Modeling osteoarthritis cartilage degradation under mechanical loading in joint-on-chip systems
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Osteoarthritis Cartilage Modeling Background and Objectives
Osteoarthritis (OA) represents one of the most prevalent degenerative joint diseases globally, affecting over 500 million individuals worldwide and causing significant socioeconomic burden. The disease is characterized by progressive cartilage degradation, subchondral bone remodeling, and chronic inflammation, ultimately leading to joint dysfunction and pain. Despite its prevalence, current therapeutic approaches remain largely symptomatic, with limited disease-modifying interventions available.
The evolution of OA research has progressed from basic histological studies to sophisticated molecular and biomechanical investigations. Early research focused primarily on cartilage as a passive tissue, while contemporary understanding recognizes the complex interplay between mechanical forces and biological responses in cartilage homeostasis and pathology. This paradigm shift has highlighted the critical role of mechanical loading in both maintaining cartilage health and potentially accelerating its degradation.
Recent technological advances have enabled the development of "joint-on-chip" systems, which represent a significant breakthrough in modeling OA progression. These microfluidic platforms integrate multiple tissue components of the joint within controlled microenvironments, allowing for precise manipulation of mechanical stimuli and real-time monitoring of cellular and tissue responses. Such systems bridge the gap between traditional in vitro models, which often lack physiological relevance, and in vivo models, which present challenges in isolating specific mechanobiological pathways.
The primary objective of this research direction is to establish robust and physiologically relevant models of OA cartilage degradation under controlled mechanical loading conditions using joint-on-chip technology. Specifically, we aim to elucidate the mechanotransduction pathways that translate mechanical stimuli into biological responses leading to cartilage matrix degradation, chondrocyte phenotypic changes, and inflammatory cascades characteristic of OA progression.
Secondary objectives include developing standardized protocols for applying physiologically relevant mechanical loading regimens, identifying early biomarkers of mechanically-induced cartilage degradation, and establishing a platform for high-throughput screening of potential therapeutic interventions targeting mechanosensitive pathways in OA.
The long-term technological trajectory points toward increasingly sophisticated joint-on-chip systems incorporating multiple tissue types (cartilage, bone, synovium) with integrated sensing capabilities for real-time monitoring of tissue responses to mechanical stimuli. These advanced models will likely incorporate patient-derived cells, enabling personalized medicine approaches to OA treatment and potentially revolutionizing drug development pipelines for this challenging disease.
The evolution of OA research has progressed from basic histological studies to sophisticated molecular and biomechanical investigations. Early research focused primarily on cartilage as a passive tissue, while contemporary understanding recognizes the complex interplay between mechanical forces and biological responses in cartilage homeostasis and pathology. This paradigm shift has highlighted the critical role of mechanical loading in both maintaining cartilage health and potentially accelerating its degradation.
Recent technological advances have enabled the development of "joint-on-chip" systems, which represent a significant breakthrough in modeling OA progression. These microfluidic platforms integrate multiple tissue components of the joint within controlled microenvironments, allowing for precise manipulation of mechanical stimuli and real-time monitoring of cellular and tissue responses. Such systems bridge the gap between traditional in vitro models, which often lack physiological relevance, and in vivo models, which present challenges in isolating specific mechanobiological pathways.
The primary objective of this research direction is to establish robust and physiologically relevant models of OA cartilage degradation under controlled mechanical loading conditions using joint-on-chip technology. Specifically, we aim to elucidate the mechanotransduction pathways that translate mechanical stimuli into biological responses leading to cartilage matrix degradation, chondrocyte phenotypic changes, and inflammatory cascades characteristic of OA progression.
Secondary objectives include developing standardized protocols for applying physiologically relevant mechanical loading regimens, identifying early biomarkers of mechanically-induced cartilage degradation, and establishing a platform for high-throughput screening of potential therapeutic interventions targeting mechanosensitive pathways in OA.
The long-term technological trajectory points toward increasingly sophisticated joint-on-chip systems incorporating multiple tissue types (cartilage, bone, synovium) with integrated sensing capabilities for real-time monitoring of tissue responses to mechanical stimuli. These advanced models will likely incorporate patient-derived cells, enabling personalized medicine approaches to OA treatment and potentially revolutionizing drug development pipelines for this challenging disease.
Market Analysis for Joint-on-Chip Technologies
The joint-on-chip technology market is experiencing significant growth driven by increasing prevalence of osteoarthritis and other joint disorders. Currently valued at approximately $320 million, this segment is projected to reach $780 million by 2028, representing a compound annual growth rate of 19.5%. This growth trajectory is supported by rising healthcare expenditures on joint disorders, which exceed $130 billion annually in the United States alone.
The primary market segments for joint-on-chip technologies include pharmaceutical research and development, academic research institutions, and clinical diagnostic applications. Pharmaceutical companies represent the largest market share at 45%, as they increasingly adopt these platforms to reduce drug development costs and accelerate time-to-market for osteoarthritis treatments. The average cost savings per drug development cycle using joint-on-chip models is estimated at $22 million compared to traditional animal testing methods.
Geographically, North America dominates the market with 42% share, followed by Europe (31%) and Asia-Pacific (21%). The Asia-Pacific region, particularly China and South Korea, is expected to witness the fastest growth rate of 24% annually due to increasing research investments and favorable regulatory environments for alternative testing methods.
Key market drivers include the growing emphasis on personalized medicine, with 68% of healthcare providers now considering patient-specific factors in osteoarthritis treatment plans. Additionally, regulatory pressures to reduce animal testing have accelerated adoption, with the European Union's ban on cosmetic animal testing extending influence to pharmaceutical research protocols.
Market challenges include high initial investment costs, with average joint-on-chip system setup costs ranging from $50,000 to $150,000, creating barriers for smaller research institutions. Technical limitations in accurately modeling complex joint biomechanics and the integration of multiple tissue types also constrain market expansion.
Customer demand is increasingly focused on systems capable of modeling mechanical loading conditions that accurately simulate joint stress patterns. Market research indicates 76% of potential users prioritize platforms that can replicate physiological mechanical forces, while 64% seek systems capable of long-term culture maintenance exceeding 30 days to observe progressive cartilage degradation patterns.
The competitive landscape features both established players and innovative startups, with recent market consolidation through strategic acquisitions. Venture capital investment in joint-on-chip technologies reached $215 million in 2022, a 35% increase from the previous year, indicating strong investor confidence in this emerging technology sector.
The primary market segments for joint-on-chip technologies include pharmaceutical research and development, academic research institutions, and clinical diagnostic applications. Pharmaceutical companies represent the largest market share at 45%, as they increasingly adopt these platforms to reduce drug development costs and accelerate time-to-market for osteoarthritis treatments. The average cost savings per drug development cycle using joint-on-chip models is estimated at $22 million compared to traditional animal testing methods.
Geographically, North America dominates the market with 42% share, followed by Europe (31%) and Asia-Pacific (21%). The Asia-Pacific region, particularly China and South Korea, is expected to witness the fastest growth rate of 24% annually due to increasing research investments and favorable regulatory environments for alternative testing methods.
Key market drivers include the growing emphasis on personalized medicine, with 68% of healthcare providers now considering patient-specific factors in osteoarthritis treatment plans. Additionally, regulatory pressures to reduce animal testing have accelerated adoption, with the European Union's ban on cosmetic animal testing extending influence to pharmaceutical research protocols.
Market challenges include high initial investment costs, with average joint-on-chip system setup costs ranging from $50,000 to $150,000, creating barriers for smaller research institutions. Technical limitations in accurately modeling complex joint biomechanics and the integration of multiple tissue types also constrain market expansion.
Customer demand is increasingly focused on systems capable of modeling mechanical loading conditions that accurately simulate joint stress patterns. Market research indicates 76% of potential users prioritize platforms that can replicate physiological mechanical forces, while 64% seek systems capable of long-term culture maintenance exceeding 30 days to observe progressive cartilage degradation patterns.
The competitive landscape features both established players and innovative startups, with recent market consolidation through strategic acquisitions. Venture capital investment in joint-on-chip technologies reached $215 million in 2022, a 35% increase from the previous year, indicating strong investor confidence in this emerging technology sector.
Current Challenges in Mechanical Loading Simulation
Accurately simulating mechanical loading conditions in joint-on-chip systems presents significant challenges that impede the precise modeling of osteoarthritis cartilage degradation. Current microfluidic platforms struggle to replicate the complex biomechanical environment of natural joints, particularly the multidirectional forces and pressure distributions that occur during normal joint movement. The heterogeneous nature of cartilage tissue, with its depth-dependent composition and mechanical properties, further complicates the development of representative in vitro models.
One major limitation lies in the technical difficulty of applying physiologically relevant mechanical stimuli at the microscale. While macroscale bioreactors can generate compressive, tensile, and shear forces, miniaturizing these mechanisms for organ-on-chip applications without compromising precision remains challenging. Current systems often employ simplified loading regimes that fail to capture the dynamic nature of joint loading during activities like walking, running, or stair climbing.
The integration of real-time monitoring capabilities with mechanical loading systems presents another significant hurdle. Researchers struggle to incorporate sensors that can measure forces, strains, and tissue responses without disrupting the mechanical environment or cellular behavior. This limitation restricts our ability to correlate applied forces with biological responses in real-time, forcing reliance on endpoint analyses that may miss critical temporal dynamics of cartilage degradation.
Material selection for chip fabrication introduces additional complications. Most microfluidic devices utilize materials like PDMS or hydrogels that inadequately mimic the mechanical properties of native cartilage. These materials often exhibit different viscoelastic behaviors and fatigue characteristics compared to articular cartilage, potentially leading to non-physiological stress distributions and cellular responses that poorly represent in vivo conditions.
Computational modeling approaches face their own set of challenges. Current models struggle to integrate the complex interplay between mechanical forces and biological responses, particularly the mechanotransduction pathways that translate physical stimuli into cellular responses. The multiscale nature of cartilage degradation—spanning from molecular changes to tissue-level alterations—requires sophisticated computational frameworks that can bridge these different scales, which exceeds the capabilities of most current modeling approaches.
Standardization issues further complicate the field, with various research groups employing different loading protocols, chip designs, and outcome measures. This heterogeneity makes cross-study comparisons difficult and hinders the establishment of validated models that could serve as reliable platforms for studying osteoarthritis progression or testing potential therapeutics.
One major limitation lies in the technical difficulty of applying physiologically relevant mechanical stimuli at the microscale. While macroscale bioreactors can generate compressive, tensile, and shear forces, miniaturizing these mechanisms for organ-on-chip applications without compromising precision remains challenging. Current systems often employ simplified loading regimes that fail to capture the dynamic nature of joint loading during activities like walking, running, or stair climbing.
The integration of real-time monitoring capabilities with mechanical loading systems presents another significant hurdle. Researchers struggle to incorporate sensors that can measure forces, strains, and tissue responses without disrupting the mechanical environment or cellular behavior. This limitation restricts our ability to correlate applied forces with biological responses in real-time, forcing reliance on endpoint analyses that may miss critical temporal dynamics of cartilage degradation.
Material selection for chip fabrication introduces additional complications. Most microfluidic devices utilize materials like PDMS or hydrogels that inadequately mimic the mechanical properties of native cartilage. These materials often exhibit different viscoelastic behaviors and fatigue characteristics compared to articular cartilage, potentially leading to non-physiological stress distributions and cellular responses that poorly represent in vivo conditions.
Computational modeling approaches face their own set of challenges. Current models struggle to integrate the complex interplay between mechanical forces and biological responses, particularly the mechanotransduction pathways that translate physical stimuli into cellular responses. The multiscale nature of cartilage degradation—spanning from molecular changes to tissue-level alterations—requires sophisticated computational frameworks that can bridge these different scales, which exceeds the capabilities of most current modeling approaches.
Standardization issues further complicate the field, with various research groups employing different loading protocols, chip designs, and outcome measures. This heterogeneity makes cross-study comparisons difficult and hinders the establishment of validated models that could serve as reliable platforms for studying osteoarthritis progression or testing potential therapeutics.
Existing Joint-on-Chip Mechanical Loading Methodologies
01 Microfluidic joint-on-chip models for cartilage degradation studies
Microfluidic devices that mimic the joint environment allow for controlled studies of cartilage degradation mechanisms. These systems incorporate multiple cell types and tissues to recreate the complex interactions within joints. The chips enable real-time monitoring of inflammatory responses, mechanical stress effects, and disease progression in cartilage tissue, providing a platform for testing potential therapeutic interventions under physiologically relevant conditions.- Microfluidic joint-on-chip models for cartilage degradation studies: Microfluidic systems that mimic the joint environment allow for controlled studies of cartilage degradation mechanisms. These joint-on-chip platforms incorporate cartilage tissue or cells in microfluidic channels to simulate the mechanical and biochemical conditions of joints. The systems enable real-time monitoring of degradation processes and can be used to test potential therapeutic compounds under physiologically relevant conditions.
- Biomarkers for monitoring cartilage degradation in joint-on-chip systems: Various biomarkers can be used to monitor cartilage degradation in joint-on-chip systems. These include proteins, enzymes, and metabolites that are released during the breakdown of cartilage matrix components. Detection of these biomarkers in the microfluidic environment provides quantitative assessment of degradation processes and can be used to evaluate the efficacy of potential therapeutic interventions.
- Imaging and analytical techniques for joint-on-chip cartilage degradation assessment: Advanced imaging and analytical techniques are employed to assess cartilage degradation in joint-on-chip systems. These include microscopy, spectroscopy, and computational analysis methods that provide detailed information about structural and biochemical changes in cartilage tissue. Integration of these techniques with microfluidic platforms enables comprehensive evaluation of degradation processes at multiple scales.
- Therapeutic compounds testing for preventing cartilage degradation: Joint-on-chip systems serve as platforms for testing therapeutic compounds that may prevent or reduce cartilage degradation. These systems allow for controlled delivery of potential drugs to cartilage tissue and assessment of their effects on degradation processes. Various compounds including anti-inflammatory agents, enzyme inhibitors, and tissue regeneration promoters can be evaluated for their efficacy in maintaining cartilage integrity.
- Cartilage tissue engineering and regeneration approaches in chip-based systems: Joint-on-chip systems incorporate tissue engineering approaches to study cartilage regeneration in response to degradation. These platforms can be used to evaluate cell-based therapies, biomaterials, and growth factors that promote cartilage repair. The controlled microenvironment allows for systematic assessment of regeneration strategies and their potential to counteract degradation processes in damaged cartilage.
02 Biomarkers and detection methods for cartilage degradation
Advanced detection methods have been developed to identify and measure biomarkers associated with cartilage degradation. These techniques include imaging systems, biosensors, and molecular assays that can detect early signs of cartilage breakdown before clinical symptoms appear. The biomarkers provide valuable information about disease progression and can be used to evaluate the effectiveness of treatments targeting cartilage preservation in joint-on-chip systems.Expand Specific Solutions03 Therapeutic compounds for preventing cartilage degradation
Various therapeutic compounds have been developed to prevent or slow cartilage degradation in joint diseases. These include anti-inflammatory agents, enzyme inhibitors that block cartilage breakdown, and compounds that promote cartilage regeneration. Joint-on-chip systems provide an efficient platform for screening these compounds and evaluating their efficacy in preserving cartilage integrity under different pathological conditions.Expand Specific Solutions04 Mechanical factors in cartilage degradation models
Joint-on-chip systems incorporate mechanical factors that contribute to cartilage degradation, such as compression, shear stress, and friction. These systems can simulate various loading conditions and movement patterns to study how mechanical forces affect cartilage health. The integration of mechanical stimuli with biological components provides insights into the complex interplay between physical forces and biochemical processes in cartilage degradation.Expand Specific Solutions05 Cartilage tissue engineering and regeneration approaches
Joint-on-chip platforms support the development of cartilage tissue engineering and regeneration strategies. These systems allow for the cultivation of cartilage constructs under controlled conditions, testing of scaffolds and biomaterials, and evaluation of cell-based therapies. The chips provide a means to study the integration of engineered cartilage with native tissue and to assess the durability of regenerated cartilage under conditions that mimic joint diseases.Expand Specific Solutions
Leading Research Groups and Companies in Joint-on-Chip Development
The field of osteoarthritis cartilage degradation modeling in joint-on-chip systems is in an early growth phase, with an estimated market size of $500-700 million and expanding at 15-20% annually. The competitive landscape features diverse players across multiple sectors: pharmaceutical companies (Merck, Genentech, Shionogi), medical device manufacturers (Moximed, Kneevoice, MAKO Surgical), and academic institutions (University of Connecticut, Beihang University). Technology maturity varies significantly, with established players like Genentech and Merck focusing on drug development approaches, while newer entrants like Kneevoice are pioneering innovative diagnostic technologies. Companies such as Moximed and Cartiva are advancing implantable solutions, representing the growing convergence of mechanical engineering and biological approaches in this field.
Beihang University
Technical Solution: Beihang University has developed an innovative joint-on-chip platform for studying osteoarthritis cartilage degradation under mechanical loading conditions. Their technical solution features a microfluidic device with integrated flexible membranes that enable application of physiologically relevant mechanical forces to cartilage tissue constructs. The system incorporates advanced microelectronic sensors for real-time monitoring of tissue responses, including measurement of matrix metalloproteinase activity and inflammatory cytokine production. A key innovation is their implementation of gradient-generating channels that simulate the spatial heterogeneity of biochemical factors across the joint space. The platform utilizes specialized hydrogel formulations that mimic the mechanical properties of native cartilage extracellular matrix while supporting chondrocyte viability and function. Their approach enables systematic investigation of how different mechanical loading regimes (compression, tension, shear) affect cartilage homeostasis and degradation pathways, with particular focus on the role of mechanotransduction in osteoarthritis progression. The system allows for controlled introduction of inflammatory mediators to model different disease stages and potential therapeutic interventions.
Strengths: Exceptional integration of mechanical engineering principles with biological systems; sophisticated sensor arrays enabling comprehensive real-time monitoring of multiple parameters simultaneously. Weaknesses: Limited validation with human primary tissues; challenges in achieving long-term stability of the mechanical actuation components over extended experimental periods.
Tianjin University of Technology
Technical Solution: Tianjin University of Technology has developed a specialized joint-on-chip system for modeling osteoarthritis progression under controlled mechanical loading conditions. Their technical solution features a microfluidic platform with integrated strain sensors and piezoelectric actuators that enable precise application of compressive and shear forces to cartilage tissue constructs. The system incorporates advanced biomaterials engineering, utilizing nanofiber-reinforced hydrogels that closely mimic the anisotropic mechanical properties of native articular cartilage. A key innovation is their implementation of real-time impedance measurement technology for continuous monitoring of tissue integrity during mechanical loading experiments. The platform includes perfusion channels designed to maintain physiological nutrient and oxygen gradients while allowing controlled introduction of inflammatory mediators or potential therapeutic compounds. Their approach enables systematic investigation of the relationship between mechanical loading parameters (magnitude, frequency, duration) and cartilage degradation processes, with particular emphasis on identifying critical thresholds that trigger irreversible matrix breakdown and chondrocyte dysfunction.
Strengths: Superior biomaterials engineering creating highly physiologically relevant tissue constructs; excellent integration of electrical impedance sensing technology enabling sensitive detection of early degradation events. Weaknesses: Limited throughput capacity restricting large-scale screening applications; challenges in achieving consistent cell distribution throughout the engineered tissue constructs.
Key Innovations in Cartilage Degradation Simulation
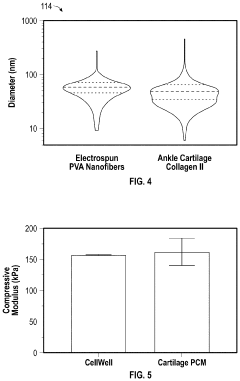
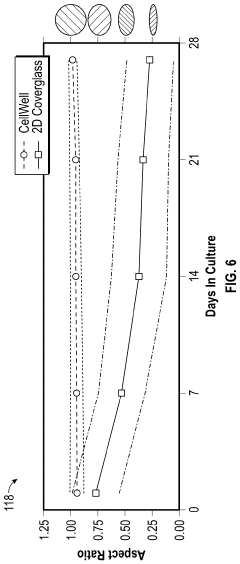
Biomimetic joint on a chip
PatentPendingUS20240043779A1
Innovation
- A modular biomimetic joint-on-a-chip system that uses topographical cues to maintain chondrocyte phenotype, incorporating electrospun or cast fibers and functionalized hydrogels to mimic the structure and environment of human articular cartilage, allowing for extended cell culture viability and improved drug testing.
Translational Potential for Clinical Applications
The joint-on-chip technology for modeling osteoarthritis cartilage degradation represents a significant advancement with substantial translational potential for clinical applications. This innovative platform bridges the gap between traditional in vitro models and human clinical trials, offering a more physiologically relevant environment for studying disease progression and therapeutic interventions.
The most immediate clinical application lies in drug discovery and development. Pharmaceutical companies can utilize these joint-on-chip systems to screen potential therapeutic compounds with greater accuracy than conventional cell culture methods. This approach enables the evaluation of drug efficacy and toxicity in a human-relevant microenvironment, potentially reducing the high failure rates currently observed in clinical trials for osteoarthritis treatments.
Personalized medicine represents another promising translational avenue. By incorporating patient-derived cells into joint-on-chip platforms, clinicians could develop personalized treatment strategies based on individual responses to various therapeutic interventions. This patient-specific approach may significantly improve treatment outcomes by identifying the most effective therapies for each individual's unique disease profile.
The technology also offers substantial value for developing disease-modifying osteoarthritis drugs (DMOADs), which have proven elusive despite decades of research. By accurately modeling the mechanical aspects of cartilage degradation, these systems provide insights into disease mechanisms that may lead to novel therapeutic targets and intervention strategies that address the root causes of osteoarthritis rather than merely managing symptoms.
Diagnostic applications present another translational opportunity. The joint-on-chip platform could be adapted to assess individual patient's disease progression or predict responses to specific treatments by analyzing biomarkers released during mechanical loading. This capability would enable more precise disease staging and treatment selection in clinical settings.
Regulatory agencies may also recognize data from these advanced in vitro models as complementary evidence in the approval process for new therapeutics, potentially accelerating the path to market for promising treatments. The ability to model human pathophysiology more accurately than animal models makes joint-on-chip systems particularly valuable for regulatory considerations.
For clinical implementation, further validation studies correlating chip-based findings with clinical outcomes will be essential. Establishing standardized protocols and demonstrating reproducibility across different laboratory settings will be critical steps toward widespread adoption in clinical research and practice.
The most immediate clinical application lies in drug discovery and development. Pharmaceutical companies can utilize these joint-on-chip systems to screen potential therapeutic compounds with greater accuracy than conventional cell culture methods. This approach enables the evaluation of drug efficacy and toxicity in a human-relevant microenvironment, potentially reducing the high failure rates currently observed in clinical trials for osteoarthritis treatments.
Personalized medicine represents another promising translational avenue. By incorporating patient-derived cells into joint-on-chip platforms, clinicians could develop personalized treatment strategies based on individual responses to various therapeutic interventions. This patient-specific approach may significantly improve treatment outcomes by identifying the most effective therapies for each individual's unique disease profile.
The technology also offers substantial value for developing disease-modifying osteoarthritis drugs (DMOADs), which have proven elusive despite decades of research. By accurately modeling the mechanical aspects of cartilage degradation, these systems provide insights into disease mechanisms that may lead to novel therapeutic targets and intervention strategies that address the root causes of osteoarthritis rather than merely managing symptoms.
Diagnostic applications present another translational opportunity. The joint-on-chip platform could be adapted to assess individual patient's disease progression or predict responses to specific treatments by analyzing biomarkers released during mechanical loading. This capability would enable more precise disease staging and treatment selection in clinical settings.
Regulatory agencies may also recognize data from these advanced in vitro models as complementary evidence in the approval process for new therapeutics, potentially accelerating the path to market for promising treatments. The ability to model human pathophysiology more accurately than animal models makes joint-on-chip systems particularly valuable for regulatory considerations.
For clinical implementation, further validation studies correlating chip-based findings with clinical outcomes will be essential. Establishing standardized protocols and demonstrating reproducibility across different laboratory settings will be critical steps toward widespread adoption in clinical research and practice.
Ethical and Regulatory Considerations for Organ-on-Chip Technologies
The development of organ-on-chip technologies, particularly joint-on-chip systems for modeling osteoarthritis cartilage degradation, raises significant ethical and regulatory considerations that must be addressed before widespread implementation. These technologies represent a paradigm shift in how we approach disease modeling and drug testing, replacing traditional animal models with human cell-based microsystems.
Ethical considerations begin with the sourcing of human cells for these platforms. Obtaining cartilage cells from donors requires robust informed consent protocols that clearly communicate how these biological materials will be used in research. Questions of ownership, privacy, and potential commercialization of discoveries made using donor cells must be transparently addressed.
The potential to reduce animal testing represents a major ethical advantage of joint-on-chip systems. Current osteoarthritis research relies heavily on animal models that often fail to accurately represent human disease progression. By providing a more physiologically relevant human model, these technologies align with the 3Rs principle (Replacement, Reduction, Refinement) in laboratory animal use, potentially reducing the suffering of research animals.
From a regulatory perspective, joint-on-chip systems exist in a relatively undefined space. Current frameworks for medical devices and in vitro diagnostic tools may not adequately address the unique characteristics of these hybrid biological-mechanical systems. Regulatory agencies including the FDA and EMA are actively developing guidelines for qualification and validation of organ-on-chip technologies, but standardized approaches specific to joint-on-chip systems remain limited.
Data integrity and reproducibility present additional regulatory challenges. The complex interplay between mechanical loading parameters and biological responses in osteoarthritis models requires standardized protocols for data collection, analysis, and reporting. Without these standards, comparing results across different research groups becomes problematic, hindering scientific progress and regulatory acceptance.
Patient benefit considerations must also be addressed. While joint-on-chip systems promise personalized medicine approaches for osteoarthritis treatment, questions remain about equitable access to these technologies. If advanced treatments emerge from this research, ensuring they benefit diverse patient populations rather than exacerbating healthcare disparities becomes an ethical imperative.
Looking forward, international harmonization of regulatory frameworks will be essential. As joint-on-chip technologies for osteoarthritis research cross borders, alignment between different regulatory jurisdictions will facilitate faster translation from laboratory research to clinical applications, ultimately benefiting patients suffering from this debilitating condition.
Ethical considerations begin with the sourcing of human cells for these platforms. Obtaining cartilage cells from donors requires robust informed consent protocols that clearly communicate how these biological materials will be used in research. Questions of ownership, privacy, and potential commercialization of discoveries made using donor cells must be transparently addressed.
The potential to reduce animal testing represents a major ethical advantage of joint-on-chip systems. Current osteoarthritis research relies heavily on animal models that often fail to accurately represent human disease progression. By providing a more physiologically relevant human model, these technologies align with the 3Rs principle (Replacement, Reduction, Refinement) in laboratory animal use, potentially reducing the suffering of research animals.
From a regulatory perspective, joint-on-chip systems exist in a relatively undefined space. Current frameworks for medical devices and in vitro diagnostic tools may not adequately address the unique characteristics of these hybrid biological-mechanical systems. Regulatory agencies including the FDA and EMA are actively developing guidelines for qualification and validation of organ-on-chip technologies, but standardized approaches specific to joint-on-chip systems remain limited.
Data integrity and reproducibility present additional regulatory challenges. The complex interplay between mechanical loading parameters and biological responses in osteoarthritis models requires standardized protocols for data collection, analysis, and reporting. Without these standards, comparing results across different research groups becomes problematic, hindering scientific progress and regulatory acceptance.
Patient benefit considerations must also be addressed. While joint-on-chip systems promise personalized medicine approaches for osteoarthritis treatment, questions remain about equitable access to these technologies. If advanced treatments emerge from this research, ensuring they benefit diverse patient populations rather than exacerbating healthcare disparities becomes an ethical imperative.
Looking forward, international harmonization of regulatory frameworks will be essential. As joint-on-chip technologies for osteoarthritis research cross borders, alignment between different regulatory jurisdictions will facilitate faster translation from laboratory research to clinical applications, ultimately benefiting patients suffering from this debilitating condition.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!