Critical Findings in Age/Sex-Dependent Cellular Responses
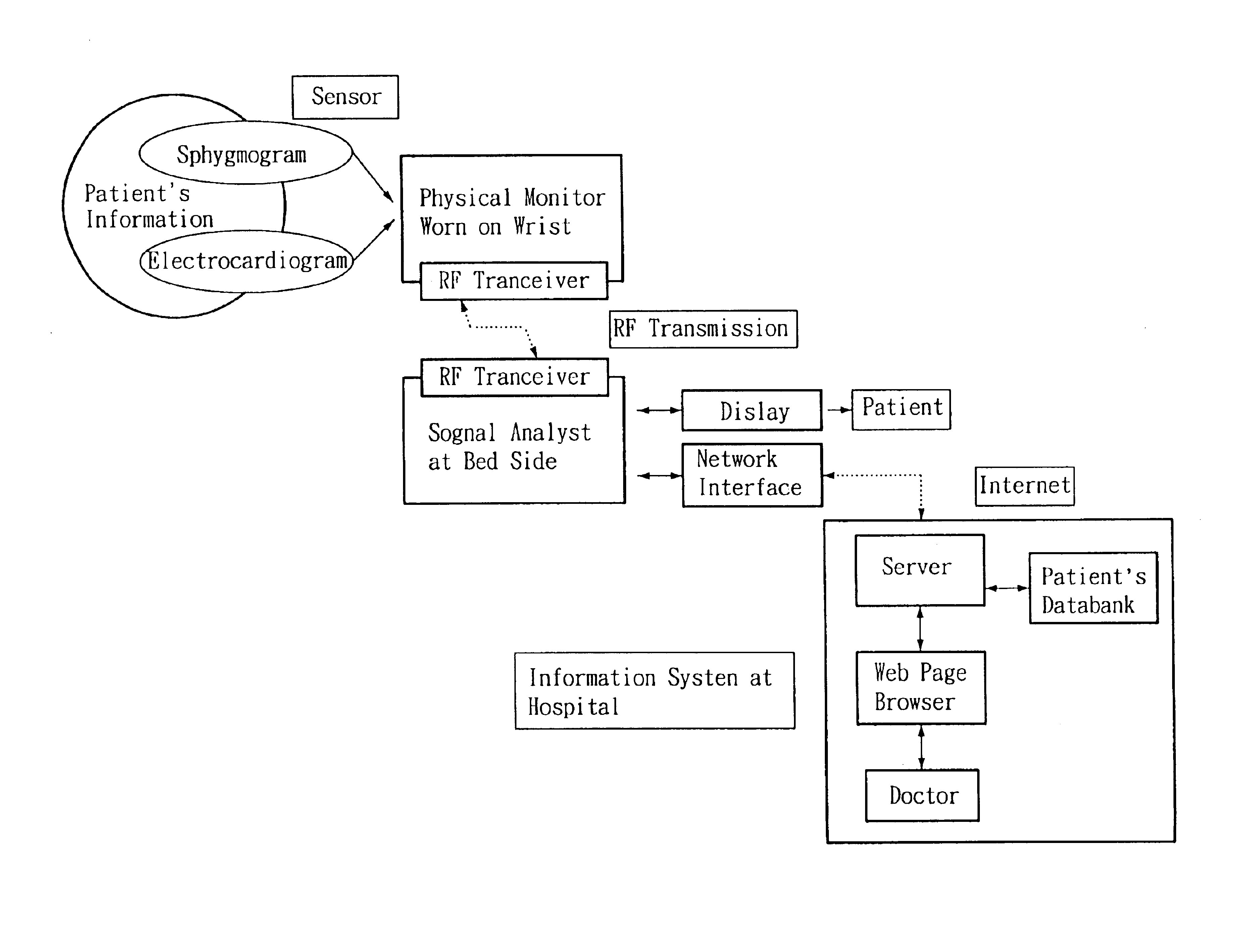
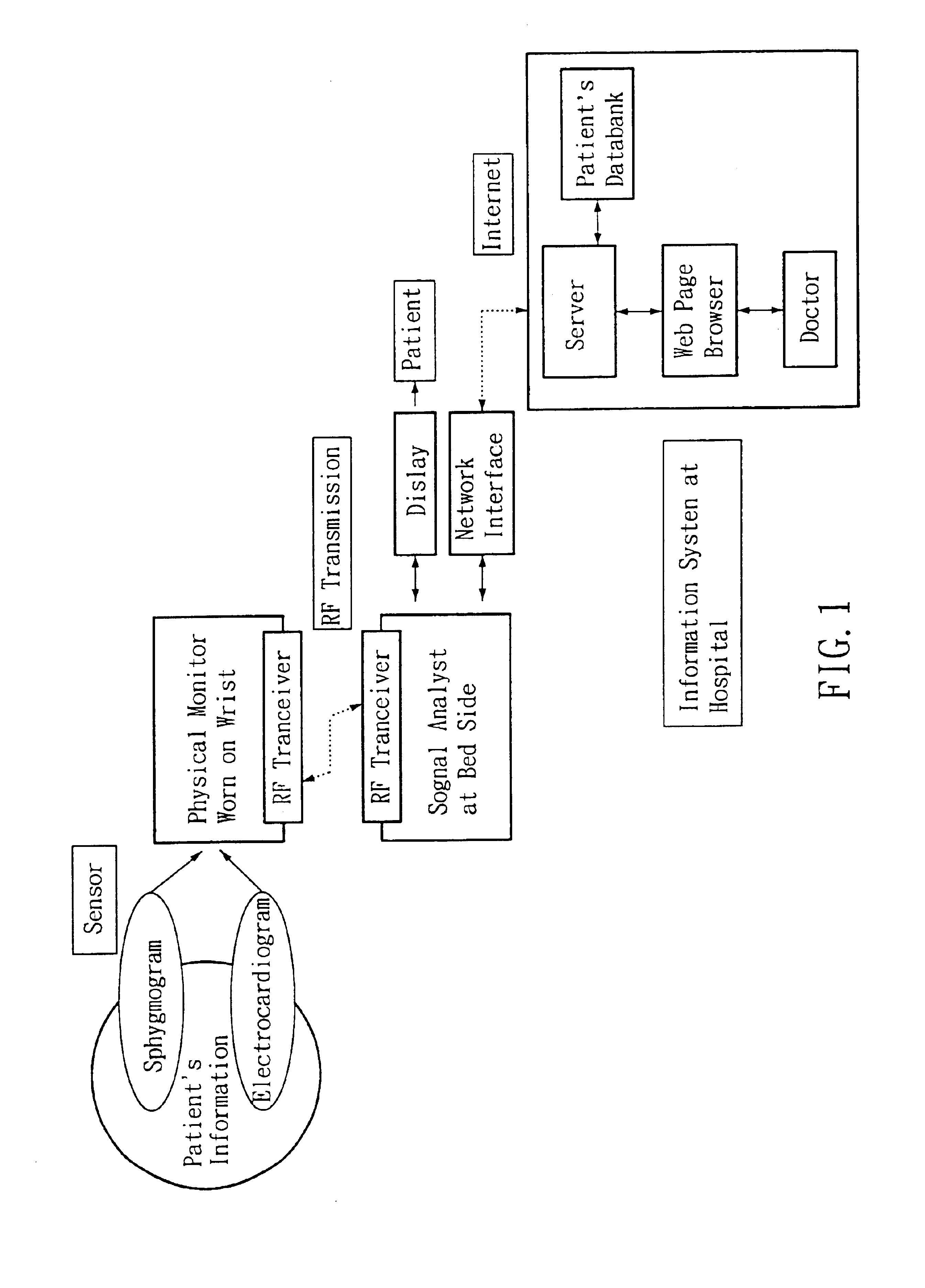
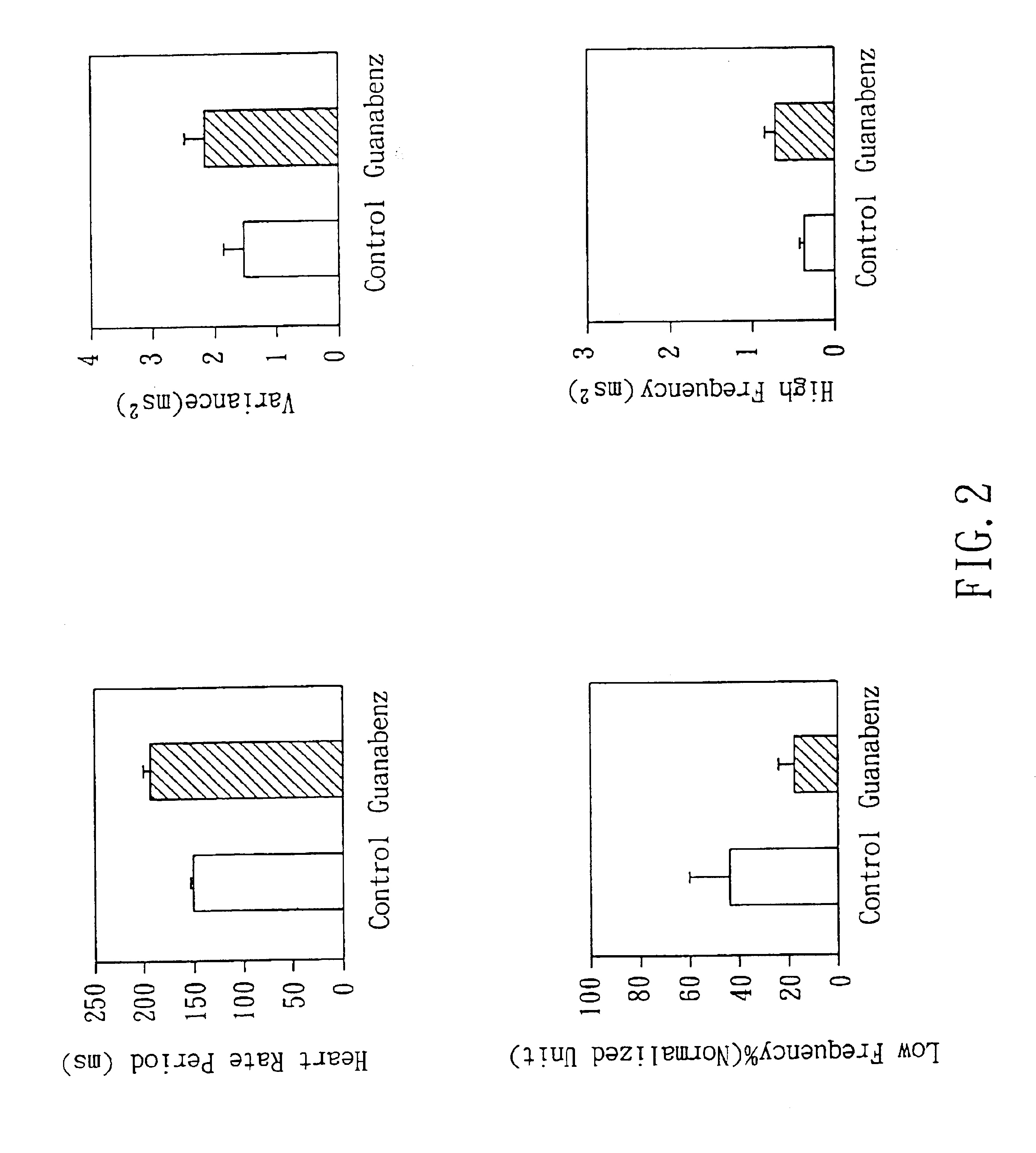
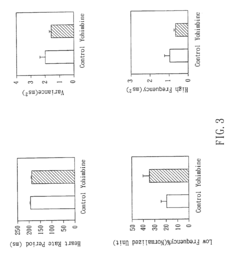
Non-invasive apparatus system for monitoring autonomic nervous system and uses thereof
PatentInactiveUS6811536B2
Innovation
- A non-invasive apparatus system using sensors to measure artery sphygmograms or heart potential signals, combined with Fourier Transform or Fast Fourier Transform analysis to calculate heart rate variability parameters, allowing for the monitoring of autonomic nervous system functions and providing early warnings for potential side effects and aging assessment.
Healthcare diagnostic
PatentInactiveUS20170233815A1
Innovation
- The use of a panel of 670 specific genes as biomarkers, selected from Table 1, to predict the likelihood of developing ageing-related diseases or assist in their diagnosis, combining gene expression levels in a linear or non-linear manner to provide a more accurate assessment of biological age.
Regulatory Considerations for Donor-Specific Models
The regulatory landscape for organ-on-chip (OOC) technologies incorporating donor-specific variables presents unique challenges that must be addressed for successful clinical and commercial implementation. Current regulatory frameworks were primarily designed for traditional drug development pathways and standardized testing models, creating a gap in guidance specifically tailored to donor-variable OOC systems. This regulatory uncertainty poses significant barriers to the translation of research findings into clinical applications.
Regulatory bodies including the FDA, EMA, and PMDA have begun recognizing the potential of OOC technologies through various innovation programs, but specific guidelines addressing donor diversity remain underdeveloped. The FDA's Predictive Toxicology Roadmap and the EMA's Innovation Task Force have acknowledged the need for considering biological variability in preclinical models, yet comprehensive regulatory pathways for donor-specific models are still evolving.
Key regulatory considerations for donor-specific OOC models include informed consent protocols that must address the unique aspects of using donor cells for long-term modeling. These protocols must clearly outline how genetic information will be handled, stored, and potentially shared, particularly when unexpected findings with clinical significance emerge during research.
Data standardization represents another critical regulatory challenge. The variability inherent in donor-specific models necessitates robust standardization protocols to ensure reproducibility and reliability across different research settings. Regulatory bodies increasingly require demonstration that donor-specific variations can be systematically documented and accounted for in data interpretation.
Privacy concerns are particularly pronounced for donor-specific models. Regulations such as GDPR in Europe and HIPAA in the United States impose strict requirements on handling identifiable biological information. OOC developers must implement comprehensive data protection measures that balance scientific utility with donor privacy rights.
Validation frameworks for donor-specific models present perhaps the most significant regulatory hurdle. Traditional validation approaches often rely on standardized cell lines and may not adequately address the inherent variability in donor-derived systems. Regulatory acceptance will require establishing clear validation protocols that account for age and sex-related differences while maintaining scientific rigor.
Moving forward, collaborative efforts between industry, academia, and regulatory agencies will be essential to develop appropriate regulatory frameworks that balance innovation with safety considerations. Regulatory science initiatives focused specifically on donor-variable OOC technologies could accelerate the development of much-needed guidance in this rapidly evolving field.
Regulatory bodies including the FDA, EMA, and PMDA have begun recognizing the potential of OOC technologies through various innovation programs, but specific guidelines addressing donor diversity remain underdeveloped. The FDA's Predictive Toxicology Roadmap and the EMA's Innovation Task Force have acknowledged the need for considering biological variability in preclinical models, yet comprehensive regulatory pathways for donor-specific models are still evolving.
Key regulatory considerations for donor-specific OOC models include informed consent protocols that must address the unique aspects of using donor cells for long-term modeling. These protocols must clearly outline how genetic information will be handled, stored, and potentially shared, particularly when unexpected findings with clinical significance emerge during research.
Data standardization represents another critical regulatory challenge. The variability inherent in donor-specific models necessitates robust standardization protocols to ensure reproducibility and reliability across different research settings. Regulatory bodies increasingly require demonstration that donor-specific variations can be systematically documented and accounted for in data interpretation.
Privacy concerns are particularly pronounced for donor-specific models. Regulations such as GDPR in Europe and HIPAA in the United States impose strict requirements on handling identifiable biological information. OOC developers must implement comprehensive data protection measures that balance scientific utility with donor privacy rights.
Validation frameworks for donor-specific models present perhaps the most significant regulatory hurdle. Traditional validation approaches often rely on standardized cell lines and may not adequately address the inherent variability in donor-derived systems. Regulatory acceptance will require establishing clear validation protocols that account for age and sex-related differences while maintaining scientific rigor.
Moving forward, collaborative efforts between industry, academia, and regulatory agencies will be essential to develop appropriate regulatory frameworks that balance innovation with safety considerations. Regulatory science initiatives focused specifically on donor-variable OOC technologies could accelerate the development of much-needed guidance in this rapidly evolving field.
Ethical Implications of Donor Demographic Representation
The ethical considerations surrounding donor demographic representation in organ-on-chip (OOC) technology present significant challenges for researchers, clinicians, and regulatory bodies. As demonstrated in the systematic study on the influence of donor age and sex on OOC cellular responses, biological variations across demographic groups can substantially impact experimental outcomes and subsequent clinical applications.
The underrepresentation of certain demographic groups in cell and tissue donation raises profound ethical concerns regarding the generalizability of research findings. When OOC models primarily utilize cells from limited demographic profiles—often young, male donors—the resulting technologies may perform suboptimally for women, elderly individuals, and ethnic minorities. This disparity potentially perpetuates existing healthcare inequities and contradicts principles of distributive justice in biomedical research.
Informed consent protocols for tissue donation must evolve to address the specific implications of demographic representation. Current consent frameworks rarely emphasize how donor characteristics might influence downstream applications in OOC technology. Donors should be made aware that their demographic attributes may affect how their biological materials contribute to model development and subsequent therapeutic applications.
Privacy concerns emerge when donor demographic data becomes integral to research interpretation. The potential for re-identification increases as more granular demographic information is linked to biological samples, particularly for donors from underrepresented groups. This necessitates robust data protection measures that balance scientific utility with donor confidentiality.
The commercialization of OOC technology introduces additional ethical tensions regarding equitable access. If development relies predominantly on certain demographic profiles, the resulting products may be optimized for specific populations, potentially limiting accessibility and effectiveness for others. This raises questions about corporate responsibility in ensuring demographic inclusivity throughout product development cycles.
Regulatory frameworks must adapt to address these ethical challenges by establishing guidelines for demographic representation in preclinical models. Current regulations rarely specify requirements for demographic diversity in cell sourcing for OOC platforms, creating a regulatory gap that may perpetuate biased technological development.
Research institutions bear responsibility for implementing policies that promote demographic diversity in tissue procurement. This includes developing targeted recruitment strategies for underrepresented donor groups and establishing transparent reporting standards for donor demographics in published research, ensuring accountability throughout the scientific community.
The underrepresentation of certain demographic groups in cell and tissue donation raises profound ethical concerns regarding the generalizability of research findings. When OOC models primarily utilize cells from limited demographic profiles—often young, male donors—the resulting technologies may perform suboptimally for women, elderly individuals, and ethnic minorities. This disparity potentially perpetuates existing healthcare inequities and contradicts principles of distributive justice in biomedical research.
Informed consent protocols for tissue donation must evolve to address the specific implications of demographic representation. Current consent frameworks rarely emphasize how donor characteristics might influence downstream applications in OOC technology. Donors should be made aware that their demographic attributes may affect how their biological materials contribute to model development and subsequent therapeutic applications.
Privacy concerns emerge when donor demographic data becomes integral to research interpretation. The potential for re-identification increases as more granular demographic information is linked to biological samples, particularly for donors from underrepresented groups. This necessitates robust data protection measures that balance scientific utility with donor confidentiality.
The commercialization of OOC technology introduces additional ethical tensions regarding equitable access. If development relies predominantly on certain demographic profiles, the resulting products may be optimized for specific populations, potentially limiting accessibility and effectiveness for others. This raises questions about corporate responsibility in ensuring demographic inclusivity throughout product development cycles.
Regulatory frameworks must adapt to address these ethical challenges by establishing guidelines for demographic representation in preclinical models. Current regulations rarely specify requirements for demographic diversity in cell sourcing for OOC platforms, creating a regulatory gap that may perpetuate biased technological development.
Research institutions bear responsibility for implementing policies that promote demographic diversity in tissue procurement. This includes developing targeted recruitment strategies for underrepresented donor groups and establishing transparent reporting standards for donor demographics in published research, ensuring accountability throughout the scientific community.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!