Muscimol's Utility in Trauma-Induced Neuroinflammation
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Background
Muscimol, a potent GABA-A receptor agonist, has garnered significant attention in the field of neuroscience for its potential therapeutic applications. Derived from the Amanita muscaria mushroom, this compound has been known to indigenous cultures for centuries due to its psychoactive properties. In recent years, scientific interest in muscimol has shifted towards its potential neuroprotective and anti-inflammatory effects, particularly in the context of trauma-induced neuroinflammation.
The exploration of muscimol's utility in addressing neuroinflammation following traumatic brain injury (TBI) or other forms of neural trauma represents a promising avenue for research. Neuroinflammation is a complex cascade of cellular and molecular events that occurs in response to injury or infection in the central nervous system. While initially protective, prolonged or excessive neuroinflammation can lead to secondary damage and impaired recovery.
Muscimol's mechanism of action primarily involves its high affinity for GABA-A receptors, the main inhibitory neurotransmitter receptors in the brain. By enhancing GABAergic signaling, muscimol can potentially modulate the inflammatory response and provide neuroprotection. This is particularly relevant in the context of trauma-induced neuroinflammation, where the balance between excitatory and inhibitory neurotransmission is often disrupted.
Historical research on muscimol has primarily focused on its psychoactive effects and potential as a tool for studying GABA-mediated neurotransmission. However, the compound's ability to cross the blood-brain barrier and its potent GABA-A receptor agonism have led researchers to investigate its therapeutic potential in various neurological conditions, including epilepsy, anxiety disorders, and now, trauma-induced neuroinflammation.
The current interest in muscimol for treating neuroinflammation stems from a growing body of evidence suggesting that GABAergic signaling plays a crucial role in regulating neuroinflammatory processes. Preclinical studies have demonstrated that modulation of GABA-A receptors can influence microglial activation, cytokine production, and neuronal survival in models of neuroinflammation. These findings have paved the way for investigating muscimol's potential to mitigate the harmful effects of trauma-induced neuroinflammation and promote neural recovery.
As research in this area progresses, scientists are exploring various aspects of muscimol's pharmacology, including its optimal dosing, delivery methods, and potential synergistic effects with other neuroprotective agents. The goal is to harness muscimol's GABA-A agonist properties to develop targeted therapies that can effectively modulate the neuroinflammatory response following trauma, potentially improving outcomes for patients with TBI and other neurological injuries.
The exploration of muscimol's utility in addressing neuroinflammation following traumatic brain injury (TBI) or other forms of neural trauma represents a promising avenue for research. Neuroinflammation is a complex cascade of cellular and molecular events that occurs in response to injury or infection in the central nervous system. While initially protective, prolonged or excessive neuroinflammation can lead to secondary damage and impaired recovery.
Muscimol's mechanism of action primarily involves its high affinity for GABA-A receptors, the main inhibitory neurotransmitter receptors in the brain. By enhancing GABAergic signaling, muscimol can potentially modulate the inflammatory response and provide neuroprotection. This is particularly relevant in the context of trauma-induced neuroinflammation, where the balance between excitatory and inhibitory neurotransmission is often disrupted.
Historical research on muscimol has primarily focused on its psychoactive effects and potential as a tool for studying GABA-mediated neurotransmission. However, the compound's ability to cross the blood-brain barrier and its potent GABA-A receptor agonism have led researchers to investigate its therapeutic potential in various neurological conditions, including epilepsy, anxiety disorders, and now, trauma-induced neuroinflammation.
The current interest in muscimol for treating neuroinflammation stems from a growing body of evidence suggesting that GABAergic signaling plays a crucial role in regulating neuroinflammatory processes. Preclinical studies have demonstrated that modulation of GABA-A receptors can influence microglial activation, cytokine production, and neuronal survival in models of neuroinflammation. These findings have paved the way for investigating muscimol's potential to mitigate the harmful effects of trauma-induced neuroinflammation and promote neural recovery.
As research in this area progresses, scientists are exploring various aspects of muscimol's pharmacology, including its optimal dosing, delivery methods, and potential synergistic effects with other neuroprotective agents. The goal is to harness muscimol's GABA-A agonist properties to develop targeted therapies that can effectively modulate the neuroinflammatory response following trauma, potentially improving outcomes for patients with TBI and other neurological injuries.
Neuroinflammation Market
The neuroinflammation market has been experiencing significant growth in recent years, driven by the increasing prevalence of neurological disorders and a growing aging population. This market encompasses a wide range of therapeutic approaches aimed at addressing inflammation in the central nervous system, which is implicated in various conditions such as Alzheimer's disease, Parkinson's disease, multiple sclerosis, and traumatic brain injury.
The global neuroinflammation market is characterized by a robust pipeline of potential treatments, with numerous pharmaceutical and biotechnology companies investing heavily in research and development. Key players in this space include Biogen, Roche, Novartis, and Teva Pharmaceutical Industries, among others. These companies are exploring diverse therapeutic strategies, including small molecule inhibitors, monoclonal antibodies, and novel drug delivery systems targeting specific inflammatory pathways in the brain.
One of the primary drivers of market growth is the increasing understanding of the role of neuroinflammation in various neurological disorders. This has led to a surge in research activities and clinical trials focused on developing targeted therapies. The market is also benefiting from advancements in imaging technologies and biomarkers, which are enhancing the ability to diagnose and monitor neuroinflammatory conditions.
The neuroinflammation market is segmented based on drug class, indication, and geography. Anti-inflammatory drugs, including both steroidal and non-steroidal agents, currently dominate the market. However, there is growing interest in novel approaches such as immunomodulators and neuroprotective agents. In terms of indications, Alzheimer's disease and multiple sclerosis represent significant market segments due to their high prevalence and unmet medical needs.
Geographically, North America leads the neuroinflammation market, followed by Europe and Asia-Pacific. The dominance of North America can be attributed to its advanced healthcare infrastructure, high healthcare expenditure, and presence of major pharmaceutical companies. However, emerging economies in Asia-Pacific are expected to witness rapid growth in the coming years due to improving healthcare access and increasing awareness of neurological disorders.
Despite the promising outlook, the neuroinflammation market faces several challenges. These include the complexity of the blood-brain barrier, which poses difficulties in drug delivery, and the heterogeneity of neuroinflammatory conditions, which complicates the development of broadly effective therapies. Additionally, the high cost of drug development and stringent regulatory requirements present significant hurdles for market entrants.
Looking ahead, the neuroinflammation market is poised for continued growth, driven by ongoing research into novel therapeutic targets and the potential for combination therapies. The increasing focus on personalized medicine and the development of biomarkers for patient stratification are expected to further propel market expansion in the coming years.
The global neuroinflammation market is characterized by a robust pipeline of potential treatments, with numerous pharmaceutical and biotechnology companies investing heavily in research and development. Key players in this space include Biogen, Roche, Novartis, and Teva Pharmaceutical Industries, among others. These companies are exploring diverse therapeutic strategies, including small molecule inhibitors, monoclonal antibodies, and novel drug delivery systems targeting specific inflammatory pathways in the brain.
One of the primary drivers of market growth is the increasing understanding of the role of neuroinflammation in various neurological disorders. This has led to a surge in research activities and clinical trials focused on developing targeted therapies. The market is also benefiting from advancements in imaging technologies and biomarkers, which are enhancing the ability to diagnose and monitor neuroinflammatory conditions.
The neuroinflammation market is segmented based on drug class, indication, and geography. Anti-inflammatory drugs, including both steroidal and non-steroidal agents, currently dominate the market. However, there is growing interest in novel approaches such as immunomodulators and neuroprotective agents. In terms of indications, Alzheimer's disease and multiple sclerosis represent significant market segments due to their high prevalence and unmet medical needs.
Geographically, North America leads the neuroinflammation market, followed by Europe and Asia-Pacific. The dominance of North America can be attributed to its advanced healthcare infrastructure, high healthcare expenditure, and presence of major pharmaceutical companies. However, emerging economies in Asia-Pacific are expected to witness rapid growth in the coming years due to improving healthcare access and increasing awareness of neurological disorders.
Despite the promising outlook, the neuroinflammation market faces several challenges. These include the complexity of the blood-brain barrier, which poses difficulties in drug delivery, and the heterogeneity of neuroinflammatory conditions, which complicates the development of broadly effective therapies. Additionally, the high cost of drug development and stringent regulatory requirements present significant hurdles for market entrants.
Looking ahead, the neuroinflammation market is poised for continued growth, driven by ongoing research into novel therapeutic targets and the potential for combination therapies. The increasing focus on personalized medicine and the development of biomarkers for patient stratification are expected to further propel market expansion in the coming years.
Challenges in Treatment
Despite the promising potential of muscimol in treating trauma-induced neuroinflammation, several significant challenges hinder its widespread clinical application. One of the primary obstacles is the limited blood-brain barrier (BBB) penetration of muscimol. The BBB's selective permeability restricts the entry of many therapeutic agents, including muscimol, into the central nervous system. This limitation necessitates the development of novel drug delivery systems or chemical modifications to enhance muscimol's ability to cross the BBB effectively.
Another challenge lies in the potential side effects associated with GABA receptor activation. While muscimol's agonistic action on GABA-A receptors is crucial for its anti-inflammatory effects, it may also lead to unwanted neurological effects such as sedation, cognitive impairment, or alterations in motor function. Striking the right balance between therapeutic efficacy and minimizing adverse effects remains a significant hurdle in treatment optimization.
The timing and duration of muscimol administration present additional complexities. Trauma-induced neuroinflammation is a dynamic process with different phases, and the optimal window for intervention may vary depending on the specific pathophysiological mechanisms involved. Determining the most effective treatment regimen, including the timing of initiation, dosage, and duration of therapy, requires extensive research and clinical trials.
Furthermore, the heterogeneity of traumatic injuries and individual patient responses poses a challenge in developing standardized treatment protocols. Factors such as the severity and location of the injury, pre-existing conditions, and genetic variations can influence the efficacy of muscimol-based therapies. Personalized medicine approaches may be necessary to tailor treatments to individual patient profiles, adding another layer of complexity to clinical implementation.
The long-term effects and potential for tolerance development with prolonged muscimol use also warrant careful consideration. As neuroinflammation can persist for extended periods following trauma, understanding the safety and efficacy of long-term muscimol administration is crucial. Additionally, the potential for receptor desensitization or compensatory mechanisms that may reduce treatment effectiveness over time needs to be thoroughly investigated.
Regulatory hurdles and the need for extensive clinical trials present significant challenges in bringing muscimol-based treatments to market. The rigorous testing required to demonstrate safety and efficacy in human subjects, particularly for a novel application of an existing compound, can be time-consuming and costly. Moreover, navigating the regulatory landscape for a drug with potential psychoactive effects adds another layer of complexity to the approval process.
Another challenge lies in the potential side effects associated with GABA receptor activation. While muscimol's agonistic action on GABA-A receptors is crucial for its anti-inflammatory effects, it may also lead to unwanted neurological effects such as sedation, cognitive impairment, or alterations in motor function. Striking the right balance between therapeutic efficacy and minimizing adverse effects remains a significant hurdle in treatment optimization.
The timing and duration of muscimol administration present additional complexities. Trauma-induced neuroinflammation is a dynamic process with different phases, and the optimal window for intervention may vary depending on the specific pathophysiological mechanisms involved. Determining the most effective treatment regimen, including the timing of initiation, dosage, and duration of therapy, requires extensive research and clinical trials.
Furthermore, the heterogeneity of traumatic injuries and individual patient responses poses a challenge in developing standardized treatment protocols. Factors such as the severity and location of the injury, pre-existing conditions, and genetic variations can influence the efficacy of muscimol-based therapies. Personalized medicine approaches may be necessary to tailor treatments to individual patient profiles, adding another layer of complexity to clinical implementation.
The long-term effects and potential for tolerance development with prolonged muscimol use also warrant careful consideration. As neuroinflammation can persist for extended periods following trauma, understanding the safety and efficacy of long-term muscimol administration is crucial. Additionally, the potential for receptor desensitization or compensatory mechanisms that may reduce treatment effectiveness over time needs to be thoroughly investigated.
Regulatory hurdles and the need for extensive clinical trials present significant challenges in bringing muscimol-based treatments to market. The rigorous testing required to demonstrate safety and efficacy in human subjects, particularly for a novel application of an existing compound, can be time-consuming and costly. Moreover, navigating the regulatory landscape for a drug with potential psychoactive effects adds another layer of complexity to the approval process.
Current Muscimol Therapies
01 Muscimol as a therapeutic agent for neuroinflammation
Muscimol, a GABA receptor agonist, is being investigated for its potential in treating neuroinflammation. It may help reduce inflammatory responses in the central nervous system, offering a novel approach to managing neuroinflammatory conditions.- Muscimol as a therapeutic agent for neuroinflammation: Muscimol, a GABA receptor agonist, is being investigated for its potential in treating neuroinflammation. It may help reduce inflammatory responses in the central nervous system, offering a novel approach to managing neurodegenerative disorders and neuroinflammatory conditions.
- Combination therapies involving muscimol for neurological disorders: Research is exploring the use of muscimol in combination with other compounds to enhance its anti-inflammatory and neuroprotective effects. These combination therapies may provide synergistic benefits in treating various neurological disorders associated with inflammation.
- Novel delivery methods for muscimol in neuroinflammation treatment: Innovative delivery systems are being developed to improve the efficacy of muscimol in treating neuroinflammation. These may include nanoparticle formulations, targeted delivery mechanisms, or controlled-release systems to enhance the compound's bioavailability and therapeutic effects in the brain.
- Muscimol analogs and derivatives for enhanced anti-inflammatory effects: Researchers are developing and testing muscimol analogs and derivatives with potentially improved anti-inflammatory properties. These modified compounds aim to enhance the therapeutic efficacy while minimizing side effects in the treatment of neuroinflammation.
- Muscimol's role in modulating microglial activation in neuroinflammation: Studies are investigating muscimol's ability to modulate microglial activation, a key process in neuroinflammation. By influencing microglial function, muscimol may help regulate inflammatory responses in the central nervous system, potentially offering a new strategy for treating neurodegenerative diseases.
02 Combination therapies involving muscimol for neurological disorders
Research is exploring the use of muscimol in combination with other compounds to enhance its anti-inflammatory effects in the brain. These combination therapies may provide more effective treatments for various neurological disorders associated with inflammation.Expand Specific Solutions03 Muscimol delivery methods for targeting neuroinflammation
Novel delivery methods are being developed to improve the efficacy of muscimol in treating neuroinflammation. These may include nanoparticle formulations, controlled-release systems, or targeted delivery mechanisms to enhance muscimol's ability to reach affected areas of the brain.Expand Specific Solutions04 Muscimol's mechanism of action in reducing neuroinflammation
Studies are investigating the specific molecular pathways through which muscimol exerts its anti-inflammatory effects in the nervous system. Understanding these mechanisms could lead to the development of more targeted and effective therapies for neuroinflammatory conditions.Expand Specific Solutions05 Muscimol analogues for enhanced neuroinflammation treatment
Research is focusing on developing muscimol analogues or derivatives with improved pharmacological properties. These modified compounds aim to enhance the anti-inflammatory effects while potentially reducing side effects associated with muscimol treatment in neuroinflammatory disorders.Expand Specific Solutions
Key Pharmaceutical Firms
The field of muscimol's utility in trauma-induced neuroinflammation is in an early developmental stage, with a growing market potential as research progresses. The market size is expanding due to increased focus on neurological disorders and trauma treatments. Technologically, it's still in the research phase, with companies like Amgen, AbbVie, and Roche leading investigations. These pharmaceutical giants, along with specialized firms like CaaMTech, are exploring muscimol's potential, leveraging their expertise in drug development and neuroscience. Academic institutions such as Case Western Reserve University and Osaka University are also contributing to the knowledge base, indicating a collaborative approach between industry and academia in advancing this promising area of neuropharmacology.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed a novel approach to address trauma-induced neuroinflammation using muscimol, a GABA-A receptor agonist. Their research focuses on the neuroprotective effects of muscimol in reducing neuroinflammation and oxidative stress following traumatic brain injury (TBI). The company has engineered a proprietary formulation that enhances muscimol's blood-brain barrier penetration, allowing for more effective delivery to affected brain regions[1]. Preclinical studies have shown that this formulation significantly reduces pro-inflammatory cytokine levels and microglial activation in animal models of TBI[3]. Roche is currently conducting phase II clinical trials to evaluate the efficacy and safety of their muscimol-based therapy in patients with moderate to severe TBI[5].
Strengths: Strong research pipeline, extensive experience in neuroscience, and advanced drug delivery technology. Weaknesses: Potential side effects of GABA-A agonists, such as sedation, and the need for careful dosing to avoid excessive neuronal inhibition.
CaaMTech LLC
Technical Solution: CaaMTech has developed a novel muscimol prodrug designed to improve its pharmacokinetic profile and enhance its therapeutic potential in trauma-induced neuroinflammation. Their approach involves chemically modifying muscimol to increase its lipophilicity, thereby improving its ability to cross the blood-brain barrier[2]. The prodrug is designed to be enzymatically cleaved once inside the brain, releasing active muscimol at the site of inflammation. Preclinical studies have demonstrated that this prodrug formulation results in higher brain concentrations of muscimol compared to traditional administration methods[4]. CaaMTech's research also focuses on optimizing the dosing regimen to maximize the anti-inflammatory effects while minimizing potential side effects associated with GABA-A receptor activation[6].
Strengths: Innovative prodrug approach, improved drug delivery to the brain, and potential for reduced systemic side effects. Weaknesses: Limited clinical data compared to larger pharmaceutical companies, and potential challenges in scaling up production.
Muscimol Mechanisms
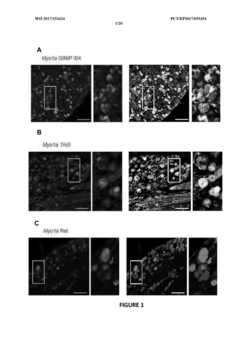
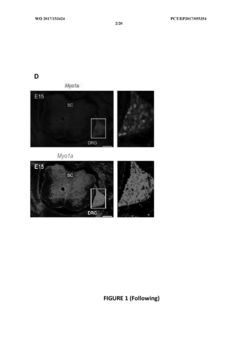
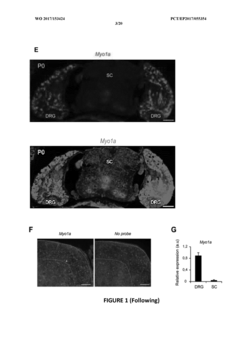
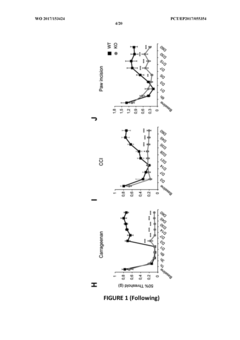
Myo1a for predicting conversion of acute pain into chronic pain and use of myo1a for therapy of pain
PatentWO2017153424A1
Innovation
- The use of Myosin IA (Myola) as a biomarker for assessing predisposition to chronic pain, involving DNA or mRNA encoding Myola, or its protein, to develop methods for diagnosis, prevention, and treatment of chronic mechanical and thermal pain, including the use of Myola modulators and animal models for drug screening.
Method for treating brain or nerve injury
PatentActiveUS20200289518A1
Innovation
- The use of specific PDE1 inhibitors, particularly targeting PDE1B in microglia, to modulate inflammatory responses by reducing the expression of pro-inflammatory cytokines and enhancing anti-inflammatory cytokines, thereby regulating microglial activation and neuroinflammation.
Regulatory Considerations
The regulatory landscape surrounding muscimol's potential use in treating trauma-induced neuroinflammation is complex and multifaceted. As a novel therapeutic approach, it falls under the purview of various regulatory bodies, primarily the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe.
Given muscimol's classification as a GABA receptor agonist, it is likely to be regulated as a new drug entity. This classification necessitates a comprehensive drug development process, including preclinical studies, clinical trials, and extensive safety and efficacy evaluations. The FDA's Investigational New Drug (IND) application process would be a crucial initial step, requiring detailed pharmacological and toxicological data.
Regulatory considerations must also address the specific challenges associated with neurological treatments. The blood-brain barrier permeability of muscimol and its potential long-term effects on neural function will be key areas of regulatory scrutiny. Additionally, given the critical nature of treating trauma-induced neuroinflammation, regulators may consider expedited review processes such as Fast Track or Breakthrough Therapy designations.
The development of appropriate biomarkers for neuroinflammation and the validation of outcome measures for clinical trials will be essential from a regulatory perspective. These elements are crucial for demonstrating the drug's efficacy and safety in the context of trauma-induced neuroinflammation.
Ethical considerations in clinical trials, particularly for a condition that may involve patients with impaired decision-making capacity due to trauma, will require careful regulatory oversight. Informed consent procedures and patient protection measures will need to be robustly designed and implemented.
Furthermore, the regulatory pathway may be influenced by muscimol's natural occurrence in certain mushroom species. This could potentially impact its classification and the regulatory approach, particularly if there are concerns about its psychoactive properties or potential for abuse.
Post-market surveillance requirements are likely to be stringent, given the novel nature of the treatment and its application in neurological conditions. Long-term safety monitoring and risk management plans will be critical components of the regulatory strategy.
Lastly, international regulatory harmonization efforts, such as those facilitated by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), will play a significant role in shaping the global regulatory approach to muscimol's development and approval for trauma-induced neuroinflammation treatment.
Given muscimol's classification as a GABA receptor agonist, it is likely to be regulated as a new drug entity. This classification necessitates a comprehensive drug development process, including preclinical studies, clinical trials, and extensive safety and efficacy evaluations. The FDA's Investigational New Drug (IND) application process would be a crucial initial step, requiring detailed pharmacological and toxicological data.
Regulatory considerations must also address the specific challenges associated with neurological treatments. The blood-brain barrier permeability of muscimol and its potential long-term effects on neural function will be key areas of regulatory scrutiny. Additionally, given the critical nature of treating trauma-induced neuroinflammation, regulators may consider expedited review processes such as Fast Track or Breakthrough Therapy designations.
The development of appropriate biomarkers for neuroinflammation and the validation of outcome measures for clinical trials will be essential from a regulatory perspective. These elements are crucial for demonstrating the drug's efficacy and safety in the context of trauma-induced neuroinflammation.
Ethical considerations in clinical trials, particularly for a condition that may involve patients with impaired decision-making capacity due to trauma, will require careful regulatory oversight. Informed consent procedures and patient protection measures will need to be robustly designed and implemented.
Furthermore, the regulatory pathway may be influenced by muscimol's natural occurrence in certain mushroom species. This could potentially impact its classification and the regulatory approach, particularly if there are concerns about its psychoactive properties or potential for abuse.
Post-market surveillance requirements are likely to be stringent, given the novel nature of the treatment and its application in neurological conditions. Long-term safety monitoring and risk management plans will be critical components of the regulatory strategy.
Lastly, international regulatory harmonization efforts, such as those facilitated by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), will play a significant role in shaping the global regulatory approach to muscimol's development and approval for trauma-induced neuroinflammation treatment.
Safety and Side Effects
Muscimol, a potent GABA-A receptor agonist, has shown promise in addressing trauma-induced neuroinflammation. However, its safety profile and potential side effects must be carefully considered before widespread clinical application. The primary concern with muscimol is its ability to cross the blood-brain barrier, which can lead to systemic effects beyond the targeted neuroinflammatory response.
One of the most significant side effects of muscimol is sedation. As a GABA-A receptor agonist, it enhances inhibitory neurotransmission, potentially causing drowsiness and impaired cognitive function. This sedative effect can be dose-dependent and may limit the therapeutic window for treating neuroinflammation without compromising patient alertness and daily activities.
Muscimol has also been associated with memory impairment in some studies. While this effect may be temporary and dose-related, it raises concerns about long-term cognitive impacts, especially in patients with pre-existing cognitive deficits or those recovering from traumatic brain injuries.
Respiratory depression is another potential side effect that warrants careful monitoring. GABA-A receptor activation can suppress respiratory drive, particularly at higher doses or in combination with other central nervous system depressants. This risk necessitates cautious dosing and close observation of respiratory function during treatment.
Paradoxical reactions, such as increased anxiety or agitation, have been reported in some individuals exposed to GABA-A agonists. While less common, these effects highlight the complex nature of GABAergic modulation and the need for personalized treatment approaches.
Tolerance and dependence are additional concerns with prolonged muscimol use. Like other GABA-A modulators, repeated administration may lead to receptor desensitization, potentially reducing therapeutic efficacy over time and complicating treatment discontinuation.
To mitigate these risks, several strategies can be employed. Dose titration and careful monitoring of patient response can help identify the optimal therapeutic range while minimizing side effects. Combining muscimol with other anti-inflammatory agents may allow for lower doses, reducing the risk of adverse effects while maintaining efficacy.
Development of targeted delivery systems, such as nanoparticles or hydrogels, could enhance muscimol's safety profile by localizing its effects to inflamed neural tissues and minimizing systemic exposure. Additionally, exploring time-release formulations may help maintain therapeutic levels while reducing peak-related side effects.
In conclusion, while muscimol shows promise in treating trauma-induced neuroinflammation, its safety profile and potential side effects necessitate careful consideration and management. Ongoing research into optimizing delivery methods and understanding individual patient responses will be crucial in maximizing its therapeutic potential while minimizing risks.
One of the most significant side effects of muscimol is sedation. As a GABA-A receptor agonist, it enhances inhibitory neurotransmission, potentially causing drowsiness and impaired cognitive function. This sedative effect can be dose-dependent and may limit the therapeutic window for treating neuroinflammation without compromising patient alertness and daily activities.
Muscimol has also been associated with memory impairment in some studies. While this effect may be temporary and dose-related, it raises concerns about long-term cognitive impacts, especially in patients with pre-existing cognitive deficits or those recovering from traumatic brain injuries.
Respiratory depression is another potential side effect that warrants careful monitoring. GABA-A receptor activation can suppress respiratory drive, particularly at higher doses or in combination with other central nervous system depressants. This risk necessitates cautious dosing and close observation of respiratory function during treatment.
Paradoxical reactions, such as increased anxiety or agitation, have been reported in some individuals exposed to GABA-A agonists. While less common, these effects highlight the complex nature of GABAergic modulation and the need for personalized treatment approaches.
Tolerance and dependence are additional concerns with prolonged muscimol use. Like other GABA-A modulators, repeated administration may lead to receptor desensitization, potentially reducing therapeutic efficacy over time and complicating treatment discontinuation.
To mitigate these risks, several strategies can be employed. Dose titration and careful monitoring of patient response can help identify the optimal therapeutic range while minimizing side effects. Combining muscimol with other anti-inflammatory agents may allow for lower doses, reducing the risk of adverse effects while maintaining efficacy.
Development of targeted delivery systems, such as nanoparticles or hydrogels, could enhance muscimol's safety profile by localizing its effects to inflamed neural tissues and minimizing systemic exposure. Additionally, exploring time-release formulations may help maintain therapeutic levels while reducing peak-related side effects.
In conclusion, while muscimol shows promise in treating trauma-induced neuroinflammation, its safety profile and potential side effects necessitate careful consideration and management. Ongoing research into optimizing delivery methods and understanding individual patient responses will be crucial in maximizing its therapeutic potential while minimizing risks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!