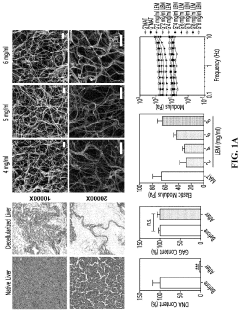
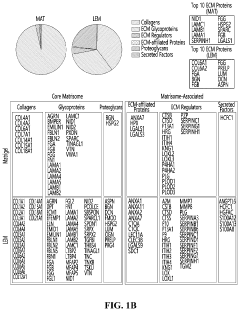
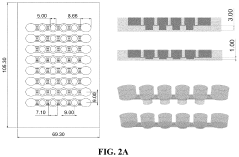
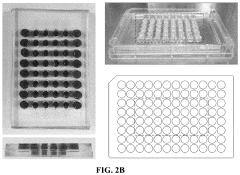
Modeling non-alcoholic fatty liver disease progression and therapeutic reversal on a liver-on-chip
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NAFLD Modeling Background and Objectives
Non-alcoholic fatty liver disease (NAFLD) has emerged as a significant global health concern, affecting approximately 25% of the world's population. The disease spectrum ranges from simple steatosis to non-alcoholic steatohepatitis (NASH), which can progress to cirrhosis and hepatocellular carcinoma. Despite its prevalence and severity, the pathophysiological mechanisms underlying NAFLD progression remain incompletely understood, hindering the development of effective therapeutic interventions.
The evolution of NAFLD research has transitioned from traditional animal models to more sophisticated in vitro systems. Early studies relied heavily on rodent models, which, while valuable, often failed to recapitulate the complex interplay of metabolic, inflammatory, and fibrotic processes characteristic of human NAFLD. The emergence of 2D cell culture systems provided initial insights but lacked the three-dimensional architecture and multicellular interactions essential for modeling liver pathophysiology.
Recent technological advances have enabled the development of liver-on-chip platforms, representing a paradigm shift in NAFLD modeling. These microfluidic devices integrate multiple cell types within a controlled microenvironment, allowing for the simulation of physiological fluid flow, nutrient gradients, and mechanical forces. The temporal evolution of this technology reflects broader trends in bioengineering and tissue engineering, with increasing emphasis on recapitulating the complex liver microenvironment.
The primary objective of liver-on-chip NAFLD modeling is to establish a physiologically relevant platform that accurately mimics disease progression and response to therapeutic interventions. Specifically, these models aim to capture the sequential events of lipid accumulation, hepatocellular injury, inflammation, and fibrosis that characterize NAFLD pathogenesis. Furthermore, they seek to incorporate key metabolic and immune components that drive disease progression.
A critical goal is to develop a standardized, reproducible system that enables high-throughput screening of potential therapeutic compounds. This would significantly accelerate drug discovery efforts by providing a more predictive preclinical model than conventional approaches. Additionally, these platforms aim to facilitate personalized medicine approaches by incorporating patient-derived cells, potentially enabling tailored therapeutic strategies.
The long-term technical objective extends beyond disease modeling to establishing predictive biomarkers of NAFLD progression and therapeutic response. By integrating advanced imaging techniques, metabolomic analyses, and computational modeling, liver-on-chip platforms aspire to identify novel diagnostic and prognostic indicators. Ultimately, these systems aim to bridge the translational gap between preclinical research and clinical applications, potentially revolutionizing our approach to NAFLD management and treatment.
The evolution of NAFLD research has transitioned from traditional animal models to more sophisticated in vitro systems. Early studies relied heavily on rodent models, which, while valuable, often failed to recapitulate the complex interplay of metabolic, inflammatory, and fibrotic processes characteristic of human NAFLD. The emergence of 2D cell culture systems provided initial insights but lacked the three-dimensional architecture and multicellular interactions essential for modeling liver pathophysiology.
Recent technological advances have enabled the development of liver-on-chip platforms, representing a paradigm shift in NAFLD modeling. These microfluidic devices integrate multiple cell types within a controlled microenvironment, allowing for the simulation of physiological fluid flow, nutrient gradients, and mechanical forces. The temporal evolution of this technology reflects broader trends in bioengineering and tissue engineering, with increasing emphasis on recapitulating the complex liver microenvironment.
The primary objective of liver-on-chip NAFLD modeling is to establish a physiologically relevant platform that accurately mimics disease progression and response to therapeutic interventions. Specifically, these models aim to capture the sequential events of lipid accumulation, hepatocellular injury, inflammation, and fibrosis that characterize NAFLD pathogenesis. Furthermore, they seek to incorporate key metabolic and immune components that drive disease progression.
A critical goal is to develop a standardized, reproducible system that enables high-throughput screening of potential therapeutic compounds. This would significantly accelerate drug discovery efforts by providing a more predictive preclinical model than conventional approaches. Additionally, these platforms aim to facilitate personalized medicine approaches by incorporating patient-derived cells, potentially enabling tailored therapeutic strategies.
The long-term technical objective extends beyond disease modeling to establishing predictive biomarkers of NAFLD progression and therapeutic response. By integrating advanced imaging techniques, metabolomic analyses, and computational modeling, liver-on-chip platforms aspire to identify novel diagnostic and prognostic indicators. Ultimately, these systems aim to bridge the translational gap between preclinical research and clinical applications, potentially revolutionizing our approach to NAFLD management and treatment.
Market Analysis for Liver-on-Chip NAFLD Models
The global market for liver-on-chip NAFLD models is experiencing significant growth, driven by the increasing prevalence of non-alcoholic fatty liver disease (NAFLD) worldwide. Currently estimated at approximately $380 million, this specialized segment of the organ-on-chip market is projected to grow at a compound annual growth rate of 25% through 2028, potentially reaching $1.2 billion by the end of the forecast period.
This robust growth is primarily fueled by the alarming rise in NAFLD cases globally, with prevalence rates reaching 25-30% in many developed countries and showing concerning upward trends in developing nations. The economic burden of NAFLD-related healthcare costs exceeds $100 billion annually in the United States alone, creating urgent demand for more effective diagnostic and therapeutic solutions.
Pharmaceutical companies represent the largest market segment, accounting for nearly 65% of current liver-on-chip NAFLD model utilization. These companies are increasingly adopting these platforms to reduce the 90% failure rate of liver disease drugs in clinical trials, potentially saving billions in development costs. Academic research institutions constitute approximately 20% of the market, while biotechnology startups and contract research organizations make up the remaining 15%.
Regionally, North America dominates with 45% market share, followed by Europe at 30% and Asia-Pacific at 20%. The Asia-Pacific region, particularly China and South Korea, is expected to show the fastest growth rate at 32% annually, driven by increasing research funding and rising NAFLD prevalence.
Key market drivers include the limitations of traditional animal models in accurately replicating human NAFLD pathophysiology, with concordance rates between animal studies and human clinical outcomes below 50% for liver diseases. Additionally, regulatory pressures to reduce animal testing, particularly in Europe under Directive 2010/63/EU, are accelerating adoption of alternative testing platforms.
The market faces several challenges, including the high cost of liver-on-chip systems, with average setup costs ranging from $50,000 to $150,000, limiting adoption in smaller research facilities. Technical challenges in achieving standardization across platforms and regulatory uncertainties regarding validation protocols also constrain market expansion.
Emerging opportunities include the integration of liver-on-chip NAFLD models with artificial intelligence for predictive analytics, potentially increasing diagnostic accuracy by 40%. The personalized medicine approach using patient-derived cells on liver chips represents another high-growth segment, expected to expand at 35% annually as precision medicine initiatives gain momentum globally.
This robust growth is primarily fueled by the alarming rise in NAFLD cases globally, with prevalence rates reaching 25-30% in many developed countries and showing concerning upward trends in developing nations. The economic burden of NAFLD-related healthcare costs exceeds $100 billion annually in the United States alone, creating urgent demand for more effective diagnostic and therapeutic solutions.
Pharmaceutical companies represent the largest market segment, accounting for nearly 65% of current liver-on-chip NAFLD model utilization. These companies are increasingly adopting these platforms to reduce the 90% failure rate of liver disease drugs in clinical trials, potentially saving billions in development costs. Academic research institutions constitute approximately 20% of the market, while biotechnology startups and contract research organizations make up the remaining 15%.
Regionally, North America dominates with 45% market share, followed by Europe at 30% and Asia-Pacific at 20%. The Asia-Pacific region, particularly China and South Korea, is expected to show the fastest growth rate at 32% annually, driven by increasing research funding and rising NAFLD prevalence.
Key market drivers include the limitations of traditional animal models in accurately replicating human NAFLD pathophysiology, with concordance rates between animal studies and human clinical outcomes below 50% for liver diseases. Additionally, regulatory pressures to reduce animal testing, particularly in Europe under Directive 2010/63/EU, are accelerating adoption of alternative testing platforms.
The market faces several challenges, including the high cost of liver-on-chip systems, with average setup costs ranging from $50,000 to $150,000, limiting adoption in smaller research facilities. Technical challenges in achieving standardization across platforms and regulatory uncertainties regarding validation protocols also constrain market expansion.
Emerging opportunities include the integration of liver-on-chip NAFLD models with artificial intelligence for predictive analytics, potentially increasing diagnostic accuracy by 40%. The personalized medicine approach using patient-derived cells on liver chips represents another high-growth segment, expected to expand at 35% annually as precision medicine initiatives gain momentum globally.
Current Challenges in NAFLD Modeling Technologies
Despite significant advancements in liver-on-chip technologies, current NAFLD modeling approaches face several critical challenges that limit their clinical and research utility. The primary obstacle remains the accurate recapitulation of the complex, multifactorial nature of NAFLD progression. Most existing models fail to integrate the dynamic interplay between hepatocytes, stellate cells, Kupffer cells, and other non-parenchymal cells that collectively drive disease pathogenesis.
Temporal limitations present another significant hurdle, as NAFLD develops over years in humans, while most in vitro models operate within timeframes of days to weeks. This compressed timeline makes it difficult to observe subtle disease progression markers and evaluate long-term therapeutic interventions. Additionally, current platforms struggle to maintain stable cellular phenotypes over extended periods, with dedifferentiation of primary hepatocytes occurring rapidly in conventional culture systems.
The incorporation of systemic metabolic factors represents another substantial challenge. NAFLD is intrinsically linked to metabolic syndrome, involving cross-talk between liver, adipose tissue, pancreas, and muscle. Current liver-on-chip models typically focus exclusively on hepatic tissue, neglecting these crucial inter-organ communications that influence disease development and progression.
Technical reproducibility issues further complicate NAFLD modeling efforts. Variations in cell sources, extracellular matrix compositions, and microfluidic parameters lead to inconsistent results across laboratories. The field lacks standardized protocols for establishing reliable NAFLD models, making cross-study comparisons and validation of therapeutic findings problematic.
Quantification and monitoring methodologies present additional challenges. Non-invasive, real-time assessment of steatosis, inflammation, and fibrosis remains difficult in microfluidic environments. Current techniques often require endpoint analyses that destroy the model, preventing longitudinal studies of disease progression or therapeutic reversal.
Scaling limitations also hinder progress, as most liver-on-chip platforms accommodate only small tissue volumes, restricting their utility for high-throughput drug screening. This constraint significantly impacts pharmaceutical development pipelines seeking efficient evaluation of candidate compounds for NAFLD treatment.
Finally, regulatory and validation challenges persist. Establishing the predictive value of liver-on-chip NAFLD models for human disease outcomes requires extensive correlation with clinical data. Regulatory agencies have yet to establish clear guidelines for qualifying these models as alternatives to animal testing in drug development pathways, creating uncertainty in their translational value.
Temporal limitations present another significant hurdle, as NAFLD develops over years in humans, while most in vitro models operate within timeframes of days to weeks. This compressed timeline makes it difficult to observe subtle disease progression markers and evaluate long-term therapeutic interventions. Additionally, current platforms struggle to maintain stable cellular phenotypes over extended periods, with dedifferentiation of primary hepatocytes occurring rapidly in conventional culture systems.
The incorporation of systemic metabolic factors represents another substantial challenge. NAFLD is intrinsically linked to metabolic syndrome, involving cross-talk between liver, adipose tissue, pancreas, and muscle. Current liver-on-chip models typically focus exclusively on hepatic tissue, neglecting these crucial inter-organ communications that influence disease development and progression.
Technical reproducibility issues further complicate NAFLD modeling efforts. Variations in cell sources, extracellular matrix compositions, and microfluidic parameters lead to inconsistent results across laboratories. The field lacks standardized protocols for establishing reliable NAFLD models, making cross-study comparisons and validation of therapeutic findings problematic.
Quantification and monitoring methodologies present additional challenges. Non-invasive, real-time assessment of steatosis, inflammation, and fibrosis remains difficult in microfluidic environments. Current techniques often require endpoint analyses that destroy the model, preventing longitudinal studies of disease progression or therapeutic reversal.
Scaling limitations also hinder progress, as most liver-on-chip platforms accommodate only small tissue volumes, restricting their utility for high-throughput drug screening. This constraint significantly impacts pharmaceutical development pipelines seeking efficient evaluation of candidate compounds for NAFLD treatment.
Finally, regulatory and validation challenges persist. Establishing the predictive value of liver-on-chip NAFLD models for human disease outcomes requires extensive correlation with clinical data. Regulatory agencies have yet to establish clear guidelines for qualifying these models as alternatives to animal testing in drug development pathways, creating uncertainty in their translational value.
Current Liver-on-Chip NAFLD Modeling Approaches
01 Liver-on-chip models for disease progression monitoring
Microfluidic liver-on-chip platforms that simulate liver tissue structure and function to study disease progression in real-time. These models incorporate hepatocytes and other liver cells in a controlled microenvironment that mimics physiological conditions, allowing researchers to observe changes during disease development. The systems enable continuous monitoring of cellular responses, biomarkers, and metabolic activities as liver diseases progress from early to advanced stages.- Liver-on-chip platforms for disease modeling: Microfluidic liver-on-chip platforms can be used to model liver diseases and their progression. These platforms incorporate hepatocytes and other liver cells in a controlled microenvironment that mimics the liver's structure and function. The chips allow for real-time monitoring of disease progression markers and can simulate various liver pathologies including fibrosis, steatosis, and inflammation. These models provide valuable insights into disease mechanisms and progression patterns.
- Therapeutic screening and drug efficacy assessment: Liver-on-chip systems serve as platforms for screening potential therapeutic compounds and assessing drug efficacy against liver diseases. These systems enable high-throughput testing of drug candidates under physiologically relevant conditions, allowing researchers to evaluate therapeutic reversal of disease states. The chips can measure key parameters such as drug metabolism, toxicity profiles, and therapeutic responses, providing valuable data for drug development and personalized medicine approaches.
- Disease progression biomarkers and monitoring: Liver-on-chip platforms facilitate the identification and monitoring of biomarkers associated with liver disease progression. These systems can detect changes in cellular morphology, secreted factors, gene expression, and metabolic profiles that indicate disease advancement or therapeutic response. By continuously monitoring these biomarkers, researchers can track disease progression in real-time and evaluate the effectiveness of interventions aimed at reversing pathological states.
- Integration of immune components for disease modeling: Advanced liver-on-chip systems incorporate immune cells to better model inflammatory and immune-mediated liver diseases. These integrated systems allow for the study of immune cell interactions with hepatocytes during disease progression and therapeutic intervention. The inclusion of immune components enables more accurate modeling of conditions such as autoimmune hepatitis, viral hepatitis, and inflammation-driven fibrosis, as well as assessment of immunomodulatory therapies for disease reversal.
- Personalized medicine applications: Liver-on-chip technology enables personalized medicine approaches by incorporating patient-derived cells to create individualized disease models. These patient-specific chips can recapitulate unique disease characteristics and predict individual responses to therapeutic interventions. By testing various treatment options on personalized liver chips, clinicians can identify optimal therapeutic strategies for reversing disease progression in individual patients, leading to more effective and tailored treatment regimens.
02 Therapeutic screening and drug efficacy assessment
Liver-on-chip platforms designed specifically for evaluating therapeutic interventions and their potential to reverse disease states. These systems allow for high-throughput screening of drug candidates against liver diseases by providing a physiologically relevant environment for testing. The platforms can measure multiple parameters simultaneously, including drug metabolism, toxicity profiles, and therapeutic efficacy, enabling researchers to identify compounds capable of halting or reversing disease progression.Expand Specific Solutions03 Disease-specific liver-on-chip models
Specialized liver-on-chip systems engineered to replicate specific liver pathologies such as fibrosis, steatosis, viral hepatitis, or cancer. These models incorporate disease-specific features like inflammatory stimuli, fat accumulation mechanisms, or viral components to accurately represent particular liver conditions. By recreating the pathophysiological environment of specific diseases, these platforms enable targeted studies of disease mechanisms and potential therapeutic interventions tailored to each condition.Expand Specific Solutions04 Integration of immune components for disease modeling
Advanced liver-on-chip systems that incorporate immune cells and components to better model inflammatory and immune-mediated liver diseases. These platforms include co-cultures of hepatocytes with various immune cells such as Kupffer cells, stellate cells, and lymphocytes to recreate immune-mediated disease processes. The integration of immune components allows for studying the complex interactions between liver cells and the immune system during disease progression and therapeutic intervention.Expand Specific Solutions05 Multi-organ chip systems for systemic disease effects
Integrated multi-organ chip platforms that connect liver-on-chip models with other organ systems to study the systemic effects of liver diseases and treatments. These systems link the liver compartment with other organs such as intestine, kidney, or heart through microfluidic channels to model organ crosstalk during disease states. The multi-organ approach enables assessment of how liver disease progression affects other organs and how therapeutic interventions targeting the liver may impact overall systemic health.Expand Specific Solutions
Key Industry Players in Organ-on-Chip NAFLD Research
The non-alcoholic fatty liver disease (NAFLD) modeling market is in an early growth phase, with increasing research focus due to rising global prevalence. The liver-on-chip technology represents an emerging segment within the estimated $2-3 billion NAFLD therapeutics market. Technical maturity varies significantly among key players: academic institutions like University of California and Virginia Commonwealth University lead fundamental research, while specialized companies such as Sagimet Biosciences, Genfit SA, and Organovo are advancing commercial applications. Pharmaceutical companies including Takeda are investing heavily in this space. The technology remains in developmental stages with most liver-on-chip models still transitioning from proof-of-concept to validated diagnostic and drug screening platforms, though recent breakthroughs suggest accelerating progress toward clinical applications.
The Regents of the University of California
Technical Solution: The University of California has developed a sophisticated liver-on-chip platform for NAFLD modeling that integrates advanced microfluidics with tissue engineering principles. Their system features a multi-compartment design that recreates the liver lobule architecture, with separate chambers for hepatocytes, stellate cells, and Kupffer cells connected by microchannels that mimic sinusoidal blood flow. The platform incorporates oxygen gradient control to replicate the zonation phenomenon observed in native liver tissue, where different metabolic activities occur in different regions. For NAFLD modeling, the UC system employs a combination of free fatty acids, insulin resistance inducers, and inflammatory stimuli to recreate the "multiple hits" pathogenesis of the disease. Their platform enables sequential observation of steatosis development, inflammatory activation, and fibrogenesis. The system includes integrated biosensors for real-time monitoring of key metabolites, inflammatory markers, and cellular stress indicators. UC researchers have validated their model against human liver biopsy samples from NAFLD patients and demonstrated its utility for testing novel therapeutic compounds targeting different aspects of disease progression.
Strengths: Comprehensive recreation of liver zonation and multiple cell type interactions; integrated biosensing capabilities for real-time monitoring; validated against human clinical samples. Weaknesses: Complex system setup requires significant expertise; higher cost and maintenance requirements compared to simpler models; limited throughput for high-volume drug screening applications.
Organovo, Inc.
Technical Solution: Organovo has pioneered the development of 3D bioprinted liver tissue models specifically designed for modeling non-alcoholic fatty liver disease (NAFLD) progression. Their ExVive™ Human Liver Tissue platform creates architecturally accurate, multicellular liver tissues that maintain key liver functions for extended periods. For NAFLD research, Organovo's technology incorporates primary human hepatocytes, stellate cells, and Kupffer cells in a defined spatial arrangement that mimics the native liver microenvironment. This allows researchers to induce steatosis through exposure to free fatty acids, observe inflammatory responses, and monitor fibrosis development over time. The platform enables real-time monitoring of disease progression markers and evaluation of therapeutic compounds' efficacy in reversing NAFLD pathology. Organovo's liver-on-chip models demonstrate dose-dependent responses to known therapeutic agents and can maintain viability for up to 28 days, allowing for chronic disease modeling and longitudinal studies of therapeutic interventions.
Strengths: Highly accurate representation of human liver architecture and cellular interactions; extended tissue viability allowing for chronic disease modeling; ability to incorporate patient-specific cells for personalized medicine applications. Weaknesses: Higher cost compared to traditional 2D cell culture systems; technical complexity requiring specialized expertise; limited throughput compared to simpler in vitro models.
Critical Technologies for NAFLD Progression Simulation
Non-alcoholic fatty liver artificial tissue model
PatentPendingUS20240228973A9
Innovation
- A non-alcoholic fatty liver artificial tissue model is developed, comprising a hydrogel with decellularized liver tissue-derived extracellular matrix, liver organoids, and a device with microchannels for free fatty acid flow, allowing for the simulation of NAFLD and screening of therapeutic drugs.
Use of Engineered Liver Tissue Constructs for Modeling Liver Disorders
PatentActiveUS20200115682A1
Innovation
- Development of three-dimensional, engineered, bioprinted liver tissue constructs that include parenchymal and non-parenchymal cells, capable of exhibiting phenotypes such as lipid accumulation, inflammation, and fibrosis, allowing for the creation of accurate in vitro models of NAFLD and NASH, enabling the assessment of therapeutic agents and biomarker discovery.
Regulatory Considerations for Preclinical NAFLD Models
The regulatory landscape for preclinical Non-Alcoholic Fatty Liver Disease (NAFLD) models presents significant challenges for researchers and pharmaceutical developers utilizing liver-on-chip technologies. Current FDA and EMA guidelines for preclinical NAFLD studies emphasize the need for models that accurately recapitulate disease progression pathways, particularly the transition from simple steatosis to non-alcoholic steatohepatitis (NASH) and fibrosis.
Liver-on-chip platforms face unique regulatory considerations compared to traditional animal models. While regulatory bodies acknowledge the potential of these platforms to reduce animal testing in accordance with 3R principles (Replacement, Reduction, Refinement), they require robust validation against established gold standards. The FDA's Alternative Methods Working Group has shown increasing interest in organ-on-chip technologies but maintains stringent requirements for demonstrating physiological relevance.
Key regulatory hurdles for liver-on-chip NAFLD models include standardization of protocols, reproducibility across laboratories, and correlation with clinical outcomes. The International Conference on Harmonisation (ICH) guidelines specifically require demonstration of metabolic functionality, inflammatory responses, and fibrogenic pathways that mirror human pathophysiology. Recent FDA draft guidance (2018) on NASH drug development emphasizes the importance of models that can predict therapeutic efficacy across multiple disease stages.
Qualification processes for novel methodologies under both FDA and EMA frameworks require extensive documentation of the model's biological relevance, technical performance, and limitations. For liver-on-chip NAFLD models, this includes validation of key disease markers such as lipid accumulation patterns, insulin resistance mechanisms, and inflammatory cascades against human biopsy samples.
Regulatory acceptance of these models is evolving through collaborative initiatives such as the Innovation and Quality Consortium (IQ) and the Critical Path Initiative. These programs facilitate dialogue between developers, regulatory agencies, and pharmaceutical companies to establish consensus on validation criteria and appropriate applications of liver-on-chip technologies in the NAFLD drug development pipeline.
Looking forward, the integration of liver-on-chip models into regulatory frameworks will likely follow a stepwise approach, initially as complementary tools alongside traditional models before potentially gaining acceptance as standalone alternatives for specific applications. Developers must engage early and frequently with regulatory authorities through programs like the FDA's Emerging Technology Program to navigate this evolving landscape effectively.
Liver-on-chip platforms face unique regulatory considerations compared to traditional animal models. While regulatory bodies acknowledge the potential of these platforms to reduce animal testing in accordance with 3R principles (Replacement, Reduction, Refinement), they require robust validation against established gold standards. The FDA's Alternative Methods Working Group has shown increasing interest in organ-on-chip technologies but maintains stringent requirements for demonstrating physiological relevance.
Key regulatory hurdles for liver-on-chip NAFLD models include standardization of protocols, reproducibility across laboratories, and correlation with clinical outcomes. The International Conference on Harmonisation (ICH) guidelines specifically require demonstration of metabolic functionality, inflammatory responses, and fibrogenic pathways that mirror human pathophysiology. Recent FDA draft guidance (2018) on NASH drug development emphasizes the importance of models that can predict therapeutic efficacy across multiple disease stages.
Qualification processes for novel methodologies under both FDA and EMA frameworks require extensive documentation of the model's biological relevance, technical performance, and limitations. For liver-on-chip NAFLD models, this includes validation of key disease markers such as lipid accumulation patterns, insulin resistance mechanisms, and inflammatory cascades against human biopsy samples.
Regulatory acceptance of these models is evolving through collaborative initiatives such as the Innovation and Quality Consortium (IQ) and the Critical Path Initiative. These programs facilitate dialogue between developers, regulatory agencies, and pharmaceutical companies to establish consensus on validation criteria and appropriate applications of liver-on-chip technologies in the NAFLD drug development pipeline.
Looking forward, the integration of liver-on-chip models into regulatory frameworks will likely follow a stepwise approach, initially as complementary tools alongside traditional models before potentially gaining acceptance as standalone alternatives for specific applications. Developers must engage early and frequently with regulatory authorities through programs like the FDA's Emerging Technology Program to navigate this evolving landscape effectively.
Ethical Implications of Reduced Animal Testing
The development of liver-on-chip technology for modeling non-alcoholic fatty liver disease (NAFLD) represents a significant advancement in reducing reliance on animal testing. This technological approach raises important ethical considerations that extend beyond the scientific benefits. The reduction of animal testing aligns with the 3Rs principle (Replacement, Reduction, and Refinement) that has become a cornerstone of ethical research practices in biomedical sciences.
The liver-on-chip platform offers a humane alternative to traditional animal models for NAFLD research. Conventional studies often require large numbers of animals subjected to specialized diets or genetic modifications over extended periods to develop disease states. These animals frequently experience discomfort, metabolic disturbances, and ultimately euthanasia for tissue analysis. By contrast, the liver-on-chip technology utilizes human cells in a controlled microenvironment, eliminating the need for animal suffering while potentially providing more translatable results.
From a utilitarian ethical perspective, the liver-on-chip approach maximizes benefits while minimizing harm. It allows for high-throughput screening of potential therapeutics without animal sacrifice, addressing a major ethical concern in pharmaceutical development. Additionally, this technology enables personalized medicine approaches by incorporating patient-derived cells, further reducing the need for animal testing in treatment optimization.
The ethical implications extend to scientific validity considerations. Animal models often fail to fully recapitulate human NAFLD pathophysiology due to species differences in metabolism and disease progression. This discrepancy has contributed to high failure rates in clinical trials despite promising preclinical results. Liver-on-chip technology addresses this ethical concern by providing a more physiologically relevant human model, potentially reducing failed clinical trials and unnecessary animal testing.
However, ethical challenges remain in the transition away from animal models. The validation of liver-on-chip platforms requires comparison with existing animal data, necessitating some continued animal use during development phases. Additionally, regulatory frameworks still largely require animal testing data for drug approval processes, creating a tension between ethical advances and regulatory compliance.
The broader societal implications include potential shifts in public perception of biomedical research. As technologies like liver-on-chip gain prominence, they may foster greater public support for research by demonstrating the scientific community's commitment to ethical advancement and animal welfare while maintaining scientific progress in addressing critical health challenges like NAFLD.
The liver-on-chip platform offers a humane alternative to traditional animal models for NAFLD research. Conventional studies often require large numbers of animals subjected to specialized diets or genetic modifications over extended periods to develop disease states. These animals frequently experience discomfort, metabolic disturbances, and ultimately euthanasia for tissue analysis. By contrast, the liver-on-chip technology utilizes human cells in a controlled microenvironment, eliminating the need for animal suffering while potentially providing more translatable results.
From a utilitarian ethical perspective, the liver-on-chip approach maximizes benefits while minimizing harm. It allows for high-throughput screening of potential therapeutics without animal sacrifice, addressing a major ethical concern in pharmaceutical development. Additionally, this technology enables personalized medicine approaches by incorporating patient-derived cells, further reducing the need for animal testing in treatment optimization.
The ethical implications extend to scientific validity considerations. Animal models often fail to fully recapitulate human NAFLD pathophysiology due to species differences in metabolism and disease progression. This discrepancy has contributed to high failure rates in clinical trials despite promising preclinical results. Liver-on-chip technology addresses this ethical concern by providing a more physiologically relevant human model, potentially reducing failed clinical trials and unnecessary animal testing.
However, ethical challenges remain in the transition away from animal models. The validation of liver-on-chip platforms requires comparison with existing animal data, necessitating some continued animal use during development phases. Additionally, regulatory frameworks still largely require animal testing data for drug approval processes, creating a tension between ethical advances and regulatory compliance.
The broader societal implications include potential shifts in public perception of biomedical research. As technologies like liver-on-chip gain prominence, they may foster greater public support for research by demonstrating the scientific community's commitment to ethical advancement and animal welfare while maintaining scientific progress in addressing critical health challenges like NAFLD.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!