Examining Bioresonance Benefits for Chronic Digestive Issues
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioresonance Background
Bioresonance therapy, a form of alternative medicine, has its roots in the early 20th century with the development of electromagnetic field theories in biology. The concept was first introduced by Dr. Franz Morell in the 1970s, who proposed that all cells and organs in the body emit electromagnetic frequencies. He theorized that these frequencies could be detected, analyzed, and manipulated to diagnose and treat various health conditions.
The fundamental principle of bioresonance is based on the idea that unhealthy cells or organs emit altered electromagnetic waves due to illness or environmental factors. Proponents of this therapy claim that by detecting and correcting these abnormal frequencies, it is possible to restore health and balance to the body. This approach aligns with the broader field of energy medicine, which posits that imbalances in the body's electromagnetic field can lead to various health issues.
Over the years, bioresonance technology has evolved from simple frequency detection devices to more sophisticated systems that claim to both diagnose and treat a wide range of conditions. These modern devices typically involve electrodes placed on the skin, which are said to both read the body's frequencies and transmit corrective frequencies back into the body. The therapy has gained popularity in some European countries and has been applied to various health concerns, including allergies, pain management, and, more recently, chronic digestive issues.
In the context of chronic digestive problems, bioresonance advocates suggest that the therapy can help identify food intolerances, reduce inflammation, and restore balance to the digestive system. They argue that by detecting and correcting abnormal frequencies associated with digestive organs and processes, bioresonance can potentially alleviate symptoms of conditions such as irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), and chronic indigestion.
However, it is important to note that the scientific community largely regards bioresonance as pseudoscience. The underlying theories of bioresonance have not been substantiated by rigorous scientific research, and there is a lack of robust clinical evidence supporting its efficacy. Many mainstream medical professionals and regulatory bodies, including the U.S. Food and Drug Administration (FDA), do not recognize bioresonance as a valid diagnostic or therapeutic tool.
Despite the skepticism from conventional medicine, interest in bioresonance and other complementary therapies continues to grow among patients seeking alternative approaches to managing chronic health conditions. This interest has led to an increase in research efforts aimed at exploring the potential benefits and mechanisms of bioresonance, particularly in the field of digestive health. As the demand for non-invasive and holistic treatment options rises, understanding the background and principles of bioresonance becomes increasingly relevant for both healthcare providers and patients exploring alternative therapies for chronic digestive issues.
The fundamental principle of bioresonance is based on the idea that unhealthy cells or organs emit altered electromagnetic waves due to illness or environmental factors. Proponents of this therapy claim that by detecting and correcting these abnormal frequencies, it is possible to restore health and balance to the body. This approach aligns with the broader field of energy medicine, which posits that imbalances in the body's electromagnetic field can lead to various health issues.
Over the years, bioresonance technology has evolved from simple frequency detection devices to more sophisticated systems that claim to both diagnose and treat a wide range of conditions. These modern devices typically involve electrodes placed on the skin, which are said to both read the body's frequencies and transmit corrective frequencies back into the body. The therapy has gained popularity in some European countries and has been applied to various health concerns, including allergies, pain management, and, more recently, chronic digestive issues.
In the context of chronic digestive problems, bioresonance advocates suggest that the therapy can help identify food intolerances, reduce inflammation, and restore balance to the digestive system. They argue that by detecting and correcting abnormal frequencies associated with digestive organs and processes, bioresonance can potentially alleviate symptoms of conditions such as irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), and chronic indigestion.
However, it is important to note that the scientific community largely regards bioresonance as pseudoscience. The underlying theories of bioresonance have not been substantiated by rigorous scientific research, and there is a lack of robust clinical evidence supporting its efficacy. Many mainstream medical professionals and regulatory bodies, including the U.S. Food and Drug Administration (FDA), do not recognize bioresonance as a valid diagnostic or therapeutic tool.
Despite the skepticism from conventional medicine, interest in bioresonance and other complementary therapies continues to grow among patients seeking alternative approaches to managing chronic health conditions. This interest has led to an increase in research efforts aimed at exploring the potential benefits and mechanisms of bioresonance, particularly in the field of digestive health. As the demand for non-invasive and holistic treatment options rises, understanding the background and principles of bioresonance becomes increasingly relevant for both healthcare providers and patients exploring alternative therapies for chronic digestive issues.
Digestive Health Market
The digestive health market has experienced significant growth in recent years, driven by increasing awareness of gut health's importance and rising prevalence of digestive disorders. This market encompasses a wide range of products and services, including dietary supplements, functional foods, probiotics, digestive enzymes, and medical devices aimed at improving gastrointestinal health.
Consumer demand for natural and holistic approaches to digestive health has been a key factor in market expansion. There is a growing preference for non-pharmaceutical solutions, with many individuals seeking alternatives to traditional medications for managing chronic digestive issues. This trend has led to a surge in the development and marketing of probiotic products, herbal supplements, and dietary interventions targeting gut health.
The global digestive health market size was valued at over $40 billion in 2020, with projections indicating continued growth at a compound annual growth rate (CAGR) of around 7% through 2027. North America currently holds the largest market share, followed by Europe and Asia-Pacific. However, emerging economies in Asia and Latin America are expected to witness the fastest growth rates due to increasing health consciousness and disposable incomes.
Key market segments include probiotics, prebiotics, digestive enzymes, and functional foods. The probiotics segment, in particular, has seen robust growth, driven by scientific advancements and increasing consumer acceptance. Functional foods and beverages fortified with digestive health ingredients are also gaining traction, appealing to health-conscious consumers seeking convenient ways to support their gut health.
The market is characterized by intense competition, with both established pharmaceutical companies and innovative startups vying for market share. Major players are investing heavily in research and development to introduce novel products and expand their product portfolios. Mergers, acquisitions, and strategic partnerships are common strategies employed to strengthen market positions and expand geographical reach.
Technological advancements, such as the development of targeted probiotics and personalized nutrition solutions, are shaping the future of the digestive health market. The integration of artificial intelligence and big data analytics in product development and marketing strategies is expected to drive further innovation in this space.
Regulatory environments vary across regions, influencing market dynamics. While some countries have established clear guidelines for digestive health products, others are still developing regulatory frameworks. This variability creates both challenges and opportunities for market players, necessitating adaptive strategies for global expansion.
Consumer demand for natural and holistic approaches to digestive health has been a key factor in market expansion. There is a growing preference for non-pharmaceutical solutions, with many individuals seeking alternatives to traditional medications for managing chronic digestive issues. This trend has led to a surge in the development and marketing of probiotic products, herbal supplements, and dietary interventions targeting gut health.
The global digestive health market size was valued at over $40 billion in 2020, with projections indicating continued growth at a compound annual growth rate (CAGR) of around 7% through 2027. North America currently holds the largest market share, followed by Europe and Asia-Pacific. However, emerging economies in Asia and Latin America are expected to witness the fastest growth rates due to increasing health consciousness and disposable incomes.
Key market segments include probiotics, prebiotics, digestive enzymes, and functional foods. The probiotics segment, in particular, has seen robust growth, driven by scientific advancements and increasing consumer acceptance. Functional foods and beverages fortified with digestive health ingredients are also gaining traction, appealing to health-conscious consumers seeking convenient ways to support their gut health.
The market is characterized by intense competition, with both established pharmaceutical companies and innovative startups vying for market share. Major players are investing heavily in research and development to introduce novel products and expand their product portfolios. Mergers, acquisitions, and strategic partnerships are common strategies employed to strengthen market positions and expand geographical reach.
Technological advancements, such as the development of targeted probiotics and personalized nutrition solutions, are shaping the future of the digestive health market. The integration of artificial intelligence and big data analytics in product development and marketing strategies is expected to drive further innovation in this space.
Regulatory environments vary across regions, influencing market dynamics. While some countries have established clear guidelines for digestive health products, others are still developing regulatory frameworks. This variability creates both challenges and opportunities for market players, necessitating adaptive strategies for global expansion.
Bioresonance Challenges
Despite the growing interest in bioresonance therapy for chronic digestive issues, several significant challenges hinder its widespread adoption and acceptance in mainstream medical practice. One of the primary obstacles is the lack of robust scientific evidence supporting its efficacy. While anecdotal reports and small-scale studies suggest potential benefits, large-scale, randomized controlled trials are scarce, making it difficult to draw definitive conclusions about the therapy's effectiveness.
The complexity of the human body's electromagnetic field and its interaction with external frequencies poses another challenge. The precise mechanisms by which bioresonance therapy influences digestive function remain poorly understood, leading to skepticism among medical professionals and researchers. This knowledge gap hampers the development of standardized protocols and treatment guidelines, resulting in inconsistent application of the therapy across practitioners.
Additionally, the variability in bioresonance devices and treatment protocols presents a significant hurdle. The lack of standardization in equipment and methodology makes it challenging to compare results across studies and replicate findings. This inconsistency also raises concerns about the reliability and reproducibility of treatment outcomes, further complicating the evaluation of bioresonance therapy's effectiveness for chronic digestive issues.
Regulatory challenges also play a crucial role in limiting the adoption of bioresonance therapy. In many countries, bioresonance devices are not approved or regulated as medical devices, leading to concerns about safety, quality control, and potential misuse. The absence of clear regulatory guidelines creates uncertainty for both practitioners and patients, potentially deterring investment in research and development in this field.
The integration of bioresonance therapy into conventional medical practice faces resistance from the medical community due to its perceived lack of scientific basis. Many healthcare professionals view bioresonance as an alternative or complementary therapy, rather than a mainstream treatment option. This perception creates barriers to collaboration between conventional and bioresonance practitioners, limiting opportunities for comprehensive patient care and interdisciplinary research.
Furthermore, the potential for placebo effects in bioresonance therapy complicates the assessment of its true therapeutic value. The subjective nature of many digestive symptoms and the holistic approach often employed in bioresonance treatment make it challenging to distinguish between specific therapeutic effects and general improvements due to increased attention to health and lifestyle factors.
Lastly, the cost and accessibility of bioresonance therapy pose challenges for widespread adoption. Many insurance providers do not cover bioresonance treatments, making them financially inaccessible to a significant portion of patients with chronic digestive issues. The specialized equipment and training required for bioresonance therapy also limit its availability, particularly in rural or underserved areas.
The complexity of the human body's electromagnetic field and its interaction with external frequencies poses another challenge. The precise mechanisms by which bioresonance therapy influences digestive function remain poorly understood, leading to skepticism among medical professionals and researchers. This knowledge gap hampers the development of standardized protocols and treatment guidelines, resulting in inconsistent application of the therapy across practitioners.
Additionally, the variability in bioresonance devices and treatment protocols presents a significant hurdle. The lack of standardization in equipment and methodology makes it challenging to compare results across studies and replicate findings. This inconsistency also raises concerns about the reliability and reproducibility of treatment outcomes, further complicating the evaluation of bioresonance therapy's effectiveness for chronic digestive issues.
Regulatory challenges also play a crucial role in limiting the adoption of bioresonance therapy. In many countries, bioresonance devices are not approved or regulated as medical devices, leading to concerns about safety, quality control, and potential misuse. The absence of clear regulatory guidelines creates uncertainty for both practitioners and patients, potentially deterring investment in research and development in this field.
The integration of bioresonance therapy into conventional medical practice faces resistance from the medical community due to its perceived lack of scientific basis. Many healthcare professionals view bioresonance as an alternative or complementary therapy, rather than a mainstream treatment option. This perception creates barriers to collaboration between conventional and bioresonance practitioners, limiting opportunities for comprehensive patient care and interdisciplinary research.
Furthermore, the potential for placebo effects in bioresonance therapy complicates the assessment of its true therapeutic value. The subjective nature of many digestive symptoms and the holistic approach often employed in bioresonance treatment make it challenging to distinguish between specific therapeutic effects and general improvements due to increased attention to health and lifestyle factors.
Lastly, the cost and accessibility of bioresonance therapy pose challenges for widespread adoption. Many insurance providers do not cover bioresonance treatments, making them financially inaccessible to a significant portion of patients with chronic digestive issues. The specialized equipment and training required for bioresonance therapy also limit its availability, particularly in rural or underserved areas.
Current Applications
01 Health assessment and diagnosis
Bioresonance technology can be used for health assessment and diagnosis by analyzing the body's electromagnetic frequencies. This non-invasive method may help identify imbalances or potential health issues, allowing for early intervention and personalized treatment plans.- Health assessment and diagnosis: Bioresonance technology can be used for health assessment and diagnosis by detecting electromagnetic frequencies emitted by the body. This non-invasive method may help identify imbalances or health issues, potentially leading to earlier detection and treatment of various conditions.
- Stress reduction and relaxation: Bioresonance therapy may help reduce stress and promote relaxation by balancing the body's energy fields. This can lead to improved overall well-being, better sleep quality, and enhanced mental clarity.
- Pain management and relief: Bioresonance techniques may be effective in managing various types of pain, including chronic pain conditions. By targeting specific frequencies associated with pain, this therapy aims to provide relief and improve quality of life for individuals suffering from pain-related issues.
- Detoxification and immune system support: Bioresonance therapy may aid in detoxification processes by identifying and addressing harmful substances in the body. Additionally, it may help support the immune system by promoting balance and optimal functioning of various bodily systems.
- Personalized treatment plans: Bioresonance technology can be used to create personalized treatment plans based on individual frequency patterns. This tailored approach may lead to more effective interventions and improved health outcomes for patients with various conditions.
02 Stress reduction and relaxation
Bioresonance therapy may help reduce stress and promote relaxation by balancing the body's energy fields. This can lead to improved overall well-being, better sleep quality, and enhanced mental clarity.Expand Specific Solutions03 Pain management and relief
Bioresonance techniques may be effective in managing various types of pain, including chronic pain conditions. By targeting specific frequencies associated with pain, this therapy aims to provide relief and improve quality of life for patients.Expand Specific Solutions04 Detoxification and immune system support
Bioresonance therapy may aid in detoxification processes and support the immune system. By identifying and addressing harmful frequencies in the body, this approach aims to enhance the body's natural ability to eliminate toxins and strengthen its defense mechanisms.Expand Specific Solutions05 Personalized treatment and wellness optimization
Bioresonance technology can be used to create personalized treatment plans and optimize overall wellness. By analyzing individual frequency patterns, practitioners can tailor interventions to address specific health concerns and promote optimal functioning of various body systems.Expand Specific Solutions
Key Industry Players
The bioresonance technology market for chronic digestive issues is in its early growth stage, with increasing interest but limited widespread adoption. The market size is expanding as more patients seek alternative therapies, though exact figures are not well-established. Technologically, bioresonance is still considered experimental by mainstream medicine. Companies like Alimetry Ltd. and Seed Health, Inc. are advancing digestive health diagnostics and therapies, while established players such as Olympus Corp. and Boston Scientific Scimed, Inc. focus on traditional medical devices for gastrointestinal issues. Research institutions like Cincinnati Children's Hospital Medical Center and the University of Porto are contributing to the scientific understanding of digestive health, potentially influencing future bioresonance applications.
Alimetry Ltd.
Technical Solution: Alimetry has developed a non-invasive wearable device called the Gastric Alimetry system for diagnosing digestive disorders. The system uses advanced algorithms to analyze electrical signals from the stomach, providing detailed insights into gastric function[1]. This technology could potentially be adapted to incorporate bioresonance principles for chronic digestive issues. The device captures high-resolution data on stomach contractions and emptying patterns, which could be correlated with bioresonance frequencies to identify potential imbalances or disruptions in the digestive system[2].
Strengths: Non-invasive approach, detailed gastric function analysis. Weaknesses: May require additional research to integrate bioresonance principles effectively.
Olympus Corp.
Technical Solution: Olympus has developed advanced endoscopic imaging technologies that could be adapted to study bioresonance effects on the digestive system. Their Narrow Band Imaging (NBI) technology enhances visualization of mucosal surfaces, potentially allowing for the detection of subtle changes in tissue resonance[3]. By combining this imaging capability with bioresonance sensors, Olympus could create a hybrid system for real-time analysis of digestive tract health. The company's expertise in minimally invasive medical devices positions them well to develop tools for assessing bioresonance benefits in chronic digestive issues[4].
Strengths: Established expertise in medical imaging and endoscopy. Weaknesses: May require significant R&D to integrate bioresonance technology with existing systems.
Core Bioresonance Tech
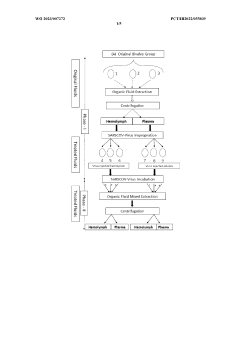
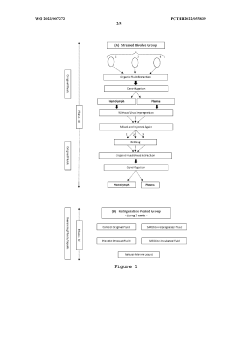
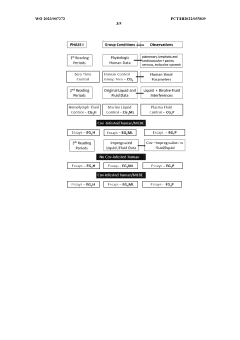
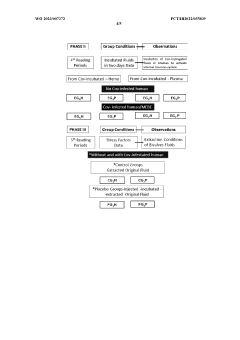
Method of obtaining electromagnetic frequencies from aquatic organisms bioactivated fluids for bioresonance therapy against a disease and/or pathogen
PatentWO2023007272A1
Innovation
- A method involving bioresonance therapy using electromagnetic frequencies obtained from aquatic non-human organisms, specifically bioactivated fluids from bivalves like Anodonta cygnea, to induce an immune response and treat diseases and pathogens in humans, utilizing a bioresonance device to transfer and record these frequencies for therapeutic applications.
Electromagnetic wave applicator
PatentWO2004047875A1
Innovation
- An electromagnetic wave irradiation device that selectively targets microorganisms by emitting frequencies matching their natural frequencies, causing resonant vibrations and destruction without affecting human tissues, using a thin tube with an electromagnetic wave generating means and an irradiation terminal, and an antenna array for precise frequency delivery.
Clinical Trial Landscape
The clinical trial landscape for bioresonance therapy in chronic digestive issues is still in its early stages, with limited large-scale, randomized controlled trials available. However, there is a growing body of smaller studies and pilot trials that are beginning to explore the potential benefits of this alternative therapy.
Several small-scale clinical trials have been conducted in Europe, particularly in Germany and Russia, where bioresonance therapy has gained more acceptance. These studies have primarily focused on conditions such as irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), and functional dyspepsia. While the results have been mixed, some trials have reported improvements in symptom severity and quality of life for patients with chronic digestive issues.
One notable study, conducted at a German university hospital, involved 60 patients with IBS. The randomized, double-blind, placebo-controlled trial found that patients receiving bioresonance therapy experienced a significant reduction in abdominal pain and bloating compared to the placebo group. However, the study's small sample size and short duration (8 weeks) limit its generalizability.
Another pilot study in Russia examined the effects of bioresonance therapy on 40 patients with ulcerative colitis. The results suggested potential benefits in reducing inflammation and improving clinical symptoms, but the lack of a control group and the small sample size warrant caution in interpreting these findings.
Despite these promising initial results, the overall quality of evidence remains low due to methodological limitations in many of the existing studies. Common issues include small sample sizes, lack of proper control groups, and potential bias in study design and reporting.
Several ongoing clinical trials are attempting to address these limitations and provide more robust evidence for the efficacy of bioresonance therapy in chronic digestive issues. A multicenter trial in the United Kingdom is currently recruiting participants for a larger-scale study on bioresonance therapy for IBS, with a planned sample size of 200 patients and a 12-week treatment period.
Additionally, a collaborative effort between research institutions in Germany and Switzerland is underway to investigate the potential of bioresonance therapy in managing symptoms of Crohn's disease. This study aims to include a larger sample size and employ more rigorous methodological standards to enhance the quality of evidence.
As the field progresses, there is a growing recognition of the need for standardized protocols and more comprehensive outcome measures in bioresonance clinical trials. Researchers are increasingly incorporating objective biomarkers, such as inflammatory markers and gut microbiome analysis, alongside subjective symptom assessments to provide a more holistic evaluation of treatment efficacy.
Several small-scale clinical trials have been conducted in Europe, particularly in Germany and Russia, where bioresonance therapy has gained more acceptance. These studies have primarily focused on conditions such as irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), and functional dyspepsia. While the results have been mixed, some trials have reported improvements in symptom severity and quality of life for patients with chronic digestive issues.
One notable study, conducted at a German university hospital, involved 60 patients with IBS. The randomized, double-blind, placebo-controlled trial found that patients receiving bioresonance therapy experienced a significant reduction in abdominal pain and bloating compared to the placebo group. However, the study's small sample size and short duration (8 weeks) limit its generalizability.
Another pilot study in Russia examined the effects of bioresonance therapy on 40 patients with ulcerative colitis. The results suggested potential benefits in reducing inflammation and improving clinical symptoms, but the lack of a control group and the small sample size warrant caution in interpreting these findings.
Despite these promising initial results, the overall quality of evidence remains low due to methodological limitations in many of the existing studies. Common issues include small sample sizes, lack of proper control groups, and potential bias in study design and reporting.
Several ongoing clinical trials are attempting to address these limitations and provide more robust evidence for the efficacy of bioresonance therapy in chronic digestive issues. A multicenter trial in the United Kingdom is currently recruiting participants for a larger-scale study on bioresonance therapy for IBS, with a planned sample size of 200 patients and a 12-week treatment period.
Additionally, a collaborative effort between research institutions in Germany and Switzerland is underway to investigate the potential of bioresonance therapy in managing symptoms of Crohn's disease. This study aims to include a larger sample size and employ more rigorous methodological standards to enhance the quality of evidence.
As the field progresses, there is a growing recognition of the need for standardized protocols and more comprehensive outcome measures in bioresonance clinical trials. Researchers are increasingly incorporating objective biomarkers, such as inflammatory markers and gut microbiome analysis, alongside subjective symptom assessments to provide a more holistic evaluation of treatment efficacy.
Regulatory Considerations
The regulatory landscape surrounding bioresonance therapy for chronic digestive issues is complex and varies significantly across different jurisdictions. In many countries, bioresonance devices are classified as medical devices, subject to stringent regulatory oversight. The United States Food and Drug Administration (FDA) has not approved bioresonance devices for diagnostic or therapeutic use, citing a lack of scientific evidence supporting their efficacy. Consequently, these devices are often marketed as general wellness products to avoid regulatory scrutiny.
In the European Union, the regulatory framework is more nuanced. Some bioresonance devices have obtained CE marking, allowing their sale as medical devices within the EU market. However, this certification primarily focuses on safety rather than efficacy. The European Medicines Agency (EMA) has not issued specific guidelines on bioresonance therapy, leaving individual member states to determine their stance on its use in medical practice.
Several countries, including Germany and Switzerland, have a more accepting approach to complementary and alternative medicine, including bioresonance. In these nations, bioresonance therapy may be offered by licensed practitioners, although it is often not covered by public health insurance schemes. This regulatory environment has led to a higher prevalence of bioresonance clinics and practitioners in these regions.
The lack of standardized regulations and clinical evidence poses challenges for manufacturers and practitioners of bioresonance therapy. To address these issues, some industry associations have established voluntary standards and best practices. These efforts aim to enhance the credibility of bioresonance therapy and potentially pave the way for more favorable regulatory treatment in the future.
As research into the potential benefits of bioresonance for chronic digestive issues continues, regulatory bodies may reassess their positions. Manufacturers and proponents of bioresonance therapy are increasingly focusing on conducting rigorous clinical trials to build a stronger evidence base. This approach could potentially lead to regulatory approvals for specific applications in digestive health management.
The global regulatory landscape for bioresonance therapy remains dynamic, with ongoing debates about its classification, efficacy, and appropriate use. As the field evolves, it is crucial for stakeholders to stay informed about regulatory changes and adapt their strategies accordingly. The future regulatory status of bioresonance therapy for chronic digestive issues will likely depend on the quality and quantity of scientific evidence supporting its use, as well as shifting attitudes towards complementary and alternative medicine in different healthcare systems worldwide.
In the European Union, the regulatory framework is more nuanced. Some bioresonance devices have obtained CE marking, allowing their sale as medical devices within the EU market. However, this certification primarily focuses on safety rather than efficacy. The European Medicines Agency (EMA) has not issued specific guidelines on bioresonance therapy, leaving individual member states to determine their stance on its use in medical practice.
Several countries, including Germany and Switzerland, have a more accepting approach to complementary and alternative medicine, including bioresonance. In these nations, bioresonance therapy may be offered by licensed practitioners, although it is often not covered by public health insurance schemes. This regulatory environment has led to a higher prevalence of bioresonance clinics and practitioners in these regions.
The lack of standardized regulations and clinical evidence poses challenges for manufacturers and practitioners of bioresonance therapy. To address these issues, some industry associations have established voluntary standards and best practices. These efforts aim to enhance the credibility of bioresonance therapy and potentially pave the way for more favorable regulatory treatment in the future.
As research into the potential benefits of bioresonance for chronic digestive issues continues, regulatory bodies may reassess their positions. Manufacturers and proponents of bioresonance therapy are increasingly focusing on conducting rigorous clinical trials to build a stronger evidence base. This approach could potentially lead to regulatory approvals for specific applications in digestive health management.
The global regulatory landscape for bioresonance therapy remains dynamic, with ongoing debates about its classification, efficacy, and appropriate use. As the field evolves, it is crucial for stakeholders to stay informed about regulatory changes and adapt their strategies accordingly. The future regulatory status of bioresonance therapy for chronic digestive issues will likely depend on the quality and quantity of scientific evidence supporting its use, as well as shifting attitudes towards complementary and alternative medicine in different healthcare systems worldwide.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!