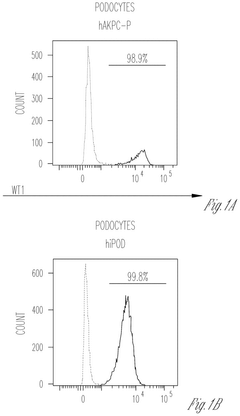
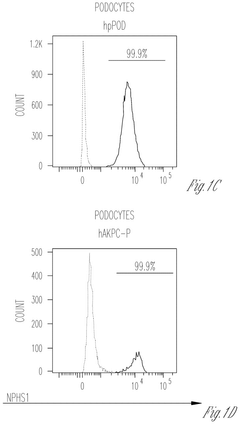
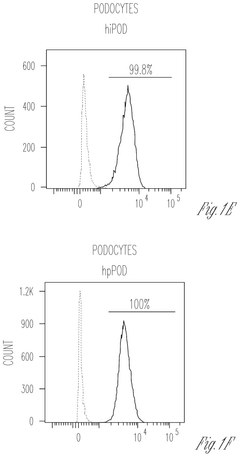
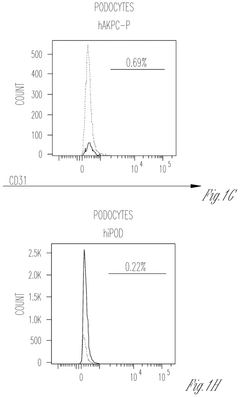
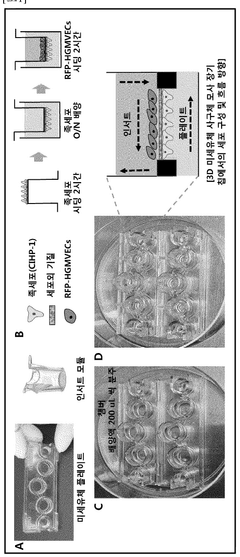
Engineering glycosaminoglycan-rich microenvironments to simulate kidney glomerular filtration on chip
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycosaminoglycan-Rich Microenvironments Background and Objectives
Glycosaminoglycans (GAGs) represent a critical class of complex polysaccharides that play fundamental roles in cellular signaling, tissue development, and maintenance of organ function. In the kidney glomerulus specifically, GAGs form an essential component of the glomerular basement membrane (GBM) and contribute significantly to the selective filtration barrier that prevents protein leakage while allowing water and small solutes to pass through. The historical trajectory of GAG research dates back to the 1970s, when their presence in the glomerular filtration barrier was first identified, but only in recent decades has their precise functional significance been elucidated.
The evolution of this technology field has accelerated dramatically with the emergence of organ-on-chip platforms, which offer unprecedented opportunities to recreate physiological microenvironments in vitro. These platforms have evolved from simple microfluidic channels to complex, multi-compartment systems capable of mimicking organ-specific functions. Within this context, engineering GAG-rich microenvironments represents a cutting-edge approach to faithfully recapitulate the kidney's filtration mechanisms.
Current technological trends point toward increasing sophistication in biomaterial design, with particular emphasis on controlling the spatial distribution, density, and specific sulfation patterns of GAGs to accurately mimic the native glomerular environment. The integration of advanced fabrication techniques such as 3D bioprinting, layer-by-layer assembly, and microfluidic patterning has opened new avenues for creating biomimetic filtration barriers with precisely controlled GAG compositions.
The primary objective of this technological pursuit is to develop a kidney-on-chip platform featuring engineered GAG-rich microenvironments that accurately simulate the glomerular filtration barrier's selective permeability. This includes recreating the charge-selective properties conferred by negatively charged GAGs, particularly heparan sulfate, which plays a crucial role in preventing albumin filtration under physiological conditions.
Secondary objectives encompass the development of standardized protocols for GAG functionalization of biomaterials, quantitative methods for characterizing GAG distribution and density, and validation approaches that correlate in vitro filtration properties with in vivo glomerular function. The ultimate goal is to establish a reliable platform for studying kidney diseases characterized by compromised filtration barriers, such as diabetic nephropathy and minimal change disease, and for screening potential therapeutic interventions targeting GAG-related pathologies.
The evolution of this technology field has accelerated dramatically with the emergence of organ-on-chip platforms, which offer unprecedented opportunities to recreate physiological microenvironments in vitro. These platforms have evolved from simple microfluidic channels to complex, multi-compartment systems capable of mimicking organ-specific functions. Within this context, engineering GAG-rich microenvironments represents a cutting-edge approach to faithfully recapitulate the kidney's filtration mechanisms.
Current technological trends point toward increasing sophistication in biomaterial design, with particular emphasis on controlling the spatial distribution, density, and specific sulfation patterns of GAGs to accurately mimic the native glomerular environment. The integration of advanced fabrication techniques such as 3D bioprinting, layer-by-layer assembly, and microfluidic patterning has opened new avenues for creating biomimetic filtration barriers with precisely controlled GAG compositions.
The primary objective of this technological pursuit is to develop a kidney-on-chip platform featuring engineered GAG-rich microenvironments that accurately simulate the glomerular filtration barrier's selective permeability. This includes recreating the charge-selective properties conferred by negatively charged GAGs, particularly heparan sulfate, which plays a crucial role in preventing albumin filtration under physiological conditions.
Secondary objectives encompass the development of standardized protocols for GAG functionalization of biomaterials, quantitative methods for characterizing GAG distribution and density, and validation approaches that correlate in vitro filtration properties with in vivo glomerular function. The ultimate goal is to establish a reliable platform for studying kidney diseases characterized by compromised filtration barriers, such as diabetic nephropathy and minimal change disease, and for screening potential therapeutic interventions targeting GAG-related pathologies.
Market Analysis for Kidney-on-Chip Technologies
The kidney-on-chip technology market is experiencing significant growth, driven by increasing prevalence of kidney diseases and the limitations of current treatment options. The global market for organ-on-chip technologies was valued at approximately $21 million in 2019 and is projected to reach $220 million by 2025, with kidney-on-chip representing a substantial segment of this market.
Kidney diseases affect over 850 million people worldwide, with chronic kidney disease (CKD) prevalence ranging from 11-13% globally. The economic burden is substantial, with Medicare spending for CKD and ESRD (End-Stage Renal Disease) patients exceeding $120 billion annually in the United States alone. This creates a strong market pull for innovative technologies that can improve disease understanding, drug development, and personalized treatment approaches.
The pharmaceutical industry represents the primary customer base for kidney-on-chip technologies, as these platforms offer significant advantages in drug discovery and development. The average cost to develop a new drug exceeds $2.6 billion, with kidney toxicity being a major cause of drug attrition during development. Kidney-on-chip models that accurately simulate glomerular filtration can potentially reduce these costs by enabling earlier detection of nephrotoxicity.
Academic research institutions constitute another significant market segment, utilizing these technologies for fundamental research into kidney physiology and pathophysiology. Government funding for kidney research has been increasing, with the NIH allocating over $600 million annually to kidney disease research initiatives.
The glycosaminoglycan-rich microenvironment approach to simulating glomerular filtration addresses a specific high-value niche within the kidney-on-chip market. The glomerular basement membrane's unique composition, rich in specific glycosaminoglycans, is critical for proper filtration function. Technologies that accurately recreate this environment can command premium pricing due to their enhanced physiological relevance.
Geographically, North America dominates the organ-on-chip market with approximately 45% market share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate due to increasing research investments and growing awareness about alternative testing methods.
Key market drivers include stringent regulatory requirements for drug safety testing, increasing R&D investments in pharmaceutical companies, and growing emphasis on reducing animal testing. The market faces challenges including high development costs, technical complexities in creating physiologically relevant models, and the need for standardization and validation of these technologies.
Kidney diseases affect over 850 million people worldwide, with chronic kidney disease (CKD) prevalence ranging from 11-13% globally. The economic burden is substantial, with Medicare spending for CKD and ESRD (End-Stage Renal Disease) patients exceeding $120 billion annually in the United States alone. This creates a strong market pull for innovative technologies that can improve disease understanding, drug development, and personalized treatment approaches.
The pharmaceutical industry represents the primary customer base for kidney-on-chip technologies, as these platforms offer significant advantages in drug discovery and development. The average cost to develop a new drug exceeds $2.6 billion, with kidney toxicity being a major cause of drug attrition during development. Kidney-on-chip models that accurately simulate glomerular filtration can potentially reduce these costs by enabling earlier detection of nephrotoxicity.
Academic research institutions constitute another significant market segment, utilizing these technologies for fundamental research into kidney physiology and pathophysiology. Government funding for kidney research has been increasing, with the NIH allocating over $600 million annually to kidney disease research initiatives.
The glycosaminoglycan-rich microenvironment approach to simulating glomerular filtration addresses a specific high-value niche within the kidney-on-chip market. The glomerular basement membrane's unique composition, rich in specific glycosaminoglycans, is critical for proper filtration function. Technologies that accurately recreate this environment can command premium pricing due to their enhanced physiological relevance.
Geographically, North America dominates the organ-on-chip market with approximately 45% market share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate due to increasing research investments and growing awareness about alternative testing methods.
Key market drivers include stringent regulatory requirements for drug safety testing, increasing R&D investments in pharmaceutical companies, and growing emphasis on reducing animal testing. The market faces challenges including high development costs, technical complexities in creating physiologically relevant models, and the need for standardization and validation of these technologies.
Current Challenges in Glomerular Filtration Simulation
Despite significant advancements in organ-on-chip technology, simulating the complex glomerular filtration process of the kidney remains a formidable challenge. Current in vitro models struggle to accurately replicate the intricate three-dimensional architecture of the glomerular filtration barrier, which consists of fenestrated endothelial cells, the glomerular basement membrane (GBM), and podocytes with their interdigitating foot processes and slit diaphragms.
The glycosaminoglycan (GAG) composition of the glomerular filtration barrier presents a particular challenge. These highly negatively charged molecules contribute significantly to the charge-selective properties of the filtration barrier, but recreating their precise spatial arrangement and density in artificial systems has proven difficult. Researchers face obstacles in engineering materials that can maintain stable GAG presentation while allowing for cellular integration and matrix remodeling.
Another major hurdle is achieving physiologically relevant filtration pressures and flow dynamics. The glomerular capillaries operate under unique hemodynamic conditions that are challenging to replicate in microfluidic systems. Current platforms often fail to generate the appropriate hydrostatic and oncotic pressure gradients necessary for accurate filtration simulation, leading to discrepancies between in vitro and in vivo filtration rates and selectivity.
Cell source and phenotype stability represent additional challenges. Primary human podocytes and glomerular endothelial cells are difficult to obtain in sufficient quantities and tend to dedifferentiate in culture. Immortalized cell lines, while more accessible, often fail to express the full complement of specialized proteins necessary for proper barrier function, particularly those related to GAG synthesis and modification.
The dynamic nature of the glomerular filtration barrier further complicates in vitro modeling. The barrier continuously responds to physiological and pathological stimuli, adjusting its permeability properties accordingly. Current static models cannot capture this adaptability, limiting their utility for studying disease progression or drug responses over time.
Analytical limitations also hinder progress in this field. Quantifying the contribution of specific GAG compositions to filtration properties requires sophisticated analytical techniques that can operate at the microscale with high sensitivity. Current methods often lack the spatial resolution needed to correlate local GAG composition with filtration function in real-time.
Interdisciplinary challenges persist as well, as developing effective glomerular filtration models requires expertise spanning nephrology, materials science, microfluidics, glycobiology, and bioengineering. The complexity of integrating these diverse knowledge domains has slowed progress toward creating truly biomimetic kidney-on-chip platforms that can accurately simulate the filtration properties of the native glomerulus.
The glycosaminoglycan (GAG) composition of the glomerular filtration barrier presents a particular challenge. These highly negatively charged molecules contribute significantly to the charge-selective properties of the filtration barrier, but recreating their precise spatial arrangement and density in artificial systems has proven difficult. Researchers face obstacles in engineering materials that can maintain stable GAG presentation while allowing for cellular integration and matrix remodeling.
Another major hurdle is achieving physiologically relevant filtration pressures and flow dynamics. The glomerular capillaries operate under unique hemodynamic conditions that are challenging to replicate in microfluidic systems. Current platforms often fail to generate the appropriate hydrostatic and oncotic pressure gradients necessary for accurate filtration simulation, leading to discrepancies between in vitro and in vivo filtration rates and selectivity.
Cell source and phenotype stability represent additional challenges. Primary human podocytes and glomerular endothelial cells are difficult to obtain in sufficient quantities and tend to dedifferentiate in culture. Immortalized cell lines, while more accessible, often fail to express the full complement of specialized proteins necessary for proper barrier function, particularly those related to GAG synthesis and modification.
The dynamic nature of the glomerular filtration barrier further complicates in vitro modeling. The barrier continuously responds to physiological and pathological stimuli, adjusting its permeability properties accordingly. Current static models cannot capture this adaptability, limiting their utility for studying disease progression or drug responses over time.
Analytical limitations also hinder progress in this field. Quantifying the contribution of specific GAG compositions to filtration properties requires sophisticated analytical techniques that can operate at the microscale with high sensitivity. Current methods often lack the spatial resolution needed to correlate local GAG composition with filtration function in real-time.
Interdisciplinary challenges persist as well, as developing effective glomerular filtration models requires expertise spanning nephrology, materials science, microfluidics, glycobiology, and bioengineering. The complexity of integrating these diverse knowledge domains has slowed progress toward creating truly biomimetic kidney-on-chip platforms that can accurately simulate the filtration properties of the native glomerulus.
Current Glycosaminoglycan Engineering Approaches
01 Role of glycosaminoglycans in glomerular filtration
Glycosaminoglycans (GAGs) play a crucial role in the glomerular filtration process by maintaining the structural integrity and charge selectivity of the glomerular basement membrane. These negatively charged molecules form a barrier that prevents the passage of certain proteins and other macromolecules while allowing the filtration of water and small solutes. Alterations in GAG composition or structure can lead to proteinuria and kidney dysfunction in various renal diseases.- Role of glycosaminoglycans in glomerular filtration barrier: Glycosaminoglycans (GAGs) play a crucial role in maintaining the integrity and function of the glomerular filtration barrier. These negatively charged molecules contribute to the charge selectivity of the glomerular basement membrane, preventing the passage of negatively charged proteins. Alterations in GAG composition or structure can lead to proteinuria and kidney disease. Research focuses on understanding how GAG-rich microenvironments influence filtration properties and kidney function.
- Therapeutic approaches targeting glycosaminoglycan metabolism: Various therapeutic strategies target glycosaminoglycan metabolism to improve glomerular filtration and treat kidney diseases. These approaches include administering exogenous GAGs, modulating endogenous GAG synthesis, and using GAG mimetics. Such interventions aim to restore the normal composition and function of the glomerular filtration barrier, reduce proteinuria, and slow disease progression in conditions like diabetic nephropathy and other glomerular diseases.
- Diagnostic methods for assessing glycosaminoglycan-related kidney function: Advanced diagnostic techniques have been developed to assess glycosaminoglycan composition and its relationship to glomerular filtration. These methods include imaging techniques, biomarker analysis, and functional tests that can detect alterations in GAG content or distribution within the kidney. Such diagnostic approaches enable early detection of kidney dysfunction, monitoring of disease progression, and evaluation of treatment efficacy in conditions affecting the glomerular filtration barrier.
- Engineered glycosaminoglycan-rich scaffolds for kidney tissue engineering: Biomaterial scaffolds enriched with glycosaminoglycans have been developed for kidney tissue engineering applications. These scaffolds mimic the natural extracellular matrix environment of the kidney, providing appropriate biochemical and mechanical cues for cell growth and function. GAG-rich scaffolds support the development of functional glomerular structures, potentially offering solutions for kidney regeneration and the creation of in vitro models for studying glomerular filtration processes.
- Glycosaminoglycan alterations in kidney disease pathophysiology: Research has revealed significant alterations in glycosaminoglycan composition and distribution in various kidney diseases. These changes contribute to the pathophysiology of conditions such as diabetic nephropathy, lupus nephritis, and other glomerular diseases. Loss of specific GAGs or changes in their sulfation patterns can compromise the glomerular filtration barrier, leading to proteinuria and progressive kidney dysfunction. Understanding these alterations provides insights into disease mechanisms and potential therapeutic targets.
02 Diagnostic methods for assessing glycosaminoglycan-related kidney function
Various diagnostic techniques have been developed to assess the relationship between glycosaminoglycans and glomerular filtration. These methods include imaging techniques, biomarker analysis, and functional tests that can evaluate the integrity of the glomerular filtration barrier. Such diagnostic approaches help in early detection of kidney diseases associated with GAG abnormalities and monitoring treatment efficacy in patients with renal disorders.Expand Specific Solutions03 Therapeutic approaches targeting glycosaminoglycan metabolism
Therapeutic interventions targeting glycosaminoglycan metabolism have shown promise in treating kidney diseases characterized by glomerular filtration dysfunction. These approaches include administration of exogenous GAGs, modulation of endogenous GAG synthesis or degradation, and use of GAG mimetics. Such therapies aim to restore the normal composition and function of the glomerular filtration barrier, thereby improving kidney function and reducing proteinuria in patients with renal disorders.Expand Specific Solutions04 Engineered glycosaminoglycan-rich microenvironments for kidney tissue engineering
Development of engineered glycosaminoglycan-rich microenvironments has emerged as a promising approach for kidney tissue engineering and regenerative medicine. These biomimetic scaffolds incorporate GAGs to recreate the natural extracellular matrix environment of the kidney glomerulus. Such engineered constructs support the growth and function of kidney cells, potentially offering new solutions for kidney repair, disease modeling, and drug testing applications related to glomerular filtration.Expand Specific Solutions05 Impact of disease states on glycosaminoglycan composition in the glomerular filtration barrier
Various disease states, including diabetes, hypertension, and inflammatory conditions, can significantly alter the glycosaminoglycan composition in the glomerular filtration barrier. These changes often involve reduced GAG content, altered sulfation patterns, or abnormal distribution within the glomerular basement membrane. Understanding these disease-induced modifications provides insights into the pathophysiology of kidney diseases and identifies potential targets for therapeutic interventions aimed at preserving or restoring normal glomerular filtration.Expand Specific Solutions
Leading Research Groups and Companies in Kidney-on-Chip
The kidney glomerular filtration on-chip technology market is currently in an early growth phase, characterized by significant research activity but limited commercial deployment. The global market for organ-on-chip technologies is projected to reach $220 million by 2025, with kidney models representing approximately 15% of this segment. From a technical maturity perspective, the field is transitioning from proof-of-concept to validation stage. Leading academic institutions (Tsinghua University, University of Washington, Yale University) are driving fundamental research, while commercial entities like Emulate, Inc. are developing scalable platforms. Pharmaceutical companies such as Agilent Technologies are increasingly adopting these technologies for drug screening applications. The integration of glycosaminoglycan-rich microenvironments represents a critical advancement in mimicking the complex kidney filtration barrier, with collaborative efforts between research institutions and biotechnology companies accelerating development toward clinical applications.
Emulate, Inc.
Technical Solution: Emulate has developed advanced Organ-on-Chip technology specifically designed to recreate kidney glomerular filtration. Their platform incorporates glycosaminoglycan-rich extracellular matrices to mimic the glomerular basement membrane's composition and function. The company's proprietary microfluidic chips feature two parallel microchannels separated by a porous membrane coated with specialized glycosaminoglycans that simulate the glomerular filtration barrier. This design enables the recreation of physiological shear stress and mechanical forces critical for proper podocyte and endothelial cell function. Emulate's technology allows for precise control of flow rates, pressure gradients, and mechanical stretching to accurately model glomerular filtration dynamics. Their system incorporates real-time monitoring capabilities to measure albumin retention, small molecule clearance, and other key parameters of kidney function.
Strengths: Industry-leading microfluidic technology with precise control over mechanical forces and flow dynamics; commercially available standardized platform with robust validation data. Weaknesses: Higher cost compared to traditional cell culture systems; requires specialized equipment and expertise to operate effectively.
The Charles Stark Draper Laboratory, Inc.
Technical Solution: Draper Laboratory has engineered a sophisticated kidney-on-chip platform specifically addressing glycosaminoglycan-rich microenvironments for glomerular filtration simulation. Their approach utilizes a multi-layered microfluidic device incorporating precisely patterned glycosaminoglycan compositions that mimic the zonal organization of the glomerular basement membrane. The system employs advanced microfabrication techniques to create physiologically relevant geometries with controlled porosity gradients. Draper's technology integrates embedded sensors for real-time monitoring of filtration parameters, including molecular sieving coefficients and hydraulic permeability. Their platform incorporates automated pressure control systems to maintain physiological pressure gradients across the filtration barrier, enabling accurate modeling of glomerular function under both normal and pathological conditions. The system allows for co-culture of podocytes, endothelial cells, and mesangial cells in their native spatial arrangement.
Strengths: Exceptional engineering precision with integrated sensing capabilities; highly customizable platform adaptable to various research questions; strong focus on physiological relevance. Weaknesses: Complex system requiring significant technical expertise; less widely adopted in academic settings compared to some competitors.
Key Innovations in Glomerular Basement Membrane Mimicry
Glomerulus on a chip to recapitulate glomerular filtration barrier
PatentActiveUS12134759B2
Innovation
- A Glomerulus on a Chip (GOAC) device with human podocytes and glomerular endothelial cells seeded on Organoplates, devoid of artificial membranes, allowing natural cell interaction and long-term culture, maintaining phenotype and forming a functional GFB with selective permeability.
Glomerulus-on-chip and use thereof
PatentWO2025058295A1
Innovation
- The development of a 3D glomerulus mimicking organ-on-a-chip using microfluidic technology, which recreates the three-dimensional glomerular filtration barrier and allows for the evaluation of kidney disease models and drug efficacy in a more human-relevant and ethically acceptable manner.
Regulatory Pathway for Organ-on-Chip Validation
The regulatory landscape for organ-on-chip (OOC) technologies, particularly those simulating kidney glomerular filtration, presents a complex pathway toward clinical and commercial validation. Current regulatory frameworks were not specifically designed for these hybrid technologies that combine microfluidics, cell biology, and tissue engineering, creating significant challenges for developers.
The FDA has begun addressing these novel platforms through its Emerging Technology Program, which provides consultation for innovative technologies that improve product quality. For kidney-on-chip devices incorporating glycosaminoglycan-rich microenvironments, developers must navigate between medical device regulations and pharmaceutical testing guidelines, as these platforms serve both as potential diagnostic tools and drug testing systems.
European regulatory bodies, through the European Medicines Agency (EMA), have established the Innovation Task Force specifically to provide guidance on emerging technologies including OOC systems. Their qualification procedure offers a pathway for novel methodologies to gain regulatory acceptance, which is crucial for kidney filtration models seeking to replace traditional animal testing methods.
Key validation milestones for glycosaminoglycan-rich kidney filtration chips include demonstrating reproducibility of the glomerular basement membrane structure, consistent filtration characteristics across batches, and correlation with human physiological responses. Regulatory agencies require extensive documentation of these performance characteristics through standardized testing protocols.
International harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) are working to establish consistent guidelines for OOC validation across global markets. These efforts are particularly relevant for kidney models, as nephrotoxicity testing is a critical component of drug development worldwide.
The path to regulatory approval typically involves a phased approach: initial validation as a research tool, followed by qualification for specific contexts of use in drug development, and potentially culminating in approval as a diagnostic or personalized medicine platform. Each phase requires increasingly rigorous evidence of reliability and clinical relevance.
Collaboration with regulatory agencies through formal consultation programs is essential for developers of kidney filtration chips. Early engagement can provide valuable guidance on validation requirements and help establish appropriate performance standards for these novel technologies, potentially accelerating their path to market and clinical implementation.
The FDA has begun addressing these novel platforms through its Emerging Technology Program, which provides consultation for innovative technologies that improve product quality. For kidney-on-chip devices incorporating glycosaminoglycan-rich microenvironments, developers must navigate between medical device regulations and pharmaceutical testing guidelines, as these platforms serve both as potential diagnostic tools and drug testing systems.
European regulatory bodies, through the European Medicines Agency (EMA), have established the Innovation Task Force specifically to provide guidance on emerging technologies including OOC systems. Their qualification procedure offers a pathway for novel methodologies to gain regulatory acceptance, which is crucial for kidney filtration models seeking to replace traditional animal testing methods.
Key validation milestones for glycosaminoglycan-rich kidney filtration chips include demonstrating reproducibility of the glomerular basement membrane structure, consistent filtration characteristics across batches, and correlation with human physiological responses. Regulatory agencies require extensive documentation of these performance characteristics through standardized testing protocols.
International harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) are working to establish consistent guidelines for OOC validation across global markets. These efforts are particularly relevant for kidney models, as nephrotoxicity testing is a critical component of drug development worldwide.
The path to regulatory approval typically involves a phased approach: initial validation as a research tool, followed by qualification for specific contexts of use in drug development, and potentially culminating in approval as a diagnostic or personalized medicine platform. Each phase requires increasingly rigorous evidence of reliability and clinical relevance.
Collaboration with regulatory agencies through formal consultation programs is essential for developers of kidney filtration chips. Early engagement can provide valuable guidance on validation requirements and help establish appropriate performance standards for these novel technologies, potentially accelerating their path to market and clinical implementation.
Translational Applications in Drug Discovery and Nephrotoxicity Testing
The development of glycosaminoglycan-rich microenvironments for kidney glomerular filtration simulation presents significant translational applications in drug discovery and nephrotoxicity testing. These organ-on-chip platforms offer unprecedented opportunities to revolutionize pharmaceutical development by providing more physiologically relevant models for drug screening and toxicity assessment.
In drug discovery, these biomimetic platforms enable high-throughput screening of drug candidates with improved predictive value compared to traditional cell culture systems. The glycosaminoglycan-rich environment accurately replicates the kidney's filtration barrier, allowing researchers to evaluate drug permeability, absorption, and excretion profiles in conditions that closely resemble in vivo scenarios. This capability significantly reduces the gap between preclinical testing and clinical outcomes.
Nephrotoxicity remains a critical concern in drug development, with approximately 20% of hospital-acquired acute kidney injuries attributed to pharmaceutical compounds. Conventional animal models often fail to predict human-specific nephrotoxic responses due to interspecies differences in kidney physiology and drug metabolism. Glomerular filtration chips address this limitation by incorporating human cells within a physiologically relevant microenvironment, providing more accurate toxicity predictions.
These platforms also enable personalized medicine approaches by incorporating patient-derived cells to create individualized kidney models. This advancement allows for patient-specific drug toxicity screening, potentially identifying adverse reactions before clinical administration and facilitating the development of tailored therapeutic regimens based on individual kidney function profiles.
For pharmaceutical companies, these systems offer substantial economic benefits by identifying nephrotoxic compounds earlier in the development pipeline. Early detection of potential kidney-damaging effects can save millions in development costs by preventing the advancement of problematic compounds to expensive clinical trials, thereby streamlining the drug development process.
Regulatory agencies have shown increasing interest in these advanced in vitro models as alternatives to animal testing. The glycosaminoglycan-rich kidney chips provide quantitative, reproducible data on nephrotoxicity mechanisms, supporting the growing regulatory emphasis on mechanism-based toxicity assessment rather than traditional descriptive toxicology.
Furthermore, these platforms enable longitudinal studies of chronic drug exposure effects on kidney function, addressing a significant gap in current testing paradigms which typically focus on acute toxicity. The ability to maintain functional kidney structures for extended periods allows researchers to evaluate cumulative nephrotoxic effects and adaptive responses that more accurately reflect clinical scenarios.
In drug discovery, these biomimetic platforms enable high-throughput screening of drug candidates with improved predictive value compared to traditional cell culture systems. The glycosaminoglycan-rich environment accurately replicates the kidney's filtration barrier, allowing researchers to evaluate drug permeability, absorption, and excretion profiles in conditions that closely resemble in vivo scenarios. This capability significantly reduces the gap between preclinical testing and clinical outcomes.
Nephrotoxicity remains a critical concern in drug development, with approximately 20% of hospital-acquired acute kidney injuries attributed to pharmaceutical compounds. Conventional animal models often fail to predict human-specific nephrotoxic responses due to interspecies differences in kidney physiology and drug metabolism. Glomerular filtration chips address this limitation by incorporating human cells within a physiologically relevant microenvironment, providing more accurate toxicity predictions.
These platforms also enable personalized medicine approaches by incorporating patient-derived cells to create individualized kidney models. This advancement allows for patient-specific drug toxicity screening, potentially identifying adverse reactions before clinical administration and facilitating the development of tailored therapeutic regimens based on individual kidney function profiles.
For pharmaceutical companies, these systems offer substantial economic benefits by identifying nephrotoxic compounds earlier in the development pipeline. Early detection of potential kidney-damaging effects can save millions in development costs by preventing the advancement of problematic compounds to expensive clinical trials, thereby streamlining the drug development process.
Regulatory agencies have shown increasing interest in these advanced in vitro models as alternatives to animal testing. The glycosaminoglycan-rich kidney chips provide quantitative, reproducible data on nephrotoxicity mechanisms, supporting the growing regulatory emphasis on mechanism-based toxicity assessment rather than traditional descriptive toxicology.
Furthermore, these platforms enable longitudinal studies of chronic drug exposure effects on kidney function, addressing a significant gap in current testing paradigms which typically focus on acute toxicity. The ability to maintain functional kidney structures for extended periods allows researchers to evaluate cumulative nephrotoxic effects and adaptive responses that more accurately reflect clinical scenarios.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!