Comparative study of oxygenation strategies for high-metabolic-demand tissues on chip
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Oxygenation Strategies Background and Objectives
Organ-on-chip technology has emerged as a revolutionary platform for modeling human physiology and disease in vitro, offering significant advantages over traditional cell culture methods and animal models. However, one of the most critical challenges in developing functional organ-on-chip systems is ensuring adequate oxygen supply to tissues with high metabolic demands. The evolution of oxygenation strategies has progressed significantly over the past decade, from simple diffusion-based approaches to sophisticated integrated systems that mimic physiological oxygen gradients.
Historically, early organ-on-chip platforms relied primarily on passive diffusion of oxygen through permeable materials such as polydimethylsiloxane (PDMS). While effective for thin tissue constructs, this approach proved insufficient for metabolically active tissues like liver, heart, and brain, which require higher oxygen tension to maintain physiological function. This limitation prompted the development of more advanced oxygenation strategies, including perfusion-based systems, oxygen-generating biomaterials, and microfluidic oxygen delivery networks.
The technological trajectory in this field has been shaped by increasing understanding of tissue-specific oxygen requirements and the recognition that physiological oxygen gradients play crucial roles in cellular function and differentiation. Recent advances in materials science, microfluidics, and biosensor technologies have accelerated innovation in this domain, enabling more precise control over oxygen delivery and monitoring in organ-on-chip systems.
Current research objectives in oxygenation strategies for high-metabolic-demand tissues focus on several key areas: developing biomimetic oxygen delivery systems that recapitulate in vivo conditions; creating tunable platforms that can simulate both normoxic and hypoxic environments; integrating real-time oxygen sensing capabilities; and designing scalable solutions compatible with high-throughput applications. Additionally, there is growing interest in strategies that can maintain stable oxygen levels over extended culture periods to support long-term tissue maturation and chronic disease modeling.
The ultimate goal of these technological developments is to create physiologically relevant tissue models that accurately reproduce human organ function and response to interventions. This would significantly enhance drug development processes by providing more predictive preclinical models, potentially reducing attrition rates in clinical trials and accelerating the path to market for new therapeutics. Furthermore, advanced oxygenation strategies could enable the creation of more complex multi-organ systems, opening new avenues for studying organ interactions and systemic responses.
As the field continues to evolve, interdisciplinary collaboration between tissue engineers, microfluidics experts, materials scientists, and computational biologists will be essential to overcome current limitations and develop next-generation oxygenation solutions for high-metabolic-demand tissues on chip.
Historically, early organ-on-chip platforms relied primarily on passive diffusion of oxygen through permeable materials such as polydimethylsiloxane (PDMS). While effective for thin tissue constructs, this approach proved insufficient for metabolically active tissues like liver, heart, and brain, which require higher oxygen tension to maintain physiological function. This limitation prompted the development of more advanced oxygenation strategies, including perfusion-based systems, oxygen-generating biomaterials, and microfluidic oxygen delivery networks.
The technological trajectory in this field has been shaped by increasing understanding of tissue-specific oxygen requirements and the recognition that physiological oxygen gradients play crucial roles in cellular function and differentiation. Recent advances in materials science, microfluidics, and biosensor technologies have accelerated innovation in this domain, enabling more precise control over oxygen delivery and monitoring in organ-on-chip systems.
Current research objectives in oxygenation strategies for high-metabolic-demand tissues focus on several key areas: developing biomimetic oxygen delivery systems that recapitulate in vivo conditions; creating tunable platforms that can simulate both normoxic and hypoxic environments; integrating real-time oxygen sensing capabilities; and designing scalable solutions compatible with high-throughput applications. Additionally, there is growing interest in strategies that can maintain stable oxygen levels over extended culture periods to support long-term tissue maturation and chronic disease modeling.
The ultimate goal of these technological developments is to create physiologically relevant tissue models that accurately reproduce human organ function and response to interventions. This would significantly enhance drug development processes by providing more predictive preclinical models, potentially reducing attrition rates in clinical trials and accelerating the path to market for new therapeutics. Furthermore, advanced oxygenation strategies could enable the creation of more complex multi-organ systems, opening new avenues for studying organ interactions and systemic responses.
As the field continues to evolve, interdisciplinary collaboration between tissue engineers, microfluidics experts, materials scientists, and computational biologists will be essential to overcome current limitations and develop next-generation oxygenation solutions for high-metabolic-demand tissues on chip.
Market Analysis for Organ-on-Chip Oxygenation Solutions
The organ-on-chip (OOC) market has experienced significant growth in recent years, with the global market value reaching $45 million in 2022 and projected to grow at a CAGR of 39.2% to reach $190 million by 2026. Within this expanding market, oxygenation solutions for high-metabolic-demand tissues represent a critical segment with specialized requirements and substantial growth potential.
The demand for advanced oxygenation strategies is primarily driven by researchers in pharmaceutical companies, academic institutions, and biotechnology firms seeking to develop more physiologically relevant in vitro models. Particularly, tissues with high metabolic demands such as liver, heart, brain, and pancreatic tissues require sophisticated oxygenation approaches to maintain viability and functionality in microfluidic environments.
Market segmentation reveals distinct categories of oxygenation solutions currently available. Direct media oxygenation systems hold approximately 35% market share, while membrane-based gas exchange platforms account for 40%. Integrated perfusion systems with oxygen sensing capabilities represent 15% of the market, with emerging technologies such as oxygen-generating biomaterials comprising the remaining 10%.
Geographically, North America dominates the market with 45% share, followed by Europe (30%), Asia-Pacific (20%), and rest of the world (5%). The Asia-Pacific region is expected to witness the fastest growth due to increasing investments in biotechnology research infrastructure and rising adoption of advanced cell culture technologies.
Key market drivers include the growing emphasis on reducing animal testing, increasing R&D investments in drug discovery, and rising prevalence of chronic diseases necessitating better disease models. The pharmaceutical industry's shift toward personalized medicine has further accelerated demand for physiologically relevant tissue models with proper oxygenation.
Market challenges include high costs associated with advanced oxygenation systems, technical complexities in maintaining optimal oxygen gradients, and lack of standardization across platforms. Additionally, regulatory uncertainties regarding the validation of organ-on-chip models present barriers to widespread commercial adoption.
Customer needs analysis indicates growing demand for integrated solutions that combine oxygenation with real-time monitoring capabilities. End-users prioritize systems that can maintain physiological oxygen gradients while allowing for experimental flexibility and ease of use. Price sensitivity varies by segment, with academic institutions showing higher price sensitivity compared to pharmaceutical companies.
The market for specialized oxygenation solutions for high-metabolic-demand tissues is expected to grow at 45% annually, outpacing the overall organ-on-chip market, as researchers increasingly recognize oxygen delivery as a critical factor in developing physiologically relevant tissue models.
The demand for advanced oxygenation strategies is primarily driven by researchers in pharmaceutical companies, academic institutions, and biotechnology firms seeking to develop more physiologically relevant in vitro models. Particularly, tissues with high metabolic demands such as liver, heart, brain, and pancreatic tissues require sophisticated oxygenation approaches to maintain viability and functionality in microfluidic environments.
Market segmentation reveals distinct categories of oxygenation solutions currently available. Direct media oxygenation systems hold approximately 35% market share, while membrane-based gas exchange platforms account for 40%. Integrated perfusion systems with oxygen sensing capabilities represent 15% of the market, with emerging technologies such as oxygen-generating biomaterials comprising the remaining 10%.
Geographically, North America dominates the market with 45% share, followed by Europe (30%), Asia-Pacific (20%), and rest of the world (5%). The Asia-Pacific region is expected to witness the fastest growth due to increasing investments in biotechnology research infrastructure and rising adoption of advanced cell culture technologies.
Key market drivers include the growing emphasis on reducing animal testing, increasing R&D investments in drug discovery, and rising prevalence of chronic diseases necessitating better disease models. The pharmaceutical industry's shift toward personalized medicine has further accelerated demand for physiologically relevant tissue models with proper oxygenation.
Market challenges include high costs associated with advanced oxygenation systems, technical complexities in maintaining optimal oxygen gradients, and lack of standardization across platforms. Additionally, regulatory uncertainties regarding the validation of organ-on-chip models present barriers to widespread commercial adoption.
Customer needs analysis indicates growing demand for integrated solutions that combine oxygenation with real-time monitoring capabilities. End-users prioritize systems that can maintain physiological oxygen gradients while allowing for experimental flexibility and ease of use. Price sensitivity varies by segment, with academic institutions showing higher price sensitivity compared to pharmaceutical companies.
The market for specialized oxygenation solutions for high-metabolic-demand tissues is expected to grow at 45% annually, outpacing the overall organ-on-chip market, as researchers increasingly recognize oxygen delivery as a critical factor in developing physiologically relevant tissue models.
Current Oxygenation Technologies and Limitations
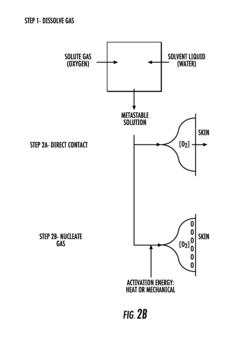
Current oxygenation strategies for organ-on-chip systems face significant limitations when supporting high-metabolic-demand tissues such as liver, heart, and brain models. Conventional approaches primarily rely on passive diffusion through polydimethylsiloxane (PDMS) membranes, which provides insufficient oxygen transfer rates for these metabolically active tissues. The oxygen diffusion distance in static culture systems typically limits effective oxygenation to approximately 100-200 μm, creating hypoxic zones in thicker tissue constructs.
Media perfusion systems represent an improvement by continuously supplying fresh, oxygenated media to tissues. However, these systems often struggle to maintain adequate oxygen levels throughout three-dimensional tissue constructs due to rapid oxygen consumption rates that exceed replenishment capabilities. Additionally, high flow rates required for sufficient oxygenation can introduce undesirable shear stress, potentially damaging delicate cell structures.
Direct oxygenation methods utilizing oxygen carriers have emerged as promising alternatives. Perfluorocarbons (PFCs) and hemoglobin-based oxygen carriers can significantly increase oxygen solubility in culture media. Nevertheless, these approaches present challenges including potential cytotoxicity, complex integration into microfluidic systems, and limited stability during extended culture periods.
Microfluidic oxygenators incorporating separate oxygen channels represent another technological approach. These systems feature oxygen-permeable membranes separating the culture chamber from oxygen-filled channels, creating localized oxygen gradients. While effective for certain applications, scaling these systems for high-density tissue constructs remains challenging, and they often require complex fabrication processes.
On-chip oxygen generation systems utilizing electrochemical or photocatalytic methods have demonstrated potential for in situ oxygen production. Electrochemical approaches split water molecules to generate oxygen directly within the culture environment. However, these systems face limitations including electrode fouling, potential pH shifts, and generation of reactive oxygen species that may damage cellular components.
Integration challenges persist across all oxygenation strategies. Maintaining sterility, achieving uniform oxygen distribution, and implementing real-time oxygen monitoring remain significant hurdles. Current oxygen sensing technologies often provide only point measurements rather than spatial oxygen distribution data, limiting the ability to detect and respond to localized hypoxic regions.
Cost considerations and manufacturing complexity further constrain widespread adoption of advanced oxygenation strategies. Many sophisticated approaches require specialized equipment and expertise, limiting accessibility for broader research applications. The field currently lacks standardized metrics for comparing oxygenation efficiency across different platforms, complicating technology assessment and optimization efforts.
Media perfusion systems represent an improvement by continuously supplying fresh, oxygenated media to tissues. However, these systems often struggle to maintain adequate oxygen levels throughout three-dimensional tissue constructs due to rapid oxygen consumption rates that exceed replenishment capabilities. Additionally, high flow rates required for sufficient oxygenation can introduce undesirable shear stress, potentially damaging delicate cell structures.
Direct oxygenation methods utilizing oxygen carriers have emerged as promising alternatives. Perfluorocarbons (PFCs) and hemoglobin-based oxygen carriers can significantly increase oxygen solubility in culture media. Nevertheless, these approaches present challenges including potential cytotoxicity, complex integration into microfluidic systems, and limited stability during extended culture periods.
Microfluidic oxygenators incorporating separate oxygen channels represent another technological approach. These systems feature oxygen-permeable membranes separating the culture chamber from oxygen-filled channels, creating localized oxygen gradients. While effective for certain applications, scaling these systems for high-density tissue constructs remains challenging, and they often require complex fabrication processes.
On-chip oxygen generation systems utilizing electrochemical or photocatalytic methods have demonstrated potential for in situ oxygen production. Electrochemical approaches split water molecules to generate oxygen directly within the culture environment. However, these systems face limitations including electrode fouling, potential pH shifts, and generation of reactive oxygen species that may damage cellular components.
Integration challenges persist across all oxygenation strategies. Maintaining sterility, achieving uniform oxygen distribution, and implementing real-time oxygen monitoring remain significant hurdles. Current oxygen sensing technologies often provide only point measurements rather than spatial oxygen distribution data, limiting the ability to detect and respond to localized hypoxic regions.
Cost considerations and manufacturing complexity further constrain widespread adoption of advanced oxygenation strategies. Many sophisticated approaches require specialized equipment and expertise, limiting accessibility for broader research applications. The field currently lacks standardized metrics for comparing oxygenation efficiency across different platforms, complicating technology assessment and optimization efforts.
Comparative Analysis of Current Oxygenation Methods
01 Medical oxygenation systems for patient care
Various medical devices and systems designed to deliver oxygen to patients in clinical settings. These include advanced oxygen delivery systems, hyperbaric oxygen therapy chambers, and specialized equipment for critical care. These technologies optimize oxygen delivery to tissues and organs, helping in the treatment of hypoxia, respiratory distress, and other medical conditions requiring oxygen supplementation.- Medical oxygenation systems for patient care: Various medical devices and systems designed to deliver oxygen to patients in clinical settings. These include advanced oxygen delivery systems, therapeutic oxygenation devices, and monitoring equipment that help maintain optimal oxygen levels in patients. These technologies are particularly important in critical care, anesthesia, and respiratory therapy applications where precise oxygen delivery is essential for patient outcomes.
- Monitoring and control systems for oxygenation: Technologies focused on monitoring oxygen levels and automatically adjusting delivery parameters. These systems incorporate sensors, feedback mechanisms, and control algorithms to maintain target oxygenation levels. They often include real-time monitoring capabilities, alert systems for abnormal conditions, and data recording for analysis of oxygenation trends over time.
- Water and environmental oxygenation methods: Systems and methods for increasing oxygen levels in water bodies and other environmental applications. These technologies include aeration systems, dissolved oxygen enhancement techniques, and circulation methods designed to improve water quality. Applications include aquaculture, wastewater treatment, and ecosystem restoration where maintaining proper oxygen levels is critical for biological processes.
- Acoustic and ultrasonic oxygenation techniques: Methods utilizing acoustic or ultrasonic energy to enhance oxygenation processes. These technologies employ sound waves to increase gas transfer efficiency, improve mixing, and enhance dissolution of oxygen in various media. The techniques can be applied in medical treatments, industrial processes, and environmental applications where conventional oxygenation methods may be less effective.
- Tissue and cellular oxygenation technologies: Specialized methods for delivering oxygen at the tissue or cellular level. These include targeted oxygen delivery systems, hyperbaric treatments, and technologies that enhance oxygen utilization at the cellular level. Applications range from wound healing and tissue preservation to specialized medical treatments where localized oxygenation is required to promote healing or maintain tissue viability.
02 Monitoring and control systems for oxygenation
Technologies focused on monitoring oxygen levels and controlling oxygen delivery in various environments. These systems include sensors, feedback mechanisms, and automated control algorithms that adjust oxygen delivery based on real-time measurements. Applications range from medical patient monitoring to environmental control systems, ensuring optimal oxygenation levels are maintained for safety and efficacy.Expand Specific Solutions03 Water oxygenation technologies
Methods and systems for increasing dissolved oxygen in water for environmental, agricultural, and aquaculture applications. These technologies include aeration systems, oxygen diffusers, and specialized equipment for enhancing water quality through improved oxygenation. Such systems help maintain aquatic ecosystems, support fish farming, and improve wastewater treatment processes by promoting aerobic biological activity.Expand Specific Solutions04 Acoustic and ultrasonic oxygenation methods
Innovative approaches using acoustic or ultrasonic technologies to enhance oxygenation processes. These methods employ sound waves to improve oxygen transfer in liquids, tissues, or other media. The technologies include specialized transducers and wave generators that create micro-bubbles or increase surface area for gas exchange, resulting in more efficient oxygenation compared to conventional methods.Expand Specific Solutions05 Tissue and cellular oxygenation techniques
Specialized methods for enhancing oxygen delivery at the tissue and cellular level. These include targeted oxygen delivery systems, perfusion technologies, and therapeutic approaches that improve cellular oxygenation. Applications range from wound healing and tissue preservation to specialized treatments for ischemic conditions, where improved oxygenation at the microscopic level can significantly enhance treatment outcomes.Expand Specific Solutions
Leading Organizations in Organ-on-Chip Oxygenation
The oxygenation strategies for high-metabolic-demand tissues on chip market is currently in its growth phase, with increasing adoption across biomedical research and pharmaceutical development. The market size is expanding rapidly, projected to reach significant value as organ-on-chip technologies gain traction for drug testing and personalized medicine applications. Technical maturity varies across approaches, with leading institutions and companies developing innovative solutions. Emulate, Inc. has established itself as a frontrunner with its commercial Organs-on-Chips platform, while academic powerhouses like Harvard, Georgia Tech, and Emory University contribute fundamental research advancements. Shanghai Jiao Tong University and Soochow University are emerging as significant players in Asia, while European contributions come from institutions like Charité Berlin and CNRS, creating a globally competitive landscape with diverse technical approaches to tissue oxygenation challenges.
The Georgia Tech Research Corp.
Technical Solution: Georgia Tech has developed an innovative oxygenation strategy for high-metabolic-demand tissues utilizing a multi-layered microfluidic platform with integrated oxygen-permeable membranes. Their approach incorporates a network of oxygen delivery microchannels positioned strategically around tissue chambers, creating physiologically relevant oxygen gradients. The system employs a unique "breathing" membrane technology that allows controlled gas exchange while maintaining liquid barriers, effectively mimicking alveolar function in lung-on-chip models[3]. For tissues with particularly high oxygen demands, such as cardiac and hepatic models, Georgia Tech researchers have implemented a dual perfusion system where oxygen carriers (perfluorocarbons) are incorporated into the media to increase oxygen carrying capacity by up to 3-fold compared to standard media formulations. Their platform also features integrated oxygen sensors based on phosphorescent quenching that provide real-time spatial mapping of oxygen concentrations across the tissue construct, allowing researchers to validate computational models and optimize flow parameters to prevent hypoxic regions. The technology has demonstrated successful maintenance of functional hepatocytes for over 14 days with preserved metabolic activity comparable to freshly isolated cells.
Strengths: Innovative "breathing" membrane technology provides superior gas exchange capabilities; dual perfusion system with oxygen carriers significantly enhances oxygen delivery capacity; integrated sensing allows for real-time optimization. Weaknesses: Complex fabrication process increases production costs; requires specialized equipment for implementation; perfluorocarbon-based oxygen carriers may affect certain cellular functions and complicate downstream analysis.
President & Fellows of Harvard College
Technical Solution: Harvard's Wyss Institute has pioneered innovative approaches to tissue oxygenation in microfluidic organ-on-chip systems. Their technology employs a multi-layered PDMS (polydimethylsiloxane) structure with integrated gas-permeable membranes that facilitate controlled oxygen diffusion to high-metabolic tissues. The Harvard team has developed specialized oxygen delivery systems that incorporate parallel microchannels with varying oxygen tensions, creating physiologically relevant oxygen gradients across tissue constructs. Their approach includes the integration of oxygen-sensing fluorescent dyes within the microfluidic channels, enabling continuous non-invasive monitoring of local oxygen concentrations[2]. For high-metabolic tissues like liver and brain, Harvard researchers have implemented pulsatile flow patterns that enhance oxygen transport efficiency by mimicking natural blood flow dynamics. Additionally, they've developed computational models that predict oxygen consumption rates and distribution patterns within the tissue constructs, allowing for optimization of channel geometries and flow rates to prevent hypoxic regions in metabolically active zones.
Strengths: Sophisticated engineering approach with precise control over oxygen gradients; integration of real-time oxygen sensing capabilities; strong foundation in computational modeling for system optimization. Weaknesses: Complex fabrication process may limit scalability; requires specialized expertise to implement; higher technical barriers for adoption by non-specialized laboratories.
Key Technical Innovations in Tissue Oxygenation
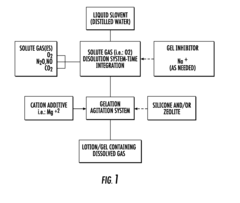
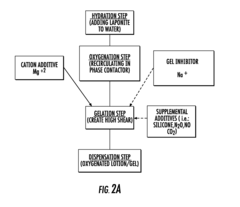
Method for producing a gas transporting rheological medium
PatentInactiveUS20150359818A1
Innovation
- A rheological medium containing molecular oxygen and other gases at supersaturated concentrations is created using elevated pressure, with a hydrated clay component to adsorb gases and micro-bubbles, allowing for high oxygen content and stable dispersion, which can be applied topically to enhance tissue regeneration and healing.
Device and method for treating tissue oxygen deficient conditions
PatentWO2023095140A1
Innovation
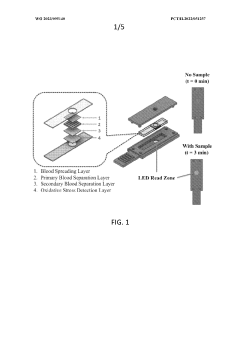
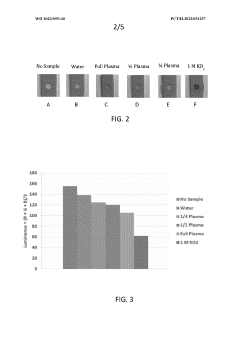
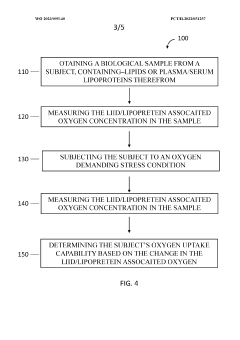
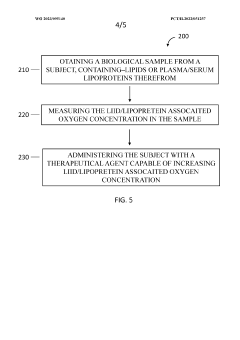
- A device and method using dry chemistry-based assays with membranes and reagents to measure lipid oxygen concentration in biological samples, allowing for rapid, non-invasive assessment of oxygen carrying capacity of plasma lipoproteins, enabling point-of-care testing without the need for specialized equipment or technical expertise.
Regulatory Considerations for Organ-on-Chip Systems
The regulatory landscape for organ-on-chip (OOC) systems presents unique challenges when considering oxygenation strategies for high-metabolic-demand tissues. Current regulatory frameworks were not specifically designed for these complex in vitro systems, creating a gap between technological innovation and regulatory oversight. The FDA and EMA have begun developing specialized guidance documents for microphysiological systems, but specific regulations addressing oxygenation parameters remain limited.
Validation and standardization of oxygenation strategies represent critical regulatory hurdles. Regulatory bodies increasingly require evidence that oxygen delivery systems can maintain physiologically relevant conditions consistently across experiments. This is particularly challenging for high-metabolic-demand tissues such as liver, heart, and brain models, where oxygen consumption rates vary significantly from standard cell cultures.
Quality control metrics for oxygenation strategies must be established to meet regulatory requirements. These include demonstrating spatial and temporal oxygen gradient stability, documenting oxygen consumption rates under various conditions, and validating sensor technologies used for real-time monitoring. Regulatory submissions increasingly require comprehensive data on how oxygenation parameters affect tissue functionality and drug responses.
Good Manufacturing Practice (GMP) considerations emerge when scaling oxygenation strategies from research to clinical applications. Materials used in oxygen delivery systems must meet biocompatibility standards, while manufacturing processes need to ensure consistent performance across production batches. This becomes especially complex when integrating advanced oxygenation technologies like oxygen-carrying perfluorocarbons or hemoglobin-based solutions.
International harmonization efforts are underway to standardize regulatory approaches to OOC technologies. The International Council for Harmonisation (ICH) has initiated discussions on microphysiological systems, with oxygenation strategies being a key consideration for high-metabolic-demand tissues. These efforts aim to establish common validation protocols and acceptance criteria across major regulatory jurisdictions.
Risk assessment frameworks for oxygenation strategies must address both technical and biological variables. Regulatory bodies increasingly require failure mode analysis for oxygen delivery systems, particularly for applications involving drug screening or toxicity testing. This includes evaluating the impact of oxygen fluctuations on cellular responses and identifying critical control points in the oxygenation system.
Future regulatory pathways will likely involve a tiered approach based on intended use. Research-only applications may face less stringent requirements, while OOC systems intended for drug development or clinical diagnostics will require comprehensive validation of oxygenation strategies. Regulatory agencies are increasingly open to collaborative approaches, working with developers to establish appropriate standards for these emerging technologies.
Validation and standardization of oxygenation strategies represent critical regulatory hurdles. Regulatory bodies increasingly require evidence that oxygen delivery systems can maintain physiologically relevant conditions consistently across experiments. This is particularly challenging for high-metabolic-demand tissues such as liver, heart, and brain models, where oxygen consumption rates vary significantly from standard cell cultures.
Quality control metrics for oxygenation strategies must be established to meet regulatory requirements. These include demonstrating spatial and temporal oxygen gradient stability, documenting oxygen consumption rates under various conditions, and validating sensor technologies used for real-time monitoring. Regulatory submissions increasingly require comprehensive data on how oxygenation parameters affect tissue functionality and drug responses.
Good Manufacturing Practice (GMP) considerations emerge when scaling oxygenation strategies from research to clinical applications. Materials used in oxygen delivery systems must meet biocompatibility standards, while manufacturing processes need to ensure consistent performance across production batches. This becomes especially complex when integrating advanced oxygenation technologies like oxygen-carrying perfluorocarbons or hemoglobin-based solutions.
International harmonization efforts are underway to standardize regulatory approaches to OOC technologies. The International Council for Harmonisation (ICH) has initiated discussions on microphysiological systems, with oxygenation strategies being a key consideration for high-metabolic-demand tissues. These efforts aim to establish common validation protocols and acceptance criteria across major regulatory jurisdictions.
Risk assessment frameworks for oxygenation strategies must address both technical and biological variables. Regulatory bodies increasingly require failure mode analysis for oxygen delivery systems, particularly for applications involving drug screening or toxicity testing. This includes evaluating the impact of oxygen fluctuations on cellular responses and identifying critical control points in the oxygenation system.
Future regulatory pathways will likely involve a tiered approach based on intended use. Research-only applications may face less stringent requirements, while OOC systems intended for drug development or clinical diagnostics will require comprehensive validation of oxygenation strategies. Regulatory agencies are increasingly open to collaborative approaches, working with developers to establish appropriate standards for these emerging technologies.
Scalability and Manufacturing Challenges
The scalability and manufacturing challenges for organ-on-chip systems with high-metabolic-demand tissues represent significant barriers to widespread adoption and commercialization. Current laboratory-scale production methods often rely on manual fabrication techniques that are time-consuming and prone to variability. When scaling these systems for high-metabolic tissues like liver, heart, or brain organoids, the complexity increases exponentially due to their enhanced oxygen requirements and intricate microarchitectures.
Mass production of these sophisticated microfluidic devices demands standardized manufacturing processes that can consistently deliver precise microchannels, membranes, and integrated sensors. Traditional soft lithography techniques using PDMS, while excellent for prototyping, present limitations for large-scale manufacturing due to material inconsistencies and labor-intensive processes. Alternative materials like thermoplastics offer better scalability but may compromise gas permeability crucial for oxygenation strategies.
The integration of oxygenation components presents particular manufacturing challenges. On-chip oxygen generators, perfluorocarbon-based oxygen carriers, and microfluidic oxygenators all require specialized fabrication steps that are difficult to standardize across large production runs. For instance, embedding oxygen-sensing elements requires precise alignment and calibration that current automated manufacturing systems struggle to achieve consistently.
Quality control represents another significant hurdle, as high-throughput testing methods for oxygen diffusion characteristics and metabolic support capacity remain underdeveloped. Each device must maintain consistent oxygen gradients and delivery rates to ensure reproducible tissue responses, yet current inspection technologies lack the sensitivity to detect subtle variations in oxygenation performance at production speeds.
Cost considerations further complicate scaling efforts. The materials required for advanced oxygenation strategies, such as perfluorocarbons or specialized membranes with controlled permeability, remain expensive when sourced at commercial scales. Additionally, the complex multi-layer fabrication processes necessary for integrated oxygenation systems drive up manufacturing costs, potentially limiting accessibility for research and clinical applications.
Regulatory considerations also impact manufacturing scalability, as production facilities must adhere to stringent quality standards when these devices are intended for drug development or clinical applications. The lack of standardized validation protocols specifically addressing oxygenation performance creates uncertainty in manufacturing specifications and quality assurance procedures.
Future advances in automated microfabrication technologies, including 3D printing of microfluidic components and roll-to-roll manufacturing of functional membranes, may address some of these challenges. However, significant investment in manufacturing process development specifically optimized for oxygenation-critical systems will be necessary to bridge the gap between laboratory prototypes and commercially viable products.
Mass production of these sophisticated microfluidic devices demands standardized manufacturing processes that can consistently deliver precise microchannels, membranes, and integrated sensors. Traditional soft lithography techniques using PDMS, while excellent for prototyping, present limitations for large-scale manufacturing due to material inconsistencies and labor-intensive processes. Alternative materials like thermoplastics offer better scalability but may compromise gas permeability crucial for oxygenation strategies.
The integration of oxygenation components presents particular manufacturing challenges. On-chip oxygen generators, perfluorocarbon-based oxygen carriers, and microfluidic oxygenators all require specialized fabrication steps that are difficult to standardize across large production runs. For instance, embedding oxygen-sensing elements requires precise alignment and calibration that current automated manufacturing systems struggle to achieve consistently.
Quality control represents another significant hurdle, as high-throughput testing methods for oxygen diffusion characteristics and metabolic support capacity remain underdeveloped. Each device must maintain consistent oxygen gradients and delivery rates to ensure reproducible tissue responses, yet current inspection technologies lack the sensitivity to detect subtle variations in oxygenation performance at production speeds.
Cost considerations further complicate scaling efforts. The materials required for advanced oxygenation strategies, such as perfluorocarbons or specialized membranes with controlled permeability, remain expensive when sourced at commercial scales. Additionally, the complex multi-layer fabrication processes necessary for integrated oxygenation systems drive up manufacturing costs, potentially limiting accessibility for research and clinical applications.
Regulatory considerations also impact manufacturing scalability, as production facilities must adhere to stringent quality standards when these devices are intended for drug development or clinical applications. The lack of standardized validation protocols specifically addressing oxygenation performance creates uncertainty in manufacturing specifications and quality assurance procedures.
Future advances in automated microfabrication technologies, including 3D printing of microfluidic components and roll-to-roll manufacturing of functional membranes, may address some of these challenges. However, significant investment in manufacturing process development specifically optimized for oxygenation-critical systems will be necessary to bridge the gap between laboratory prototypes and commercially viable products.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!