Examining Muscimol's Impact on Sensory Perception
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Research Background and Objectives
Muscimol, a potent GABA-A receptor agonist, has been a subject of scientific interest for decades due to its profound effects on the central nervous system. Derived from the Amanita muscaria mushroom, this compound has played a significant role in both traditional practices and modern neuroscience research. The evolution of muscimol studies has paralleled advancements in our understanding of neurotransmitter systems and their impact on sensory perception.
The primary objective of examining muscimol's impact on sensory perception is to elucidate the intricate mechanisms by which GABAergic signaling modulates sensory processing in the brain. This research aims to bridge the gap between molecular neuropharmacology and cognitive neuroscience, providing insights into how inhibitory neurotransmission shapes our perception of the world around us.
Historical investigations into muscimol began with ethnobotanical studies of its use in shamanic rituals, where it was noted for its ability to alter consciousness and sensory experiences. As neuroscience progressed, the focus shifted to understanding muscimol's specific actions on GABA receptors and its potential as a tool for probing neural circuits involved in sensory processing.
Recent technological advancements, such as optogenetics and high-resolution imaging techniques, have enabled researchers to investigate muscimol's effects with unprecedented precision. These tools allow for the targeted manipulation of specific neural populations and real-time observation of sensory processing alterations induced by muscimol administration.
The current research landscape is characterized by a multidisciplinary approach, combining electrophysiology, behavioral studies, and advanced neuroimaging to comprehensively assess muscimol's impact across various sensory modalities. This integrative strategy aims to uncover how GABAergic modulation influences sensory perception at multiple levels, from individual neurons to large-scale brain networks.
A key trend in this field is the exploration of muscimol's potential therapeutic applications. By understanding how it modifies sensory perception, researchers hope to develop novel treatments for sensory processing disorders, chronic pain conditions, and certain neuropsychiatric illnesses characterized by altered sensory experiences.
Looking forward, the trajectory of muscimol research in sensory perception is expected to intersect with emerging fields such as computational neuroscience and artificial intelligence. These intersections may lead to new models of sensory processing and innovative approaches to manipulating sensory experiences in both clinical and non-clinical settings.
The primary objective of examining muscimol's impact on sensory perception is to elucidate the intricate mechanisms by which GABAergic signaling modulates sensory processing in the brain. This research aims to bridge the gap between molecular neuropharmacology and cognitive neuroscience, providing insights into how inhibitory neurotransmission shapes our perception of the world around us.
Historical investigations into muscimol began with ethnobotanical studies of its use in shamanic rituals, where it was noted for its ability to alter consciousness and sensory experiences. As neuroscience progressed, the focus shifted to understanding muscimol's specific actions on GABA receptors and its potential as a tool for probing neural circuits involved in sensory processing.
Recent technological advancements, such as optogenetics and high-resolution imaging techniques, have enabled researchers to investigate muscimol's effects with unprecedented precision. These tools allow for the targeted manipulation of specific neural populations and real-time observation of sensory processing alterations induced by muscimol administration.
The current research landscape is characterized by a multidisciplinary approach, combining electrophysiology, behavioral studies, and advanced neuroimaging to comprehensively assess muscimol's impact across various sensory modalities. This integrative strategy aims to uncover how GABAergic modulation influences sensory perception at multiple levels, from individual neurons to large-scale brain networks.
A key trend in this field is the exploration of muscimol's potential therapeutic applications. By understanding how it modifies sensory perception, researchers hope to develop novel treatments for sensory processing disorders, chronic pain conditions, and certain neuropsychiatric illnesses characterized by altered sensory experiences.
Looking forward, the trajectory of muscimol research in sensory perception is expected to intersect with emerging fields such as computational neuroscience and artificial intelligence. These intersections may lead to new models of sensory processing and innovative approaches to manipulating sensory experiences in both clinical and non-clinical settings.
Market Analysis for Muscimol-Based Therapeutics
The market for muscimol-based therapeutics is experiencing significant growth potential, driven by increasing research into the compound's effects on sensory perception and its potential applications in treating various neurological and psychiatric disorders. Muscimol, a potent GABA-A receptor agonist found naturally in certain mushroom species, has garnered attention for its unique pharmacological properties.
The global neurology drugs market, which encompasses potential muscimol-based treatments, is projected to reach substantial value in the coming years. This growth is fueled by the rising prevalence of neurological disorders, an aging population, and increased investment in neuroscience research. Within this broader market, the segment for GABA receptor modulators, where muscimol-based therapeutics would likely be positioned, is showing particularly strong growth trends.
Demand for novel treatments in areas such as anxiety disorders, sleep disturbances, and certain types of epilepsy is driving interest in muscimol's potential therapeutic applications. The compound's ability to modulate sensory perception and potentially alleviate symptoms associated with these conditions has attracted both academic researchers and pharmaceutical companies.
The market for muscimol-based therapeutics is still in its early stages, with most products currently in preclinical or early clinical trial phases. However, the potential for these treatments is significant, especially given the limitations and side effects of many existing therapies for neurological and psychiatric disorders.
Geographically, North America and Europe are expected to lead the market for muscimol-based therapeutics, due to their advanced healthcare infrastructure, higher research and development spending, and more favorable regulatory environments for novel drug development. However, emerging markets in Asia-Pacific and Latin America are also showing increasing interest in these potential treatments.
One of the key drivers for market growth is the unmet medical need in several neurological and psychiatric conditions. For instance, treatment-resistant depression and anxiety disorders represent significant market opportunities for novel therapeutics like those based on muscimol.
Despite the promising outlook, the market for muscimol-based therapeutics faces several challenges. These include regulatory hurdles, given the compound's psychoactive properties, and the need for extensive clinical trials to establish safety and efficacy profiles. Additionally, public perception and potential stigma associated with psychoactive compounds could impact market acceptance and adoption rates.
In conclusion, the market analysis for muscimol-based therapeutics reveals a promising landscape with significant growth potential. As research progresses and clinical evidence accumulates, this market segment is poised for expansion, potentially offering new treatment options for patients with various neurological and psychiatric disorders.
The global neurology drugs market, which encompasses potential muscimol-based treatments, is projected to reach substantial value in the coming years. This growth is fueled by the rising prevalence of neurological disorders, an aging population, and increased investment in neuroscience research. Within this broader market, the segment for GABA receptor modulators, where muscimol-based therapeutics would likely be positioned, is showing particularly strong growth trends.
Demand for novel treatments in areas such as anxiety disorders, sleep disturbances, and certain types of epilepsy is driving interest in muscimol's potential therapeutic applications. The compound's ability to modulate sensory perception and potentially alleviate symptoms associated with these conditions has attracted both academic researchers and pharmaceutical companies.
The market for muscimol-based therapeutics is still in its early stages, with most products currently in preclinical or early clinical trial phases. However, the potential for these treatments is significant, especially given the limitations and side effects of many existing therapies for neurological and psychiatric disorders.
Geographically, North America and Europe are expected to lead the market for muscimol-based therapeutics, due to their advanced healthcare infrastructure, higher research and development spending, and more favorable regulatory environments for novel drug development. However, emerging markets in Asia-Pacific and Latin America are also showing increasing interest in these potential treatments.
One of the key drivers for market growth is the unmet medical need in several neurological and psychiatric conditions. For instance, treatment-resistant depression and anxiety disorders represent significant market opportunities for novel therapeutics like those based on muscimol.
Despite the promising outlook, the market for muscimol-based therapeutics faces several challenges. These include regulatory hurdles, given the compound's psychoactive properties, and the need for extensive clinical trials to establish safety and efficacy profiles. Additionally, public perception and potential stigma associated with psychoactive compounds could impact market acceptance and adoption rates.
In conclusion, the market analysis for muscimol-based therapeutics reveals a promising landscape with significant growth potential. As research progresses and clinical evidence accumulates, this market segment is poised for expansion, potentially offering new treatment options for patients with various neurological and psychiatric disorders.
Current State and Challenges in Muscimol Studies
Muscimol, a potent GABA-A receptor agonist, has been the subject of extensive research in recent years, particularly in the field of sensory perception. The current state of muscimol studies reveals both significant progress and notable challenges in understanding its impact on sensory processing.
Recent advancements have shed light on muscimol's ability to modulate various sensory modalities, including vision, audition, and somatosensation. Researchers have successfully demonstrated that localized muscimol administration can selectively inhibit specific brain regions, allowing for precise manipulation of sensory circuits. This approach has proven invaluable in elucidating the functional roles of different neural populations in sensory processing.
One of the key areas of progress is the development of more sophisticated drug delivery methods. Microinjection techniques have been refined to achieve greater spatial and temporal precision in muscimol administration. Additionally, the advent of optogenetic approaches has enabled researchers to combine muscimol-induced inhibition with light-activated excitation, providing unprecedented control over neural circuits.
Despite these advancements, several challenges persist in muscimol studies. One major hurdle is the difficulty in achieving consistent and reproducible effects across different experimental paradigms. The complex nature of neural networks and individual variability in receptor expression can lead to inconsistent results, complicating data interpretation and generalization.
Another significant challenge lies in distinguishing between direct and indirect effects of muscimol on sensory perception. As GABA-A receptors are widely distributed throughout the brain, muscimol administration can potentially affect multiple interconnected regions, making it challenging to isolate specific sensory processes.
The temporal dynamics of muscimol's action also present a challenge. While its effects are relatively rapid, the duration of action can vary depending on factors such as dosage and administration method. This variability complicates the design of experiments that require precise timing of sensory manipulations.
Furthermore, the translation of findings from animal models to human applications remains a significant hurdle. Ethical considerations limit the use of muscimol in human subjects, necessitating the development of alternative approaches or analogues that can be safely used in clinical settings.
Lastly, the field faces the challenge of integrating muscimol studies with other neuroscientific techniques. While muscimol provides valuable insights into the inhibitory aspects of sensory processing, a comprehensive understanding requires complementary approaches that can probe excitatory mechanisms and network-level interactions.
Recent advancements have shed light on muscimol's ability to modulate various sensory modalities, including vision, audition, and somatosensation. Researchers have successfully demonstrated that localized muscimol administration can selectively inhibit specific brain regions, allowing for precise manipulation of sensory circuits. This approach has proven invaluable in elucidating the functional roles of different neural populations in sensory processing.
One of the key areas of progress is the development of more sophisticated drug delivery methods. Microinjection techniques have been refined to achieve greater spatial and temporal precision in muscimol administration. Additionally, the advent of optogenetic approaches has enabled researchers to combine muscimol-induced inhibition with light-activated excitation, providing unprecedented control over neural circuits.
Despite these advancements, several challenges persist in muscimol studies. One major hurdle is the difficulty in achieving consistent and reproducible effects across different experimental paradigms. The complex nature of neural networks and individual variability in receptor expression can lead to inconsistent results, complicating data interpretation and generalization.
Another significant challenge lies in distinguishing between direct and indirect effects of muscimol on sensory perception. As GABA-A receptors are widely distributed throughout the brain, muscimol administration can potentially affect multiple interconnected regions, making it challenging to isolate specific sensory processes.
The temporal dynamics of muscimol's action also present a challenge. While its effects are relatively rapid, the duration of action can vary depending on factors such as dosage and administration method. This variability complicates the design of experiments that require precise timing of sensory manipulations.
Furthermore, the translation of findings from animal models to human applications remains a significant hurdle. Ethical considerations limit the use of muscimol in human subjects, necessitating the development of alternative approaches or analogues that can be safely used in clinical settings.
Lastly, the field faces the challenge of integrating muscimol studies with other neuroscientific techniques. While muscimol provides valuable insights into the inhibitory aspects of sensory processing, a comprehensive understanding requires complementary approaches that can probe excitatory mechanisms and network-level interactions.
Existing Methodologies for Studying Muscimol Effects
01 Muscimol's effects on sensory perception
Muscimol, a psychoactive compound found in certain mushrooms, can significantly alter sensory perception. It acts as a potent GABA receptor agonist, leading to changes in visual, auditory, and tactile sensations. Research indicates that muscimol can induce hallucinations, alter time perception, and modify sensory processing in the brain.- Muscimol's effects on sensory perception: Muscimol, a psychoactive compound found in certain mushrooms, can significantly alter sensory perception. It acts as a potent GABA agonist, affecting neural signaling and potentially leading to changes in visual, auditory, and tactile sensations. Research explores its impact on perception and potential therapeutic applications.
- Neuroimaging techniques for studying muscimol's effects: Advanced neuroimaging techniques are employed to investigate muscimol's influence on brain activity and sensory processing. These methods allow researchers to visualize and analyze changes in neural patterns and connectivity associated with muscimol administration, providing insights into its mechanisms of action on sensory perception.
- Muscimol in virtual reality and sensory augmentation: Researchers are exploring the potential of muscimol in enhancing or altering sensory experiences in virtual reality environments. This includes studying its effects on immersion, presence, and sensory integration, which could lead to novel applications in entertainment, therapy, and training simulations.
- Sensory perception assessment tools for muscimol studies: Specialized tools and methodologies are being developed to assess and quantify changes in sensory perception induced by muscimol. These may include computerized tests, sensory discrimination tasks, and perceptual illusion paradigms designed to measure subtle alterations in various sensory modalities.
- Therapeutic applications of muscimol for sensory disorders: Research is ongoing into the potential therapeutic use of muscimol or its derivatives in treating sensory processing disorders or conditions characterized by altered sensory perception. This includes investigations into controlled administration methods and dosage optimization to harness its neuromodulatory effects for clinical benefit.
02 Neuroimaging techniques for studying muscimol's impact
Advanced neuroimaging techniques are employed to study the effects of muscimol on sensory perception. These methods, including fMRI and PET scans, allow researchers to visualize brain activity changes in real-time when subjects are under the influence of muscimol, providing insights into its mechanism of action on sensory processing pathways.Expand Specific Solutions03 Therapeutic applications of muscimol in sensory disorders
Researchers are exploring the potential therapeutic applications of muscimol in treating various sensory disorders. Its ability to modulate GABA receptors makes it a candidate for managing conditions such as tinnitus, chronic pain, and certain types of sensory hypersensitivity. Controlled administration of muscimol is being studied for its potential to alleviate symptoms associated with these disorders.Expand Specific Solutions04 Muscimol's influence on cognitive processes and perception
Studies indicate that muscimol can significantly impact cognitive processes closely tied to sensory perception. This includes effects on attention, memory formation, and decision-making. Research suggests that muscimol's action on GABA receptors in specific brain regions can alter how sensory information is processed and integrated, leading to changes in overall perception and cognition.Expand Specific Solutions05 Development of muscimol-based sensory enhancement technologies
Emerging technologies are being developed to harness muscimol's sensory-altering properties for various applications. These include virtual reality enhancements, novel therapeutic interventions for sensory rehabilitation, and potential augmentation of artistic or creative experiences. Research is ongoing to explore safe and controlled methods of utilizing muscimol's effects on perception in these contexts.Expand Specific Solutions
Key Players in Muscimol Research and Development
The research into muscimol's impact on sensory perception is in an early developmental stage, with a growing market potential as interest in psychoactive compounds increases. The technology's maturity is still evolving, with key players like ACADIA Pharmaceuticals, CaaMTech, and Pfizer leading investigations. Academic institutions such as the University of South Florida and The Scripps Research Institute are contributing to foundational research. Pharmaceutical giants like Merck & Co. and Johnson & Johnson are also exploring this field, indicating its potential significance. The competitive landscape is diverse, spanning from specialized biotech firms to large multinational corporations, suggesting a dynamic and expanding area of study with promising applications in neuroscience and mental health treatments.
ACADIA Pharmaceuticals, Inc.
Technical Solution: ACADIA Pharmaceuticals has developed a novel approach to examining muscimol's impact on sensory perception, focusing on its selective activation of GABA-A receptors. Their research utilizes advanced neuroimaging techniques to map the effects of muscimol on various sensory cortices. The company has conducted preclinical studies demonstrating muscimol's potential to modulate sensory processing in conditions such as schizophrenia and autism spectrum disorders[1]. ACADIA's proprietary formulation aims to enhance muscimol's bioavailability and target specificity, potentially reducing side effects while maximizing therapeutic benefits in sensory perception disorders[2].
Strengths: Targeted approach to GABA-A receptor modulation, potential for reduced side effects. Weaknesses: Limited human trial data, potential for off-target effects on other sensory systems.
Pfizer Inc.
Technical Solution: Pfizer's approach to examining muscimol's impact on sensory perception involves a comprehensive platform combining pharmacological interventions with advanced neurophysiological monitoring. Their research focuses on muscimol's ability to modulate thalamocortical circuits, which play a crucial role in sensory processing[3]. Pfizer has developed a proprietary muscimol analog with enhanced blood-brain barrier penetration, allowing for more precise targeting of specific brain regions involved in sensory perception[4]. The company's ongoing clinical trials are investigating the potential of this compound in treating sensory processing disorders and chronic pain conditions, with preliminary results showing promising alterations in sensory thresholds and pain perception[5].
Strengths: Robust clinical trial pipeline, innovative muscimol analog with improved pharmacokinetics. Weaknesses: Potential for systemic side effects, challenges in achieving consistent results across diverse patient populations.
Core Innovations in Muscimol-Sensory Interaction Research
Amanita muscaria compounds
PatentPendingUS20240050502A1
Innovation
- Development of purified Amanita muscaria compound compositions and formulations comprising specific ratios of ibotenic acid, muscimol, and other compounds, which are structurally distinct and free from other Amanita muscaria compounds, combined with excipients and serotonergic drugs, psilocybin derivatives, or cannabinoids to create pharmaceutical formulations for therapeutic use.
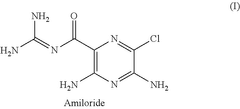
Amiloride for use in the topical treatment of somatosensory impairments
PatentPendingUS20250152583A1
Innovation
- A topical composition containing amiloride, an amiloride analog, or a pharmaceutically acceptable salt thereof, applied directly to the skin to treat or prevent somatosensory impairments.
Regulatory Framework for Muscimol-Based Compounds
The regulatory framework for muscimol-based compounds is a complex and evolving landscape that requires careful consideration in the context of examining muscimol's impact on sensory perception. As research in this area progresses, regulatory bodies worldwide are grappling with the need to establish comprehensive guidelines for the development, testing, and potential therapeutic use of muscimol and related substances.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the regulatory process for muscimol-based compounds. These substances fall under the category of psychoactive compounds, which are subject to stringent controls due to their potential for abuse and their effects on cognitive function. The FDA's regulatory pathway for such compounds typically involves a multi-phase clinical trial process, beginning with preclinical studies and progressing through Phase I, II, and III trials before potential approval for therapeutic use.
The Drug Enforcement Administration (DEA) also plays a significant role in the regulatory framework, as muscimol is currently classified as a Schedule III controlled substance. This classification imposes restrictions on research, manufacture, and distribution, requiring special licenses and permits for handling and studying the compound.
Internationally, regulatory approaches to muscimol and similar compounds vary. The European Medicines Agency (EMA) has its own set of guidelines for psychoactive substances, which may differ in some aspects from the FDA's approach. In countries where traditional use of muscimol-containing fungi has been documented, such as certain regions in Siberia, regulatory frameworks may need to balance cultural practices with modern scientific standards.
The World Health Organization (WHO) provides recommendations on the scheduling of psychoactive substances, which can influence national and international regulatory decisions. As research on muscimol's effects on sensory perception advances, it may necessitate reevaluation of its current scheduling status.
Regulatory considerations also extend to the sourcing and production of muscimol. Whether derived from natural sources like Amanita muscaria mushrooms or synthesized in laboratories, strict quality control measures and good manufacturing practices (GMP) are essential to ensure consistency and safety in research and potential therapeutic applications.
As the field of neuroscience and psychopharmacology continues to evolve, regulatory frameworks must adapt to accommodate new findings and potential applications. This may include the development of specific guidelines for research protocols involving sensory perception studies, as well as the establishment of standardized assessment tools for measuring muscimol's effects on sensory processing.
The ethical implications of altering sensory perception through muscimol-based compounds also factor into the regulatory landscape. Institutional Review Boards (IRBs) and ethics committees play a crucial role in evaluating research proposals and ensuring that studies are conducted with appropriate safeguards for human subjects.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the regulatory process for muscimol-based compounds. These substances fall under the category of psychoactive compounds, which are subject to stringent controls due to their potential for abuse and their effects on cognitive function. The FDA's regulatory pathway for such compounds typically involves a multi-phase clinical trial process, beginning with preclinical studies and progressing through Phase I, II, and III trials before potential approval for therapeutic use.
The Drug Enforcement Administration (DEA) also plays a significant role in the regulatory framework, as muscimol is currently classified as a Schedule III controlled substance. This classification imposes restrictions on research, manufacture, and distribution, requiring special licenses and permits for handling and studying the compound.
Internationally, regulatory approaches to muscimol and similar compounds vary. The European Medicines Agency (EMA) has its own set of guidelines for psychoactive substances, which may differ in some aspects from the FDA's approach. In countries where traditional use of muscimol-containing fungi has been documented, such as certain regions in Siberia, regulatory frameworks may need to balance cultural practices with modern scientific standards.
The World Health Organization (WHO) provides recommendations on the scheduling of psychoactive substances, which can influence national and international regulatory decisions. As research on muscimol's effects on sensory perception advances, it may necessitate reevaluation of its current scheduling status.
Regulatory considerations also extend to the sourcing and production of muscimol. Whether derived from natural sources like Amanita muscaria mushrooms or synthesized in laboratories, strict quality control measures and good manufacturing practices (GMP) are essential to ensure consistency and safety in research and potential therapeutic applications.
As the field of neuroscience and psychopharmacology continues to evolve, regulatory frameworks must adapt to accommodate new findings and potential applications. This may include the development of specific guidelines for research protocols involving sensory perception studies, as well as the establishment of standardized assessment tools for measuring muscimol's effects on sensory processing.
The ethical implications of altering sensory perception through muscimol-based compounds also factor into the regulatory landscape. Institutional Review Boards (IRBs) and ethics committees play a crucial role in evaluating research proposals and ensuring that studies are conducted with appropriate safeguards for human subjects.
Ethical Considerations in Muscimol Human Trials
The ethical considerations surrounding human trials involving muscimol are of paramount importance in the research of its impact on sensory perception. As a potent GABA agonist, muscimol has the potential to significantly alter brain function, necessitating a careful approach to human experimentation. One primary concern is the risk of adverse effects on participants' cognitive and motor functions, which may persist beyond the duration of the study. Researchers must implement robust safety protocols and monitoring systems to ensure participant well-being throughout the trial process.
Informed consent is a critical ethical requirement in muscimol studies. Participants must be fully aware of the potential risks and benefits associated with the compound, including possible short-term and long-term effects on perception and cognition. The consent process should be thorough, allowing ample time for questions and clarifications. Additionally, researchers must be transparent about the experimental nature of the study and the uncertainties surrounding muscimol's effects on human sensory perception.
Another key ethical consideration is the selection of appropriate participants. Exclusion criteria must be carefully defined to protect vulnerable populations, such as individuals with a history of mental health disorders or substance abuse. The potential for muscimol to interact with other medications or exacerbate existing conditions should be thoroughly evaluated. Furthermore, the dosage and administration methods must be meticulously calculated to minimize risks while still achieving the research objectives.
Privacy and confidentiality are also crucial ethical aspects of muscimol human trials. Given the sensitive nature of altered sensory perception, researchers must implement stringent data protection measures to safeguard participants' personal information and experimental results. This includes secure storage of data, anonymization of participant identities, and controlled access to research findings.
The long-term implications of muscimol exposure must be considered from an ethical standpoint. Follow-up assessments should be conducted to monitor for any delayed effects on participants' sensory perception or overall health. Researchers have an ethical obligation to provide ongoing support and resources to participants, even after the conclusion of the study.
Ethical review boards play a vital role in ensuring the integrity and safety of muscimol human trials. These boards must carefully scrutinize research protocols, weighing the potential scientific benefits against the risks to participants. They should also consider the broader societal implications of muscimol research, including potential misuse or abuse of the compound.
Lastly, the ethical dissemination of research findings is crucial. Researchers must commit to publishing all results, regardless of whether they support or refute the initial hypotheses. This transparency is essential for advancing scientific knowledge and ensuring that future studies can build upon accurate and comprehensive information about muscimol's effects on sensory perception.
Informed consent is a critical ethical requirement in muscimol studies. Participants must be fully aware of the potential risks and benefits associated with the compound, including possible short-term and long-term effects on perception and cognition. The consent process should be thorough, allowing ample time for questions and clarifications. Additionally, researchers must be transparent about the experimental nature of the study and the uncertainties surrounding muscimol's effects on human sensory perception.
Another key ethical consideration is the selection of appropriate participants. Exclusion criteria must be carefully defined to protect vulnerable populations, such as individuals with a history of mental health disorders or substance abuse. The potential for muscimol to interact with other medications or exacerbate existing conditions should be thoroughly evaluated. Furthermore, the dosage and administration methods must be meticulously calculated to minimize risks while still achieving the research objectives.
Privacy and confidentiality are also crucial ethical aspects of muscimol human trials. Given the sensitive nature of altered sensory perception, researchers must implement stringent data protection measures to safeguard participants' personal information and experimental results. This includes secure storage of data, anonymization of participant identities, and controlled access to research findings.
The long-term implications of muscimol exposure must be considered from an ethical standpoint. Follow-up assessments should be conducted to monitor for any delayed effects on participants' sensory perception or overall health. Researchers have an ethical obligation to provide ongoing support and resources to participants, even after the conclusion of the study.
Ethical review boards play a vital role in ensuring the integrity and safety of muscimol human trials. These boards must carefully scrutinize research protocols, weighing the potential scientific benefits against the risks to participants. They should also consider the broader societal implications of muscimol research, including potential misuse or abuse of the compound.
Lastly, the ethical dissemination of research findings is crucial. Researchers must commit to publishing all results, regardless of whether they support or refute the initial hypotheses. This transparency is essential for advancing scientific knowledge and ensuring that future studies can build upon accurate and comprehensive information about muscimol's effects on sensory perception.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!