Environmental Impact of Sulfamic Acid in Industrial Use
JUL 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulfamic Acid Background and Objectives
Sulfamic acid, a versatile compound with the chemical formula H3NSO3, has been widely used in various industrial applications since its discovery in the early 20th century. This strong, yet relatively safe acid has gained prominence due to its unique properties and diverse range of applications. The evolution of sulfamic acid usage in industry has been driven by its effectiveness in descaling, cleaning, and as a key ingredient in many chemical processes.
The primary objective of this technical research report is to comprehensively examine the environmental impact of sulfamic acid in industrial use. As industries worldwide strive for more sustainable practices, understanding the ecological footprint of commonly used chemicals becomes paramount. Sulfamic acid, despite its widespread use, has raised concerns regarding its potential effects on aquatic ecosystems and soil quality.
Throughout its history, sulfamic acid has been employed in various sectors, including metal finishing, water treatment, and household cleaning products. Its popularity stems from its ability to effectively remove mineral deposits and rust without the corrosive nature of stronger acids. However, as environmental awareness has grown, so has the need to assess the long-term implications of its use on the environment.
The technical evolution of sulfamic acid applications has seen significant advancements in recent years. Innovations in formulation and application methods have aimed to enhance its efficiency while minimizing environmental impact. These developments include the creation of more environmentally friendly sulfamic acid-based products and improved waste management techniques in industrial processes.
As we delve deeper into the environmental aspects of sulfamic acid use, it is crucial to consider the global perspective. Different regions have varying regulations and standards regarding the use and disposal of sulfamic acid, which directly influences its environmental impact. This report aims to provide a comprehensive overview of these regional differences and their implications for global environmental sustainability.
Furthermore, this research will explore the current technological landscape surrounding sulfamic acid, including emerging alternatives and potential substitutes that may offer similar benefits with reduced environmental risks. By examining these aspects, we aim to provide valuable insights into the future direction of sulfamic acid use in industry and its role in sustainable chemical practices.
The primary objective of this technical research report is to comprehensively examine the environmental impact of sulfamic acid in industrial use. As industries worldwide strive for more sustainable practices, understanding the ecological footprint of commonly used chemicals becomes paramount. Sulfamic acid, despite its widespread use, has raised concerns regarding its potential effects on aquatic ecosystems and soil quality.
Throughout its history, sulfamic acid has been employed in various sectors, including metal finishing, water treatment, and household cleaning products. Its popularity stems from its ability to effectively remove mineral deposits and rust without the corrosive nature of stronger acids. However, as environmental awareness has grown, so has the need to assess the long-term implications of its use on the environment.
The technical evolution of sulfamic acid applications has seen significant advancements in recent years. Innovations in formulation and application methods have aimed to enhance its efficiency while minimizing environmental impact. These developments include the creation of more environmentally friendly sulfamic acid-based products and improved waste management techniques in industrial processes.
As we delve deeper into the environmental aspects of sulfamic acid use, it is crucial to consider the global perspective. Different regions have varying regulations and standards regarding the use and disposal of sulfamic acid, which directly influences its environmental impact. This report aims to provide a comprehensive overview of these regional differences and their implications for global environmental sustainability.
Furthermore, this research will explore the current technological landscape surrounding sulfamic acid, including emerging alternatives and potential substitutes that may offer similar benefits with reduced environmental risks. By examining these aspects, we aim to provide valuable insights into the future direction of sulfamic acid use in industry and its role in sustainable chemical practices.
Industrial Demand Analysis
The industrial demand for sulfamic acid has been steadily increasing due to its versatile applications across various sectors. This compound, known for its strong acidic properties and relative stability, finds extensive use in cleaning, descaling, and metal finishing processes. The global market for sulfamic acid is projected to grow significantly in the coming years, driven by the expansion of industries such as pulp and paper, textiles, and water treatment.
In the cleaning industry, sulfamic acid is highly valued for its effectiveness in removing limescale, rust, and other mineral deposits. Its non-fuming nature and lower corrosivity compared to alternatives like hydrochloric acid make it a preferred choice for both industrial and household cleaning products. The growing awareness of hygiene and sanitation, particularly in developing countries, is expected to fuel the demand for sulfamic acid-based cleaners.
The water treatment sector represents another major market for sulfamic acid. As water scarcity becomes a pressing global issue, the need for efficient water treatment solutions is escalating. Sulfamic acid is used in water treatment plants for pH adjustment, scale removal, and as a disinfectant. The increasing implementation of stringent water quality regulations worldwide is likely to boost the demand for sulfamic acid in this sector.
In the metal finishing industry, sulfamic acid plays a crucial role in electroplating processes, particularly for nickel plating. The automotive and electronics industries, which heavily rely on metal finishing, are experiencing robust growth, consequently driving the demand for sulfamic acid. The compound's ability to produce bright, smooth finishes while minimizing hydrogen embrittlement makes it indispensable in these applications.
The pulp and paper industry also contributes significantly to the industrial demand for sulfamic acid. It is used in the production of specialty papers and for cleaning paper-making equipment. As the global demand for paper products continues to rise, particularly in emerging economies, the need for sulfamic acid in this sector is expected to grow correspondingly.
However, the increasing focus on environmental sustainability poses both challenges and opportunities for the sulfamic acid market. While the compound is biodegradable and less harmful than some alternatives, concerns about its potential environmental impact persist. This has led to a growing demand for eco-friendly formulations and more sustainable production methods, driving innovation in the sulfamic acid industry.
The geographical distribution of sulfamic acid demand is closely tied to industrial activities. Asia-Pacific region, particularly China and India, is expected to dominate the market due to rapid industrialization and urbanization. North America and Europe also maintain significant market shares, primarily driven by established industries and stringent quality standards in manufacturing processes.
In the cleaning industry, sulfamic acid is highly valued for its effectiveness in removing limescale, rust, and other mineral deposits. Its non-fuming nature and lower corrosivity compared to alternatives like hydrochloric acid make it a preferred choice for both industrial and household cleaning products. The growing awareness of hygiene and sanitation, particularly in developing countries, is expected to fuel the demand for sulfamic acid-based cleaners.
The water treatment sector represents another major market for sulfamic acid. As water scarcity becomes a pressing global issue, the need for efficient water treatment solutions is escalating. Sulfamic acid is used in water treatment plants for pH adjustment, scale removal, and as a disinfectant. The increasing implementation of stringent water quality regulations worldwide is likely to boost the demand for sulfamic acid in this sector.
In the metal finishing industry, sulfamic acid plays a crucial role in electroplating processes, particularly for nickel plating. The automotive and electronics industries, which heavily rely on metal finishing, are experiencing robust growth, consequently driving the demand for sulfamic acid. The compound's ability to produce bright, smooth finishes while minimizing hydrogen embrittlement makes it indispensable in these applications.
The pulp and paper industry also contributes significantly to the industrial demand for sulfamic acid. It is used in the production of specialty papers and for cleaning paper-making equipment. As the global demand for paper products continues to rise, particularly in emerging economies, the need for sulfamic acid in this sector is expected to grow correspondingly.
However, the increasing focus on environmental sustainability poses both challenges and opportunities for the sulfamic acid market. While the compound is biodegradable and less harmful than some alternatives, concerns about its potential environmental impact persist. This has led to a growing demand for eco-friendly formulations and more sustainable production methods, driving innovation in the sulfamic acid industry.
The geographical distribution of sulfamic acid demand is closely tied to industrial activities. Asia-Pacific region, particularly China and India, is expected to dominate the market due to rapid industrialization and urbanization. North America and Europe also maintain significant market shares, primarily driven by established industries and stringent quality standards in manufacturing processes.
Environmental Challenges and Limitations
The widespread use of sulfamic acid in industrial applications has raised significant environmental concerns. One of the primary challenges is its potential to contribute to water pollution. When sulfamic acid is discharged into water bodies, it can lower the pH, leading to acidification of aquatic ecosystems. This acidification can have detrimental effects on aquatic life, disrupting the delicate balance of flora and fauna in affected areas.
Another environmental limitation of sulfamic acid is its impact on soil quality. When sulfamic acid or its residues contaminate soil, it can alter the soil's pH, potentially affecting plant growth and microbial activity. This alteration can lead to reduced soil fertility and negatively impact agricultural productivity in affected regions.
The production and disposal of sulfamic acid also present environmental challenges. The manufacturing process of sulfamic acid involves the use of various chemicals and energy-intensive procedures, contributing to greenhouse gas emissions and resource depletion. Improper disposal of sulfamic acid waste can lead to soil and groundwater contamination, posing long-term environmental risks.
Sulfamic acid's corrosive nature presents additional environmental concerns. Its use in industrial cleaning and descaling operations can lead to the degradation of metal surfaces and infrastructure. This degradation not only shortens the lifespan of equipment but also increases the risk of leaks and spills, potentially releasing harmful substances into the environment.
The transportation and storage of sulfamic acid also pose environmental risks. Accidental spills during transport or storage can result in localized environmental damage, affecting soil, water, and surrounding ecosystems. The need for specialized containment and handling procedures adds to the environmental footprint of its industrial use.
Furthermore, the interaction of sulfamic acid with other chemicals in industrial settings can lead to the formation of harmful byproducts. These byproducts may have their own set of environmental impacts, potentially contributing to air pollution or the formation of toxic compounds in water and soil.
Lastly, the cumulative effect of long-term sulfamic acid use in industrial applications raises concerns about its potential to contribute to broader environmental issues such as acid rain and the overall acidification of ecosystems. While individual instances of sulfamic acid use may have limited impact, the collective industrial usage over time could contribute to more significant environmental challenges on a regional or global scale.
Another environmental limitation of sulfamic acid is its impact on soil quality. When sulfamic acid or its residues contaminate soil, it can alter the soil's pH, potentially affecting plant growth and microbial activity. This alteration can lead to reduced soil fertility and negatively impact agricultural productivity in affected regions.
The production and disposal of sulfamic acid also present environmental challenges. The manufacturing process of sulfamic acid involves the use of various chemicals and energy-intensive procedures, contributing to greenhouse gas emissions and resource depletion. Improper disposal of sulfamic acid waste can lead to soil and groundwater contamination, posing long-term environmental risks.
Sulfamic acid's corrosive nature presents additional environmental concerns. Its use in industrial cleaning and descaling operations can lead to the degradation of metal surfaces and infrastructure. This degradation not only shortens the lifespan of equipment but also increases the risk of leaks and spills, potentially releasing harmful substances into the environment.
The transportation and storage of sulfamic acid also pose environmental risks. Accidental spills during transport or storage can result in localized environmental damage, affecting soil, water, and surrounding ecosystems. The need for specialized containment and handling procedures adds to the environmental footprint of its industrial use.
Furthermore, the interaction of sulfamic acid with other chemicals in industrial settings can lead to the formation of harmful byproducts. These byproducts may have their own set of environmental impacts, potentially contributing to air pollution or the formation of toxic compounds in water and soil.
Lastly, the cumulative effect of long-term sulfamic acid use in industrial applications raises concerns about its potential to contribute to broader environmental issues such as acid rain and the overall acidification of ecosystems. While individual instances of sulfamic acid use may have limited impact, the collective industrial usage over time could contribute to more significant environmental challenges on a regional or global scale.
Current Mitigation Strategies
01 Biodegradability and environmental fate
Sulfamic acid is generally considered biodegradable and has a relatively low environmental impact. It breaks down in the environment into sulfate and ammonium ions, which are naturally occurring substances. However, its impact on aquatic ecosystems should be monitored, as high concentrations may affect pH levels and aquatic life.- Biodegradability and environmental fate: Sulfamic acid is generally considered biodegradable and has a relatively low environmental impact. It breaks down into sulfate and ammonium ions in the environment, which are naturally occurring substances. However, its impact on aquatic ecosystems should be monitored, especially in cases of high concentration discharge.
- Use in eco-friendly cleaning products: Sulfamic acid is increasingly used in environmentally friendly cleaning products as an alternative to more harmful chemicals. Its effectiveness in removing limescale and rust, combined with its lower environmental impact, makes it a preferred choice in green cleaning formulations.
- Water treatment applications: Sulfamic acid is used in water treatment processes, particularly for pH adjustment and scale removal in industrial systems. Its use in this context can help reduce the need for more environmentally harmful chemicals, though proper dosage and disposal practices are crucial to minimize any potential negative impacts on aquatic environments.
- Agricultural use and soil impact: In agriculture, sulfamic acid is sometimes used as a nitrogen fertilizer or herbicide. While it can provide benefits in terms of plant growth, its impact on soil pH and microbial communities should be carefully managed to prevent potential long-term soil degradation or ecosystem disruption.
- Industrial waste management: The use of sulfamic acid in various industrial processes necessitates proper waste management practices. While less harmful than many alternatives, high concentrations in industrial effluents can still pose environmental risks. Implementing effective treatment and neutralization methods before discharge is essential to minimize its environmental footprint.
02 Use in eco-friendly cleaning products
Sulfamic acid is increasingly used in environmentally friendly cleaning products as an alternative to more harmful chemicals. Its effectiveness in removing limescale and mineral deposits, combined with its lower environmental impact, makes it a preferred choice for green cleaning solutions.Expand Specific Solutions03 Wastewater treatment applications
Sulfamic acid is utilized in wastewater treatment processes to adjust pH levels and remove scale buildup in treatment systems. Its use can help improve the efficiency of water treatment plants while minimizing negative environmental impacts associated with more aggressive chemicals.Expand Specific Solutions04 Impact on soil and agriculture
When used in agricultural applications, sulfamic acid can have both positive and negative effects on soil. It can help adjust soil pH and improve nutrient availability for plants. However, excessive use may lead to soil acidification and affect microbial communities. Proper application and dosage are crucial to minimize potential environmental impacts.Expand Specific Solutions05 Eco-toxicological considerations
While sulfamic acid is generally considered to have a lower environmental impact compared to many other acids, its potential effects on aquatic organisms and ecosystems should not be overlooked. Studies have shown that high concentrations can be harmful to certain aquatic species. Proper handling, disposal, and dilution practices are essential to mitigate potential eco-toxicological risks.Expand Specific Solutions
Key Industry Players
The environmental impact of sulfamic acid in industrial use presents a complex competitive landscape. The market is in a growth phase, driven by increasing industrial applications and environmental concerns. The global market size for sulfamic acid is expanding, with projections indicating continued growth. Technologically, the field is evolving, with companies like China Petroleum & Chemical Corp., ExxonMobil Technology & Engineering Co., and Chevron Phillips Chemical Co. LP leading in process innovations. These industry giants are investing in research to improve efficiency and reduce environmental impact. Smaller players like TDA Research, Inc. and Solugen, Inc. are focusing on developing eco-friendly alternatives, potentially disrupting the traditional sulfamic acid market.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative approach to mitigate the environmental impact of sulfamic acid in industrial use. Their method involves a two-step process: first, they employ a novel catalytic decomposition technique that breaks down sulfamic acid into less harmful components[1]. This is followed by an advanced wastewater treatment system that utilizes membrane technology and biological treatment to further reduce the concentration of sulfur-containing compounds[3]. The company has also implemented a closed-loop system in their refineries, which allows for the recycling and reuse of sulfamic acid, significantly reducing overall consumption and waste[5]. Additionally, Sinopec has invested in research to develop alternative cleaning agents that can replace sulfamic acid in certain applications, further minimizing its environmental footprint[7].
Strengths: Comprehensive approach addressing both decomposition and waste treatment; Closed-loop system for recycling; Research into alternative cleaning agents. Weaknesses: High initial implementation costs; May require significant modifications to existing industrial processes.
ExxonMobil Technology & Engineering Co.
Technical Solution: ExxonMobil Technology & Engineering Co. has developed a multi-faceted approach to address the environmental impact of sulfamic acid in industrial use. Their strategy includes the implementation of advanced oxidation processes (AOPs) to degrade sulfamic acid in wastewater streams[2]. This technology utilizes a combination of UV light and hydrogen peroxide to generate highly reactive hydroxyl radicals, which effectively break down sulfamic acid into less harmful compounds. ExxonMobil has also pioneered a novel adsorption technique using specially engineered nanomaterials that can selectively remove sulfamic acid from industrial effluents[4]. Furthermore, the company has developed a proprietary chemical neutralization process that converts sulfamic acid into environmentally benign sulfates, which can be safely disposed of or potentially repurposed for other industrial applications[6]. To complement these end-of-pipe solutions, ExxonMobil has also focused on source reduction by optimizing cleaning processes to minimize the use of sulfamic acid without compromising efficiency[8].
Strengths: Diverse range of treatment technologies; Focus on both end-of-pipe solutions and source reduction; Potential for by-product repurposing. Weaknesses: High energy consumption for AOP technology; Potential for secondary pollution from chemical neutralization process.
Innovative Environmental Solutions
Weak ion exchange particulate medium prepared from phenol-containing organic matter for anions contained in aqueous solutions
PatentActiveUS20200324281A1
Innovation
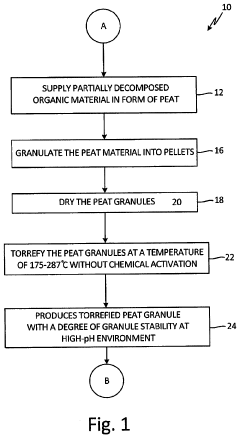
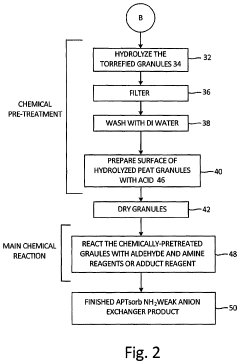
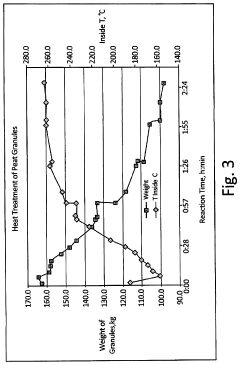
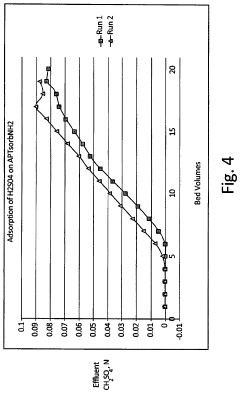
- A process to produce torrefied and chemically modified peat granules with amine functional groups, enhancing their physical stability and anion exchange capacity, involving torrefaction at 175-300°C, hydrolysis with sodium carbonate, and surface treatment with acidic reagents to create a weak anion exchange medium.
Surface modification of inorganic metal oxides for enhanced sulfur selectivity in transportion fuels
PatentInactiveUS20100163456A1
Innovation
- A fuel filter using a surface-modified inorganic oxide adsorbent with a pKa of less than or equal to −3, which selectively adsorbs sulfur compounds without requiring heat or hydrogen, allowing for efficient desulfurization in normal temperatures and compatibility with various fuel additives.
Regulatory Framework
The regulatory framework surrounding the use of sulfamic acid in industrial applications is complex and multifaceted, reflecting the growing concern for environmental protection and sustainable practices. At the international level, organizations such as the United Nations Environment Programme (UNEP) and the Organisation for Economic Co-operation and Development (OECD) have established guidelines and recommendations for the safe handling and disposal of chemicals, including sulfamic acid.
In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating the use of sulfamic acid under the Toxic Substances Control Act (TSCA). The EPA has set specific guidelines for the manufacture, import, and use of sulfamic acid, including reporting requirements and risk assessment protocols. Additionally, the Occupational Safety and Health Administration (OSHA) has established workplace safety standards for handling sulfamic acid, including exposure limits and personal protective equipment requirements.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which applies to sulfamic acid and other chemical substances. Under REACH, manufacturers and importers are required to register sulfamic acid with the European Chemicals Agency (ECHA) and provide detailed information on its properties, uses, and potential risks. The EU has also established specific classification and labeling requirements for sulfamic acid under the Classification, Labeling, and Packaging (CLP) Regulation.
Many countries have adopted their own regulatory frameworks for chemical management, often aligning with international standards. For example, Canada's Chemical Management Plan includes sulfamic acid in its assessment of existing substances, while Australia's National Industrial Chemicals Notification and Assessment Scheme (NICNAS) regulates its import and manufacture.
Environmental regulations specifically addressing the impact of sulfamic acid on aquatic ecosystems have been implemented in various jurisdictions. These regulations often set limits on the discharge of sulfamic acid and its byproducts into water bodies, requiring industrial users to implement appropriate treatment and disposal methods. Some countries have also established monitoring programs to assess the presence and concentration of sulfamic acid in surface waters and sediments.
As awareness of environmental issues continues to grow, regulatory frameworks are evolving to address emerging concerns. This includes the development of more stringent environmental impact assessment requirements for industrial processes involving sulfamic acid, as well as the promotion of cleaner production technologies and alternative substances with reduced environmental footprints.
In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating the use of sulfamic acid under the Toxic Substances Control Act (TSCA). The EPA has set specific guidelines for the manufacture, import, and use of sulfamic acid, including reporting requirements and risk assessment protocols. Additionally, the Occupational Safety and Health Administration (OSHA) has established workplace safety standards for handling sulfamic acid, including exposure limits and personal protective equipment requirements.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which applies to sulfamic acid and other chemical substances. Under REACH, manufacturers and importers are required to register sulfamic acid with the European Chemicals Agency (ECHA) and provide detailed information on its properties, uses, and potential risks. The EU has also established specific classification and labeling requirements for sulfamic acid under the Classification, Labeling, and Packaging (CLP) Regulation.
Many countries have adopted their own regulatory frameworks for chemical management, often aligning with international standards. For example, Canada's Chemical Management Plan includes sulfamic acid in its assessment of existing substances, while Australia's National Industrial Chemicals Notification and Assessment Scheme (NICNAS) regulates its import and manufacture.
Environmental regulations specifically addressing the impact of sulfamic acid on aquatic ecosystems have been implemented in various jurisdictions. These regulations often set limits on the discharge of sulfamic acid and its byproducts into water bodies, requiring industrial users to implement appropriate treatment and disposal methods. Some countries have also established monitoring programs to assess the presence and concentration of sulfamic acid in surface waters and sediments.
As awareness of environmental issues continues to grow, regulatory frameworks are evolving to address emerging concerns. This includes the development of more stringent environmental impact assessment requirements for industrial processes involving sulfamic acid, as well as the promotion of cleaner production technologies and alternative substances with reduced environmental footprints.
Life Cycle Assessment
Life Cycle Assessment (LCA) is a crucial tool for evaluating the environmental impact of sulfamic acid in industrial use. This comprehensive approach examines the entire lifecycle of sulfamic acid, from raw material extraction to disposal, providing valuable insights into its overall environmental footprint.
The production phase of sulfamic acid involves the reaction of urea with sulfuric acid or oleum. This process requires significant energy inputs and generates various emissions. The extraction and processing of raw materials, particularly sulfur and ammonia, contribute to resource depletion and potential environmental degradation. Manufacturing facilities may release air pollutants, including sulfur oxides and nitrogen oxides, which can lead to acid rain and smog formation.
During the use phase, sulfamic acid's environmental impact varies depending on its specific industrial application. In descaling and cleaning processes, it may contribute to water pollution if not properly managed. However, its biodegradability and lower toxicity compared to some alternatives can be advantageous. The acid's efficiency in removing scale and mineral deposits may lead to improved energy efficiency in industrial equipment, potentially offsetting some of its production-related impacts.
The disposal and end-of-life stage of sulfamic acid presents both challenges and opportunities. Proper neutralization and treatment of waste streams containing sulfamic acid are essential to prevent environmental contamination. However, its biodegradability facilitates natural breakdown in the environment, reducing long-term ecological risks.
Transportation throughout the lifecycle of sulfamic acid also contributes to its environmental impact. The movement of raw materials, finished products, and waste generates greenhouse gas emissions and consumes fossil fuels. Optimizing logistics and considering local production can help mitigate these impacts.
LCA studies have shown that the most significant environmental impacts of sulfamic acid often occur during the production phase. However, its use in certain applications can lead to net positive environmental outcomes by improving industrial process efficiency and reducing the need for more harmful alternatives.
To fully assess the environmental impact, it is crucial to consider the potential for sulfamic acid to form sulfate ions in aquatic environments. While sulfates are generally less harmful than other industrial pollutants, high concentrations can affect water quality and aquatic ecosystems. Proper wastewater treatment and responsible use practices are essential to mitigate these risks.
In conclusion, the life cycle assessment of sulfamic acid in industrial use reveals a complex interplay of environmental impacts across its lifecycle stages. While production and transportation contribute significantly to its environmental footprint, the acid's efficiency and relatively low toxicity in use can offer environmental benefits in certain applications. Ongoing research and innovation in production methods, coupled with responsible use and disposal practices, are key to minimizing the overall environmental impact of sulfamic acid in industrial settings.
The production phase of sulfamic acid involves the reaction of urea with sulfuric acid or oleum. This process requires significant energy inputs and generates various emissions. The extraction and processing of raw materials, particularly sulfur and ammonia, contribute to resource depletion and potential environmental degradation. Manufacturing facilities may release air pollutants, including sulfur oxides and nitrogen oxides, which can lead to acid rain and smog formation.
During the use phase, sulfamic acid's environmental impact varies depending on its specific industrial application. In descaling and cleaning processes, it may contribute to water pollution if not properly managed. However, its biodegradability and lower toxicity compared to some alternatives can be advantageous. The acid's efficiency in removing scale and mineral deposits may lead to improved energy efficiency in industrial equipment, potentially offsetting some of its production-related impacts.
The disposal and end-of-life stage of sulfamic acid presents both challenges and opportunities. Proper neutralization and treatment of waste streams containing sulfamic acid are essential to prevent environmental contamination. However, its biodegradability facilitates natural breakdown in the environment, reducing long-term ecological risks.
Transportation throughout the lifecycle of sulfamic acid also contributes to its environmental impact. The movement of raw materials, finished products, and waste generates greenhouse gas emissions and consumes fossil fuels. Optimizing logistics and considering local production can help mitigate these impacts.
LCA studies have shown that the most significant environmental impacts of sulfamic acid often occur during the production phase. However, its use in certain applications can lead to net positive environmental outcomes by improving industrial process efficiency and reducing the need for more harmful alternatives.
To fully assess the environmental impact, it is crucial to consider the potential for sulfamic acid to form sulfate ions in aquatic environments. While sulfates are generally less harmful than other industrial pollutants, high concentrations can affect water quality and aquatic ecosystems. Proper wastewater treatment and responsible use practices are essential to mitigate these risks.
In conclusion, the life cycle assessment of sulfamic acid in industrial use reveals a complex interplay of environmental impacts across its lifecycle stages. While production and transportation contribute significantly to its environmental footprint, the acid's efficiency and relatively low toxicity in use can offer environmental benefits in certain applications. Ongoing research and innovation in production methods, coupled with responsible use and disposal practices, are key to minimizing the overall environmental impact of sulfamic acid in industrial settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!