Evaluating Hypochlorous Acid Within Animal Skin Care Regimens
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HOCl in Animal Skincare: Background and Objectives
Hypochlorous acid (HOCl) has emerged as a promising solution in animal skincare, drawing attention from veterinarians, pet owners, and the animal care industry. This naturally occurring molecule, produced by the mammalian immune system, has been recognized for its potent antimicrobial properties and potential to address various skin conditions in animals.
The evolution of animal skincare has seen a shift towards more natural, effective, and safe alternatives to traditional chemical-based treatments. HOCl represents a significant milestone in this progression, offering a bio-compatible option that mimics the body's own defense mechanisms. Its ability to combat a wide range of pathogens while being gentle on skin tissue has positioned it as a versatile solution for various animal species.
The primary objective of exploring HOCl in animal skincare is to evaluate its efficacy, safety, and practical applications across different species and skin conditions. This includes assessing its potential in treating common issues such as bacterial and fungal infections, wounds, and inflammatory skin disorders. Additionally, there is a focus on understanding HOCl's role in maintaining overall skin health and hygiene in animals, potentially revolutionizing preventive care practices.
Another crucial aim is to investigate the scalability and stability of HOCl formulations for commercial use in the animal care sector. This involves addressing challenges related to shelf life, packaging, and delivery methods to ensure the product remains effective from production to application. The goal is to develop stable, easy-to-use HOCl-based products that can be integrated into regular animal grooming and healthcare routines.
Furthermore, the research seeks to compare HOCl with existing skincare solutions in terms of efficacy, cost-effectiveness, and environmental impact. This comparative analysis is essential for positioning HOCl within the current market landscape and identifying its unique value proposition in animal skincare.
Lastly, the exploration of HOCl in animal skincare aims to establish standardized protocols for its use across different animal species and skin conditions. This includes determining optimal concentrations, application methods, and treatment durations for various scenarios. The ultimate goal is to provide veterinarians, animal care professionals, and pet owners with clear, evidence-based guidelines for incorporating HOCl into animal skincare regimens effectively and safely.
The evolution of animal skincare has seen a shift towards more natural, effective, and safe alternatives to traditional chemical-based treatments. HOCl represents a significant milestone in this progression, offering a bio-compatible option that mimics the body's own defense mechanisms. Its ability to combat a wide range of pathogens while being gentle on skin tissue has positioned it as a versatile solution for various animal species.
The primary objective of exploring HOCl in animal skincare is to evaluate its efficacy, safety, and practical applications across different species and skin conditions. This includes assessing its potential in treating common issues such as bacterial and fungal infections, wounds, and inflammatory skin disorders. Additionally, there is a focus on understanding HOCl's role in maintaining overall skin health and hygiene in animals, potentially revolutionizing preventive care practices.
Another crucial aim is to investigate the scalability and stability of HOCl formulations for commercial use in the animal care sector. This involves addressing challenges related to shelf life, packaging, and delivery methods to ensure the product remains effective from production to application. The goal is to develop stable, easy-to-use HOCl-based products that can be integrated into regular animal grooming and healthcare routines.
Furthermore, the research seeks to compare HOCl with existing skincare solutions in terms of efficacy, cost-effectiveness, and environmental impact. This comparative analysis is essential for positioning HOCl within the current market landscape and identifying its unique value proposition in animal skincare.
Lastly, the exploration of HOCl in animal skincare aims to establish standardized protocols for its use across different animal species and skin conditions. This includes determining optimal concentrations, application methods, and treatment durations for various scenarios. The ultimate goal is to provide veterinarians, animal care professionals, and pet owners with clear, evidence-based guidelines for incorporating HOCl into animal skincare regimens effectively and safely.
Market Analysis for HOCl-based Animal Skincare Products
The market for HOCl-based animal skincare products is experiencing significant growth, driven by increasing awareness of pet health and the demand for effective, safe, and natural solutions. The global pet care market, valued at $190 billion in 2022, is projected to reach $325 billion by 2028, with skincare products playing a crucial role in this expansion.
HOCl-based products are gaining traction in the animal skincare segment due to their antimicrobial properties and gentle nature. Pet owners are increasingly seeking alternatives to traditional chemical-based treatments, favoring products that mimic natural defense mechanisms. This shift is particularly evident in developed markets such as North America and Europe, where pet humanization trends are most pronounced.
The market for HOCl animal skincare products is segmented into various applications, including wound care, dermatitis treatment, and general hygiene. Wound care represents the largest segment, driven by the product's ability to promote healing without causing irritation. The dermatitis treatment segment is expected to show the highest growth rate, as HOCl offers an effective solution for managing common skin conditions in pets.
Veterinary clinics and hospitals currently dominate the distribution channels for HOCl-based animal skincare products. However, the e-commerce sector is rapidly gaining market share, offering convenience and a wide range of product options to pet owners. This trend is expected to accelerate, particularly in the wake of the COVID-19 pandemic, which has shifted consumer behavior towards online purchasing.
Regionally, North America holds the largest market share for HOCl animal skincare products, followed by Europe and Asia-Pacific. The Asia-Pacific region is anticipated to witness the fastest growth, driven by rising pet ownership rates and increasing disposable incomes in countries like China and India.
Key market drivers include the growing pet population, increasing pet healthcare expenditure, and rising awareness of zoonotic diseases. The trend towards natural and eco-friendly pet care solutions also favors the adoption of HOCl-based products. However, challenges such as regulatory hurdles and the need for consumer education about the benefits of HOCl may impact market growth.
The competitive landscape of the HOCl animal skincare market is characterized by a mix of established pet care companies and innovative startups. Major players are investing in research and development to expand their product portfolios and gain a competitive edge. Strategic partnerships between HOCl manufacturers and pet care brands are becoming increasingly common, aiming to leverage complementary strengths and expand market reach.
HOCl-based products are gaining traction in the animal skincare segment due to their antimicrobial properties and gentle nature. Pet owners are increasingly seeking alternatives to traditional chemical-based treatments, favoring products that mimic natural defense mechanisms. This shift is particularly evident in developed markets such as North America and Europe, where pet humanization trends are most pronounced.
The market for HOCl animal skincare products is segmented into various applications, including wound care, dermatitis treatment, and general hygiene. Wound care represents the largest segment, driven by the product's ability to promote healing without causing irritation. The dermatitis treatment segment is expected to show the highest growth rate, as HOCl offers an effective solution for managing common skin conditions in pets.
Veterinary clinics and hospitals currently dominate the distribution channels for HOCl-based animal skincare products. However, the e-commerce sector is rapidly gaining market share, offering convenience and a wide range of product options to pet owners. This trend is expected to accelerate, particularly in the wake of the COVID-19 pandemic, which has shifted consumer behavior towards online purchasing.
Regionally, North America holds the largest market share for HOCl animal skincare products, followed by Europe and Asia-Pacific. The Asia-Pacific region is anticipated to witness the fastest growth, driven by rising pet ownership rates and increasing disposable incomes in countries like China and India.
Key market drivers include the growing pet population, increasing pet healthcare expenditure, and rising awareness of zoonotic diseases. The trend towards natural and eco-friendly pet care solutions also favors the adoption of HOCl-based products. However, challenges such as regulatory hurdles and the need for consumer education about the benefits of HOCl may impact market growth.
The competitive landscape of the HOCl animal skincare market is characterized by a mix of established pet care companies and innovative startups. Major players are investing in research and development to expand their product portfolios and gain a competitive edge. Strategic partnerships between HOCl manufacturers and pet care brands are becoming increasingly common, aiming to leverage complementary strengths and expand market reach.
Current State and Challenges of HOCl in Veterinary Dermatology
Hypochlorous acid (HOCl) has gained significant attention in veterinary dermatology due to its potent antimicrobial properties and potential for skin care applications. The current state of HOCl in veterinary dermatology is characterized by a growing body of research and clinical evidence supporting its efficacy in treating various skin conditions in animals.
HOCl is naturally produced by the immune system's neutrophils as part of the body's defense mechanism against pathogens. In recent years, stable formulations of HOCl have been developed for topical use in veterinary medicine. These formulations have demonstrated broad-spectrum antimicrobial activity against bacteria, viruses, and fungi, making them valuable tools in managing skin infections and promoting wound healing in animals.
One of the key advantages of HOCl in veterinary dermatology is its safety profile. Unlike many traditional antiseptics, HOCl is non-toxic, non-irritating, and does not contribute to antimicrobial resistance. This makes it particularly suitable for use in sensitive areas and for long-term management of chronic skin conditions in animals.
Clinical studies have shown promising results in the use of HOCl for various dermatological conditions in animals, including pyoderma, dermatitis, and otitis externa. Its ability to reduce bacterial load, decrease inflammation, and promote tissue repair has made it an attractive option for veterinarians seeking alternatives to conventional treatments.
Despite the growing adoption of HOCl in veterinary dermatology, several challenges remain. One significant hurdle is the stability of HOCl solutions. The compound is inherently unstable and can degrade rapidly, limiting its shelf life and effectiveness. Manufacturers have made progress in developing more stable formulations, but further improvements are needed to enhance the practicality of HOCl products in clinical settings.
Another challenge lies in standardizing the concentration and application protocols for different skin conditions and animal species. The optimal concentration and frequency of application may vary depending on the specific condition being treated and the animal's size and species. Establishing evidence-based guidelines for HOCl use in various veterinary dermatological scenarios is an ongoing process.
Additionally, while HOCl has shown promise in many areas of veterinary dermatology, more comprehensive, long-term studies are needed to fully understand its efficacy and potential limitations. This includes investigating its effects on the skin microbiome and its long-term impact on skin health in animals.
The integration of HOCl into existing treatment regimens also presents a challenge. Veterinarians must determine how to best incorporate HOCl alongside other topical and systemic treatments to maximize therapeutic outcomes without compromising the efficacy of other medications or causing adverse interactions.
HOCl is naturally produced by the immune system's neutrophils as part of the body's defense mechanism against pathogens. In recent years, stable formulations of HOCl have been developed for topical use in veterinary medicine. These formulations have demonstrated broad-spectrum antimicrobial activity against bacteria, viruses, and fungi, making them valuable tools in managing skin infections and promoting wound healing in animals.
One of the key advantages of HOCl in veterinary dermatology is its safety profile. Unlike many traditional antiseptics, HOCl is non-toxic, non-irritating, and does not contribute to antimicrobial resistance. This makes it particularly suitable for use in sensitive areas and for long-term management of chronic skin conditions in animals.
Clinical studies have shown promising results in the use of HOCl for various dermatological conditions in animals, including pyoderma, dermatitis, and otitis externa. Its ability to reduce bacterial load, decrease inflammation, and promote tissue repair has made it an attractive option for veterinarians seeking alternatives to conventional treatments.
Despite the growing adoption of HOCl in veterinary dermatology, several challenges remain. One significant hurdle is the stability of HOCl solutions. The compound is inherently unstable and can degrade rapidly, limiting its shelf life and effectiveness. Manufacturers have made progress in developing more stable formulations, but further improvements are needed to enhance the practicality of HOCl products in clinical settings.
Another challenge lies in standardizing the concentration and application protocols for different skin conditions and animal species. The optimal concentration and frequency of application may vary depending on the specific condition being treated and the animal's size and species. Establishing evidence-based guidelines for HOCl use in various veterinary dermatological scenarios is an ongoing process.
Additionally, while HOCl has shown promise in many areas of veterinary dermatology, more comprehensive, long-term studies are needed to fully understand its efficacy and potential limitations. This includes investigating its effects on the skin microbiome and its long-term impact on skin health in animals.
The integration of HOCl into existing treatment regimens also presents a challenge. Veterinarians must determine how to best incorporate HOCl alongside other topical and systemic treatments to maximize therapeutic outcomes without compromising the efficacy of other medications or causing adverse interactions.
Existing HOCl Applications in Animal Skin Care
01 Production methods of hypochlorous acid
Various methods are employed to produce hypochlorous acid, including electrolysis of salt solutions, chemical reactions involving chlorine and water, and the use of specialized equipment for on-site generation. These methods aim to create stable and effective hypochlorous acid solutions for different applications.- Production methods of hypochlorous acid: Various methods are employed to produce hypochlorous acid, including electrolysis of salt solutions, chemical reactions involving chlorine and water, and controlled mixing of precursor chemicals. These production methods aim to create stable and effective hypochlorous acid solutions for different applications.
- Applications in disinfection and sterilization: Hypochlorous acid is widely used as a powerful disinfectant and sterilizing agent. It is effective against a broad spectrum of microorganisms, including bacteria, viruses, and fungi. Applications include water treatment, surface disinfection, and medical sterilization.
- Formulations and stability enhancement: Research focuses on developing stable formulations of hypochlorous acid to extend its shelf life and maintain its effectiveness. This includes the use of stabilizers, pH adjustments, and packaging innovations to prevent degradation and ensure consistent potency over time.
- Medical and therapeutic applications: Hypochlorous acid is explored for various medical and therapeutic uses due to its antimicrobial properties and low toxicity to human cells. Applications include wound care, eye care, respiratory treatments, and dermatological therapies.
- Environmental and industrial uses: Hypochlorous acid finds applications in environmental remediation and industrial processes. It is used for water treatment, air purification, and as a green alternative in various industrial cleaning and sanitization processes, offering eco-friendly solutions to traditional chemical treatments.
02 Applications in disinfection and sanitization
Hypochlorous acid is widely used as a powerful disinfectant and sanitizing agent. It is effective against a broad spectrum of pathogens, including bacteria, viruses, and fungi. Applications range from water treatment and surface disinfection to medical sterilization and food safety.Expand Specific Solutions03 Formulations and stability enhancement
Research focuses on developing stable formulations of hypochlorous acid to extend its shelf life and maintain its efficacy. This includes the use of stabilizers, pH adjustments, and packaging innovations to prevent degradation and ensure consistent performance over time.Expand Specific Solutions04 Medical and therapeutic applications
Hypochlorous acid is explored for various medical and therapeutic uses due to its antimicrobial properties and low toxicity to human cells. Applications include wound care, eye care, respiratory treatments, and dermatological therapies.Expand Specific Solutions05 Environmental and industrial uses
Hypochlorous acid finds applications in environmental remediation and industrial processes. It is used in wastewater treatment, air purification systems, and as a green alternative in various industrial cleaning and sanitization processes, offering an eco-friendly option for many applications.Expand Specific Solutions
Key Players in Veterinary Skincare Industry
The evaluation of hypochlorous acid in animal skin care regimens is currently in an emerging phase, with a growing market driven by increasing demand for safe and effective animal care products. The global market size for animal skin care is expanding, though precise figures for hypochlorous acid applications are not readily available. Technologically, the field is advancing rapidly, with companies like Annihilare Medical Systems, Realm Therapeutics, and Aquaox leading innovation in hypochlorous acid generation and application. These firms are developing proprietary technologies for on-site production and specialized formulations, indicating a moderate level of technological maturity with significant room for further advancements and market penetration in the animal care sector.
ANNIHILARE MEDICAL SYSTEMS, INC.
Technical Solution: ANNIHILARE MEDICAL SYSTEMS has developed a proprietary Hypochlorous Acid (HOCl) formulation for animal skin care. Their technology focuses on producing stable, pH-balanced HOCl solutions that maintain efficacy over extended periods. The company's approach involves a unique electrolysis process that generates HOCl at specific concentrations suitable for various animal skin conditions. Their formulation is designed to be non-irritating and safe for frequent use on animals, addressing issues such as bacterial and fungal infections, wound healing, and general skin hygiene[1][3]. The company has also invested in packaging innovations to preserve the stability of their HOCl solutions during storage and application.
Strengths: High stability of HOCl formulation, pH-balanced for animal skin, versatile applications in veterinary care. Weaknesses: May require specialized equipment for production, potential higher cost compared to traditional antiseptics.
Realm Therapeutics, Inc.
Technical Solution: Realm Therapeutics has pioneered the use of stabilized Hypochlorous Acid (HOCl) in animal skin care regimens. Their proprietary technology, known as HOCI-Tech, focuses on maintaining the stability and efficacy of HOCl for extended periods. The company has developed a range of products specifically tailored for animal dermatological conditions, including sprays, gels, and wipes. Their formulations are designed to be non-toxic, non-sensitizing, and effective against a broad spectrum of pathogens commonly found in animal skin infections[2][5]. Realm's approach also includes the development of delivery systems that ensure the HOCl remains active at the site of application, enhancing its therapeutic potential in treating various skin conditions in animals.
Strengths: Patented stabilization technology, diverse product range for different applications, proven efficacy in animal studies. Weaknesses: May be more expensive than conventional treatments, requires education of veterinarians and pet owners on benefits.
Core Innovations in HOCl Formulation for Animal Use
Mixing device
PatentWO2013121295A2
Innovation
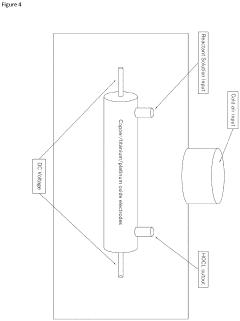
- A mixing device that produces fluidic vortices within a chamber with strategically placed apertures to stabilize hypochlorous acid by controlling proton concentration and pH through the use of buffering agents like acetic acid, allowing for its production and storage for extended periods without the need for onsite generation.
solution
PatentInactiveEP4332272A1
Innovation
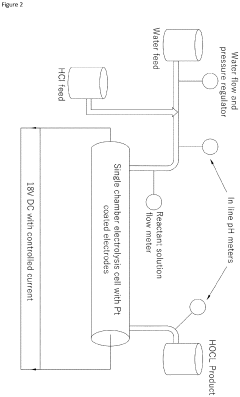
- A continuous electrolysis process using hydrochloric acid and water in a single chamber with platinum-coated electrodes, where the pH is precisely controlled to produce an ultrapure and stable hypochlorous acid solution without the need for buffers or stabilizers, eliminating chlorine gas and hypochlorite ions.
Safety and Efficacy Studies of HOCl in Animal Use
The safety and efficacy of hypochlorous acid (HOCl) in animal skin care regimens have been extensively studied, providing valuable insights into its potential applications. Numerous research efforts have focused on evaluating the effects of HOCl on various animal species, including companion animals, livestock, and laboratory animals.
In terms of safety, studies have consistently demonstrated that HOCl is well-tolerated when applied topically to animal skin. Research conducted on dogs, cats, horses, and other domesticated animals has shown minimal to no adverse reactions when HOCl solutions are used at appropriate concentrations. These findings are particularly significant given the sensitive nature of animal skin and the potential for irritation or allergic responses to topical treatments.
Efficacy studies have yielded promising results across a range of skin conditions in animals. HOCl has been found to be effective against a variety of pathogens, including bacteria, fungi, and viruses, making it a versatile option for treating and preventing skin infections. In particular, research on canine pyoderma, a common bacterial skin infection in dogs, has shown that HOCl can significantly reduce bacterial load and improve clinical symptoms.
Furthermore, studies on wound healing in animals have demonstrated the potential of HOCl as an adjunct therapy. Experiments involving both acute and chronic wounds in various animal models have shown that HOCl can accelerate wound closure, reduce inflammation, and promote tissue regeneration. These findings suggest that HOCl may be beneficial in veterinary wound care protocols.
In livestock applications, research has explored the use of HOCl for managing skin conditions and improving overall hygiene. Studies on dairy cattle have shown that HOCl-based teat dips can effectively reduce the incidence of mastitis, a common inflammatory condition of the udder. Similarly, investigations in the poultry industry have demonstrated the efficacy of HOCl in controlling bacterial populations on bird skin, potentially improving food safety.
While the majority of studies report positive outcomes, it is important to note that some research has highlighted the need for careful consideration of HOCl concentration and application methods. Overly high concentrations or prolonged exposure may lead to skin irritation or disruption of the natural skin microbiome in some animals.
Overall, the body of research on HOCl in animal skin care regimens supports its potential as a safe and effective treatment option. However, as with any medical intervention, further studies are needed to optimize protocols for specific animal species and skin conditions, ensuring the best possible outcomes in veterinary dermatology and animal health management.
In terms of safety, studies have consistently demonstrated that HOCl is well-tolerated when applied topically to animal skin. Research conducted on dogs, cats, horses, and other domesticated animals has shown minimal to no adverse reactions when HOCl solutions are used at appropriate concentrations. These findings are particularly significant given the sensitive nature of animal skin and the potential for irritation or allergic responses to topical treatments.
Efficacy studies have yielded promising results across a range of skin conditions in animals. HOCl has been found to be effective against a variety of pathogens, including bacteria, fungi, and viruses, making it a versatile option for treating and preventing skin infections. In particular, research on canine pyoderma, a common bacterial skin infection in dogs, has shown that HOCl can significantly reduce bacterial load and improve clinical symptoms.
Furthermore, studies on wound healing in animals have demonstrated the potential of HOCl as an adjunct therapy. Experiments involving both acute and chronic wounds in various animal models have shown that HOCl can accelerate wound closure, reduce inflammation, and promote tissue regeneration. These findings suggest that HOCl may be beneficial in veterinary wound care protocols.
In livestock applications, research has explored the use of HOCl for managing skin conditions and improving overall hygiene. Studies on dairy cattle have shown that HOCl-based teat dips can effectively reduce the incidence of mastitis, a common inflammatory condition of the udder. Similarly, investigations in the poultry industry have demonstrated the efficacy of HOCl in controlling bacterial populations on bird skin, potentially improving food safety.
While the majority of studies report positive outcomes, it is important to note that some research has highlighted the need for careful consideration of HOCl concentration and application methods. Overly high concentrations or prolonged exposure may lead to skin irritation or disruption of the natural skin microbiome in some animals.
Overall, the body of research on HOCl in animal skin care regimens supports its potential as a safe and effective treatment option. However, as with any medical intervention, further studies are needed to optimize protocols for specific animal species and skin conditions, ensuring the best possible outcomes in veterinary dermatology and animal health management.
Regulatory Framework for Veterinary Skincare Products
The regulatory framework for veterinary skincare products is a complex and evolving landscape that plays a crucial role in ensuring the safety and efficacy of products used in animal skin care regimens. In the United States, the Food and Drug Administration (FDA) and the Environmental Protection Agency (EPA) are the primary regulatory bodies overseeing these products.
The FDA regulates animal drugs and medical devices, including those used for skin care, under the Federal Food, Drug, and Cosmetic Act (FD&C Act). Products containing hypochlorous acid may fall under this jurisdiction if they are marketed with claims to treat, prevent, or mitigate disease. The FDA requires manufacturers to demonstrate the safety and efficacy of their products through rigorous testing and clinical trials before granting approval.
The EPA, on the other hand, regulates pesticides and antimicrobial products under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA). Hypochlorous acid solutions used as disinfectants or sanitizers in animal care settings may be subject to EPA registration requirements. This process involves submitting detailed information about the product's composition, efficacy, and safety profile.
In the European Union, the regulatory framework is governed by the Veterinary Medicinal Products Regulation (Regulation (EU) 2019/6). This regulation sets standards for the authorization, manufacture, and distribution of veterinary medicinal products, including those used in skin care. The European Medicines Agency (EMA) plays a central role in evaluating and monitoring these products.
Internationally, the World Organisation for Animal Health (OIE) provides guidelines and standards for veterinary products, which many countries use as a reference for their national regulations. These standards aim to harmonize regulatory approaches and facilitate international trade while maintaining high safety standards.
For hypochlorous acid specifically, its classification can vary depending on its intended use and marketing claims. When used as a topical antiseptic or wound care product, it may be regulated as a drug. However, if marketed as a general cleansing solution, it might be classified as a cosmetic or grooming product, subject to less stringent regulations.
Manufacturers must navigate these regulatory frameworks carefully, ensuring compliance with labeling requirements, good manufacturing practices (GMP), and post-market surveillance obligations. The regulatory landscape also influences product development strategies, as companies must balance innovation with regulatory feasibility.
As the field of animal skin care continues to advance, regulatory bodies are adapting their frameworks to address new technologies and formulations. This ongoing evolution requires stakeholders to stay informed and engaged with regulatory developments to ensure continued compliance and market access for their products.
The FDA regulates animal drugs and medical devices, including those used for skin care, under the Federal Food, Drug, and Cosmetic Act (FD&C Act). Products containing hypochlorous acid may fall under this jurisdiction if they are marketed with claims to treat, prevent, or mitigate disease. The FDA requires manufacturers to demonstrate the safety and efficacy of their products through rigorous testing and clinical trials before granting approval.
The EPA, on the other hand, regulates pesticides and antimicrobial products under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA). Hypochlorous acid solutions used as disinfectants or sanitizers in animal care settings may be subject to EPA registration requirements. This process involves submitting detailed information about the product's composition, efficacy, and safety profile.
In the European Union, the regulatory framework is governed by the Veterinary Medicinal Products Regulation (Regulation (EU) 2019/6). This regulation sets standards for the authorization, manufacture, and distribution of veterinary medicinal products, including those used in skin care. The European Medicines Agency (EMA) plays a central role in evaluating and monitoring these products.
Internationally, the World Organisation for Animal Health (OIE) provides guidelines and standards for veterinary products, which many countries use as a reference for their national regulations. These standards aim to harmonize regulatory approaches and facilitate international trade while maintaining high safety standards.
For hypochlorous acid specifically, its classification can vary depending on its intended use and marketing claims. When used as a topical antiseptic or wound care product, it may be regulated as a drug. However, if marketed as a general cleansing solution, it might be classified as a cosmetic or grooming product, subject to less stringent regulations.
Manufacturers must navigate these regulatory frameworks carefully, ensuring compliance with labeling requirements, good manufacturing practices (GMP), and post-market surveillance obligations. The regulatory landscape also influences product development strategies, as companies must balance innovation with regulatory feasibility.
As the field of animal skin care continues to advance, regulatory bodies are adapting their frameworks to address new technologies and formulations. This ongoing evolution requires stakeholders to stay informed and engaged with regulatory developments to ensure continued compliance and market access for their products.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!